Abstract
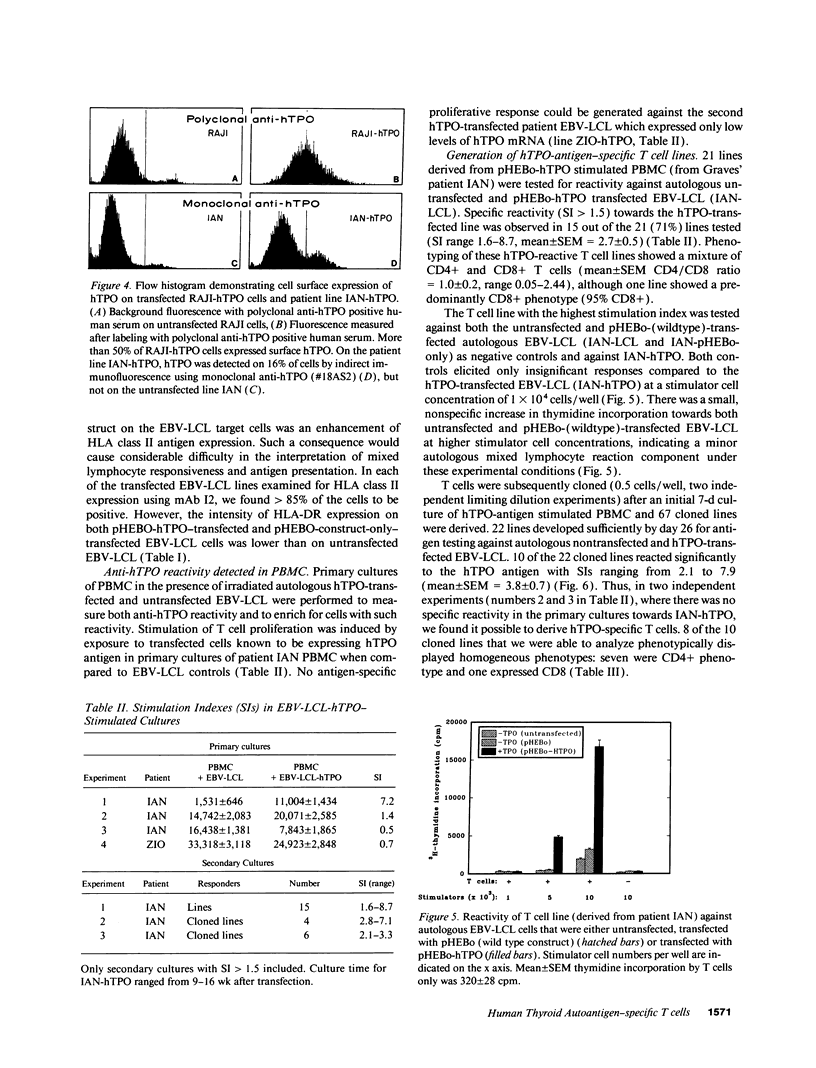
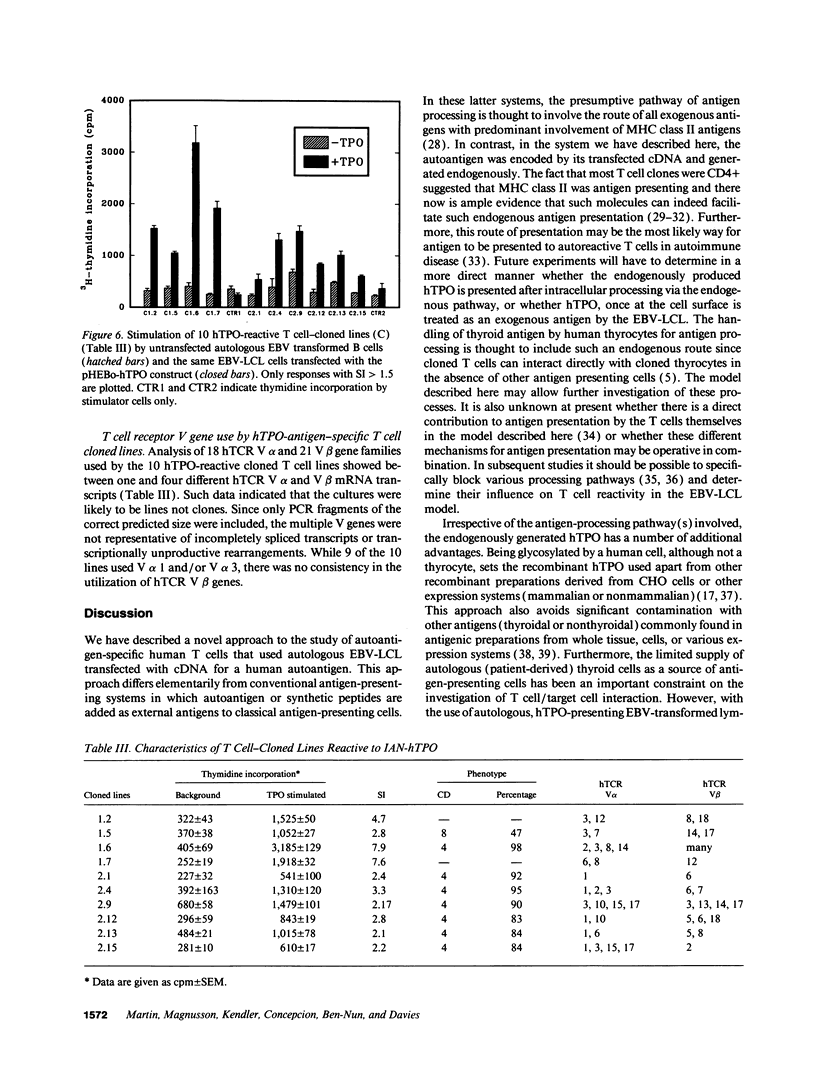
To develop a model for endogenous thyroid autoantigen presentation, we transfected EBV-transformed B lymphoblastoid cell lines (EBV-LCL), established from patients with autoimmune thyroid disease and normal controls, with cDNA for the human thyroid autoantigen thyroid peroxidase (hTPO). hTPO-antigen presentation to patient peripheral blood T cells was demonstrated after stimulation in vitro for 7 d with irradiated hTPO-transfected or untransfected autologous EBV-LCL. Anti-hTPO-reactive T cells were subsequently cloned in the presence of irradiated, autologous hTPO-transfected EBV-LCL and IL-2.10 T cell-cloned lines exhibited specific hTPO-induced proliferation (stimulation indices of 2.1-7.9) towards autologous hTPO-transfected EBV-LCL, and were subjected to human T cell receptor (hTCR) V gene analysis, using the PCR for the detection of V alpha and V beta hTcR gene families. The results indicated a preferential use of hTCR V alpha 1 and/or V alpha 3 in 9 of the 10 lines. In contrast, hTCR V beta gene family use was more variable. These data demonstrate a model for the endogenous presentation of human thyroid peroxidase in the absence of other thyroid specific antigens. The high frequency of antigen-specific T cells obtained from PBMC using this technique will facilitate further studies at both the functional and hTCR V gene level.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorini L., Moreno J., Momburg F., Hämmerling G. J., Guéry J. C., Valli A., Fuchs S. Exogenous peptides compete for the presentation of endogenous antigens to major histocompatibility complex class II-restricted T cells. J Exp Med. 1991 Oct 1;174(4):945–948. doi: 10.1084/jem.174.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adorini L., Ullrich S. J., Appella E., Fuchs S. Inhibition by brefeldin A of presentation of exogenous protein antigens to MHC class II-restricted T cells. Nature. 1990 Jul 5;346(6279):63–66. doi: 10.1038/346063a0. [DOI] [PubMed] [Google Scholar]
- Altmann D. M., Hogg N., Trowsdale J., Wilkinson D. Cotransfection of ICAM-1 and HLA-DR reconstitutes human antigen-presenting cell function in mouse L cells. Nature. 1989 Apr 6;338(6215):512–514. doi: 10.1038/338512a0. [DOI] [PubMed] [Google Scholar]
- Ben-Nun A., Liblau R. S., Cohen L., Lehmann D., Tournier-Lasserve E., Rosenzweig A., Zhang J. W., Raus J. C., Bach M. A. Restricted T-cell receptor V beta gene usage by myelin basic protein-specific T-cell clones in multiple sclerosis: predominant genes vary in individuals. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2466–2470. doi: 10.1073/pnas.88.6.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottazzo G. F., Pujol-Borrell R., Hanafusa T., Feldmann M. Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet. 1983 Nov 12;2(8359):1115–1119. doi: 10.1016/s0140-6736(83)90629-3. [DOI] [PubMed] [Google Scholar]
- Brooks A., Hartley S., Kjer-Nielsen L., Perera J., Goodnow C. C., Basten A., McCluskey J. Class II-restricted presentation of an endogenously derived immunodominant T-cell determinant of hen egg lysozyme. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3290–3294. doi: 10.1073/pnas.88.8.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cone R. D., Platzer M., Piccinini L. A., Jaramillo M., Davies T. F. HLA-DR gene expression in a proliferating human thyroid cell clone (12S). Endocrinology. 1988 Oct;123(4):2067–2074. doi: 10.1210/endo-123-4-2067. [DOI] [PubMed] [Google Scholar]
- Czarnocka B., Ruf J., Ferrand M., Carayon P., Lissitzky S. Purification of the human thyroid peroxidase and its identification as the microsomal antigen involved in autoimmune thyroid diseases. FEBS Lett. 1985 Oct 7;190(1):147–152. doi: 10.1016/0014-5793(85)80446-4. [DOI] [PubMed] [Google Scholar]
- Davies T. F., Martin A., Concepcion E. S., Graves P., Cohen L., Ben-Nun A. Evidence of limited variability of antigen receptors on intrathyroidal T cells in autoimmune thyroid disease. N Engl J Med. 1991 Jul 25;325(4):238–244. doi: 10.1056/NEJM199107253250404. [DOI] [PubMed] [Google Scholar]
- Davies T. F., Martin A., Concepcion E. S., Graves P., Lahat N., Cohen W. L., Ben-Nun A. Evidence for selective accumulation of intrathyroidal T lymphocytes in human autoimmune thyroid disease based on T cell receptor V gene usage. J Clin Invest. 1992 Jan;89(1):157–162. doi: 10.1172/JCI115556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies T. F., Martin A., Graves P. Human autoimmune thyroid disease: cellular and molecular aspects. Baillieres Clin Endocrinol Metab. 1988 Nov;2(4):911–939. doi: 10.1016/s0950-351x(88)80024-7. [DOI] [PubMed] [Google Scholar]
- Dayan C. M., Londei M., Corcoran A. E., Grubeck-Loebenstein B., James R. F., Rapoport B., Feldmann M. Autoantigen recognition by thyroid-infiltrating T cells in Graves disease. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7415–7419. doi: 10.1073/pnas.88.16.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Staunton D. E., Springer T. A. Supergene families meet in the immune system. Immunol Today. 1988 Jul-Aug;9(7-8):213–215. doi: 10.1016/0167-5699(88)91216-9. [DOI] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Fisfalen M. E., DeGroot L. J., Quintans J., Franklin W. A., Soltani K. Microsomal antigen-reactive lymphocyte lines and clones derived from thyroid tissue of patients with Graves' disease. J Clin Endocrinol Metab. 1988 Apr;66(4):776–784. doi: 10.1210/jcem-66-4-776. [DOI] [PubMed] [Google Scholar]
- Fowler P. D., Tacker M., Whitley G. S., Meager A., Nussey S. S., Johnstone A. P. Expression of intercellular adhesion molecule-1 (ICAM-1) on human thyroid cells lines correlated with their binding of lymphoblasts. Mol Cell Endocrinol. 1990 May 28;71(1):55–61. doi: 10.1016/0303-7207(90)90075-j. [DOI] [PubMed] [Google Scholar]
- Hamada N., Grimm C., Mori H., DeGroot L. J. Identification of a thyroid microsomal antigen by Western blot and immunoprecipitation. J Clin Endocrinol Metab. 1985 Jul;61(1):120–128. doi: 10.1210/jcem-61-1-120. [DOI] [PubMed] [Google Scholar]
- Issekutz T., Chu E., Geha R. S. Antigen presentation by human B cells: T cell proliferation induced by Epstein Barr virus B lymphoblastoid cells. J Immunol. 1982 Oct;129(4):1446–1450. [PubMed] [Google Scholar]
- Jacobson S., Sekaly R. P., Jacobson C. L., McFarland H. F., Long E. O. HLA class II-restricted presentation of cytoplasmic measles virus antigens to cytotoxic T cells. J Virol. 1989 Apr;63(4):1756–1762. doi: 10.1128/jvi.63.4.1756-1762.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Shih W. K., Berkower I. Human T cell response to the surface antigen of hepatitis B virus (HBsAg). Endosomal and nonendosomal processing pathways are accessible to both endogenous and exogenous antigen. J Exp Med. 1988 Jul 1;168(1):293–306. doi: 10.1084/jem.168.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenty G., Michel-Bechet M., Charreire J. Monoclonal human thyroid cell line GEJ expressing human thyrotropin receptors. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2120–2124. doi: 10.1073/pnas.82.7.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman K. D., Rapoport B., Seto P., Chazenbalk G. D., Magnusson R. P. Generation of recombinant, enzymatically active human thyroid peroxidase and its recognition by antibodies in the sera of patients with Hashimoto's thyroiditis. J Clin Invest. 1989 Aug;84(2):394–403. doi: 10.1172/JCI114179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler D. L., Martin A., Magnusson R. P., Davies T. F. Detection of autoantibodies to recombinant human thyroid peroxidase by sensitive enzyme immunoassay. Clin Endocrinol (Oxf) 1990 Dec;33(6):751–760. doi: 10.1111/j.1365-2265.1990.tb03912.x. [DOI] [PubMed] [Google Scholar]
- Kendler D. L., Rootman J., Huber G. K., Davies T. F. A 64 kDa membrane antigen is a recurrent epitope for natural autoantibodies in patients with Graves' thyroid and ophthalmic diseases. Clin Endocrinol (Oxf) 1991 Dec;35(6):539–547. doi: 10.1111/j.1365-2265.1991.tb00941.x. [DOI] [PubMed] [Google Scholar]
- Kimura H., Davies T. F. Thyroid-specific T cells in the normal Wistar rat. II. T cell clones interact with cloned wistar rat thyroid cells and provide direct evidence for autoantigen presentation by thyroid epithelial cells. Clin Immunol Immunopathol. 1991 Feb;58(2):195–206. doi: 10.1016/0090-1229(91)90136-x. [DOI] [PubMed] [Google Scholar]
- LaSalle J. M., Ota K., Hafler D. A. Presentation of autoantigen by human T cells. J Immunol. 1991 Aug 1;147(3):774–780. [PubMed] [Google Scholar]
- Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985 Apr 11;314(6011):537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- Londei M., Bottazzo G. F., Feldmann M. Human T-cell clones from autoimmune thyroid glands: specific recognition of autologous thyroid cells. Science. 1985 Apr 5;228(4695):85–89. doi: 10.1126/science.3871967. [DOI] [PubMed] [Google Scholar]
- Lotteau V., Teyton L., Peleraux A., Nilsson T., Karlsson L., Schmid S. L., Quaranta V., Peterson P. A. Intracellular transport of class II MHC molecules directed by invariant chain. Nature. 1990 Dec 13;348(6302):600–605. doi: 10.1038/348600a0. [DOI] [PubMed] [Google Scholar]
- Ludgate M., Vassart G. The molecular genetics of three thyroid autoantigens: thyroglobulin, thyroid peroxidase and the thyrotropin receptor. Autoimmunity. 1990;7(2-3):201–211. doi: 10.3109/08916939008993392. [DOI] [PubMed] [Google Scholar]
- MacKenzie W. A., Schwartz A. E., Friedman E. W., Davies T. F. Intrathyroidal T cell clones from patients with autoimmune thyroid disease. J Clin Endocrinol Metab. 1987 Apr;64(4):818–824. doi: 10.1210/jcem-64-4-818. [DOI] [PubMed] [Google Scholar]
- Mackenzie W. A., Davies T. F. An intrathyroidal T-cell clone specifically cytotoxic for human thyroid cells. Immunology. 1987 May;61(1):101–103. [PMC free article] [PubMed] [Google Scholar]
- Martin A., Huber G. K., Davies T. F. Induction of human thyroid cell ICAM-1 (CD54) antigen expression and ICAM-1-mediated lymphocyte binding. Endocrinology. 1990 Aug;127(2):651–657. doi: 10.1210/endo-127-2-651. [DOI] [PubMed] [Google Scholar]
- Martin A., Platzer M., Davies T. F. Retention of cyclic AMP response to TSH in a cloned human thyrocyte/T cell hybridoma (HY2-15). Mol Cell Endocrinol. 1988 Dec;60(2-3):233–238. doi: 10.1016/0303-7207(88)90183-9. [DOI] [PubMed] [Google Scholar]
- Martin A., Schwartz A. E., Friedman E. W., Davies T. F. Successful production of intrathyroidal human T cell hybridomas: evidence for intact helper T cell function in Graves' disease. J Clin Endocrinol Metab. 1989 Dec;69(6):1104–1108. doi: 10.1210/jcem-69-6-1104. [DOI] [PubMed] [Google Scholar]
- Mueller D. L., Jenkins M. K., Schwartz R. H. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- Piccinini L. A., Goldsmith N. K., Roman S. H., Davies T. F. HLA-DP, DQ and DR gene expression in Graves' disease and normal thyroid epithelium. Tissue Antigens. 1987 Oct;30(4):145–154. doi: 10.1111/j.1399-0039.1987.tb01613.x. [DOI] [PubMed] [Google Scholar]
- Piccinini L. A., Goldsmith N. K., Schachter B. S., Davies T. F. Localization of HLA-DR alpha-chain messenger ribonucleic acid in normal and autoimmune human thyroid using in situ hybridization. J Clin Endocrinol Metab. 1988 Jun;66(6):1307–1315. doi: 10.1210/jcem-66-6-1307. [DOI] [PubMed] [Google Scholar]
- Rees Smith B., McLachlan S. M., Furmaniak J. Autoantibodies to the thyrotropin receptor. Endocr Rev. 1988 Feb;9(1):106–121. doi: 10.1210/edrv-9-1-106. [DOI] [PubMed] [Google Scholar]
- Reid P. A., Watts C. Cycling of cell-surface MHC glycoproteins through primaquine-sensitive intracellular compartments. Nature. 1990 Aug 16;346(6285):655–657. doi: 10.1038/346655a0. [DOI] [PubMed] [Google Scholar]
- Roep B. O., Arden S. D., de Vries R. R., Hutton J. C. T-cell clones from a type-1 diabetes patient respond to insulin secretory granule proteins. Nature. 1990 Jun 14;345(6276):632–634. doi: 10.1038/345632a0. [DOI] [PubMed] [Google Scholar]
- Sugden B., Marsh K., Yates J. A vector that replicates as a plasmid and can be efficiently selected in B-lymphoblasts transformed by Epstein-Barr virus. Mol Cell Biol. 1985 Feb;5(2):410–413. doi: 10.1128/mcb.5.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weetman A. P., Cohen S., Makgoba M. W., Borysiewicz L. K. Expression of an intercellular adhesion molecule, ICAM-1, by human thyroid cells. J Endocrinol. 1989 Jul;122(1):185–191. doi: 10.1677/joe.0.1220185. [DOI] [PubMed] [Google Scholar]
- Weetman A. P., Freeman M., Borysiewicz L. K., Makgoba M. W. Functional analysis of intercellular adhesion molecule-1-expressing human thyroid cells. Eur J Immunol. 1990 Feb;20(2):271–275. doi: 10.1002/eji.1830200207. [DOI] [PubMed] [Google Scholar]
- Weiss S., Bogen B. MHC class II-restricted presentation of intracellular antigen. Cell. 1991 Feb 22;64(4):767–776. doi: 10.1016/0092-8674(91)90506-t. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig K. W., Ota K., Endo N., Seidman J. G., Rosenzweig A., Weiner H. L., Hafler D. A. Shared human T cell receptor V beta usage to immunodominant regions of myelin basic protein. Science. 1990 May 25;248(4958):1016–1019. doi: 10.1126/science.1693015. [DOI] [PubMed] [Google Scholar]
- Zeevi A., Duquesnoy R. J. Specificity of alloactivated human T lymphocyte clones in secondary proliferation, cell-mediated lympholysis and interleukin-2 release. J Immunogenet. 1985 Feb;12(1):17–31. doi: 10.1111/j.1744-313x.1985.tb00826.x. [DOI] [PubMed] [Google Scholar]