Abstract
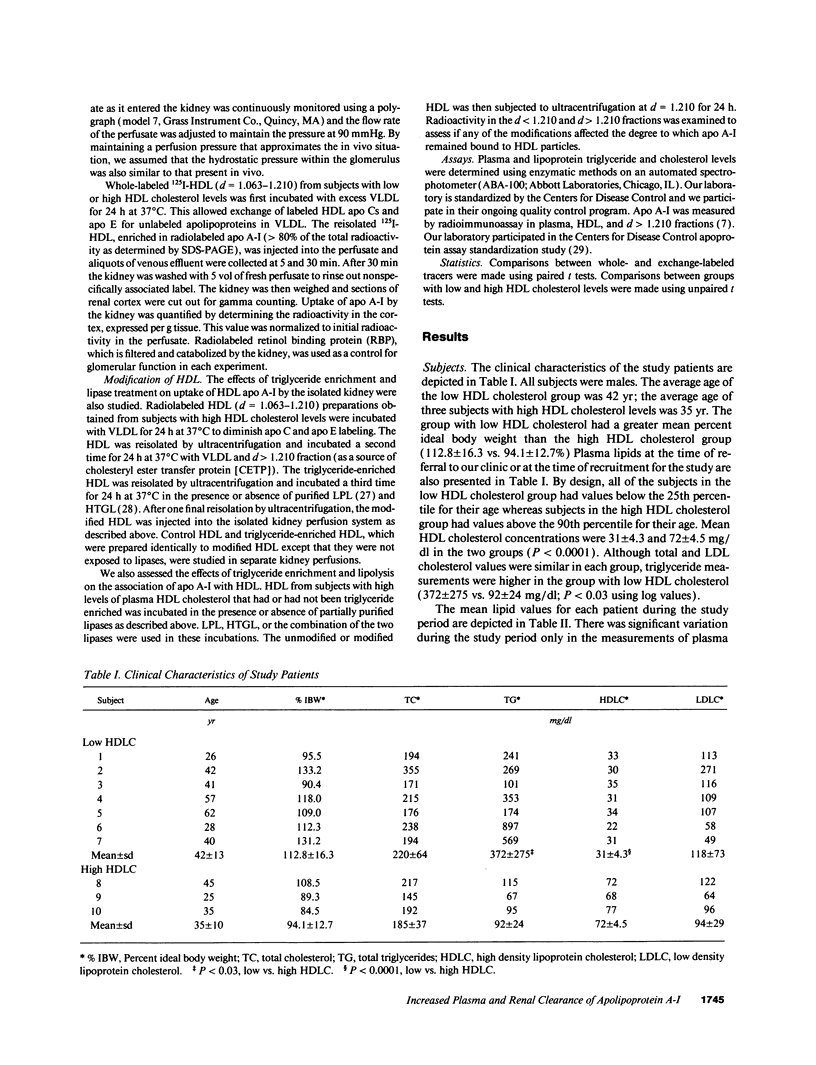
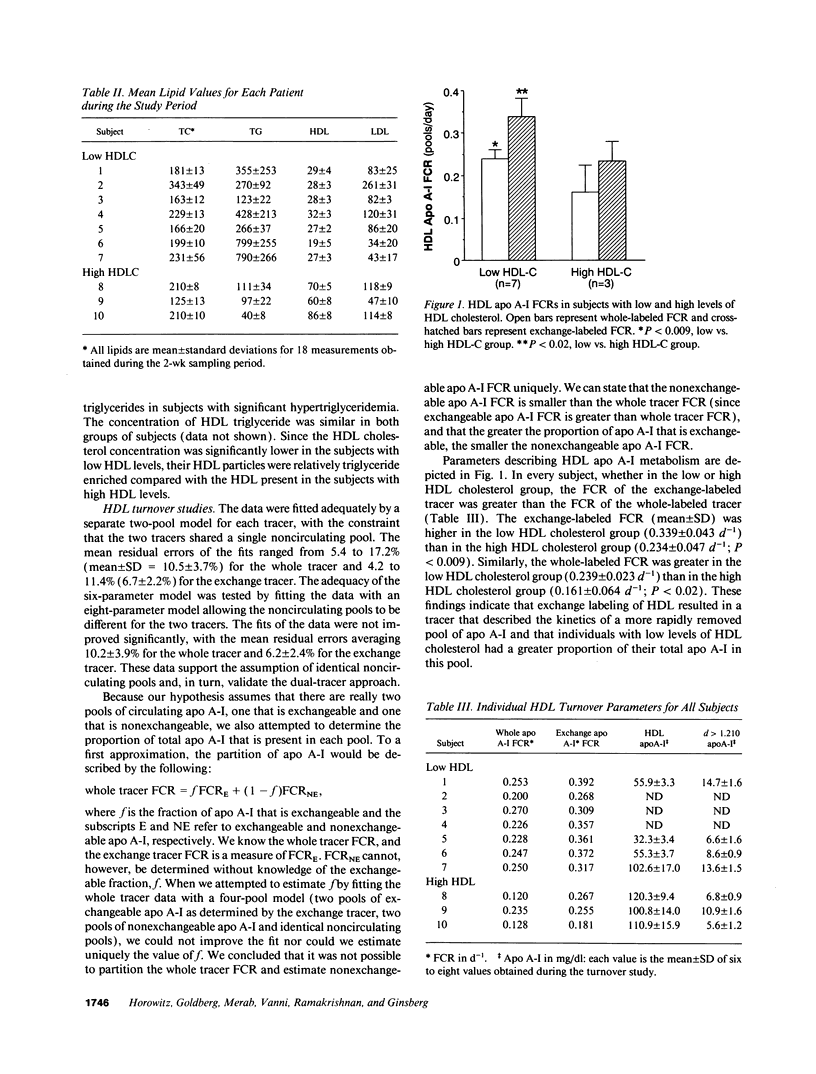
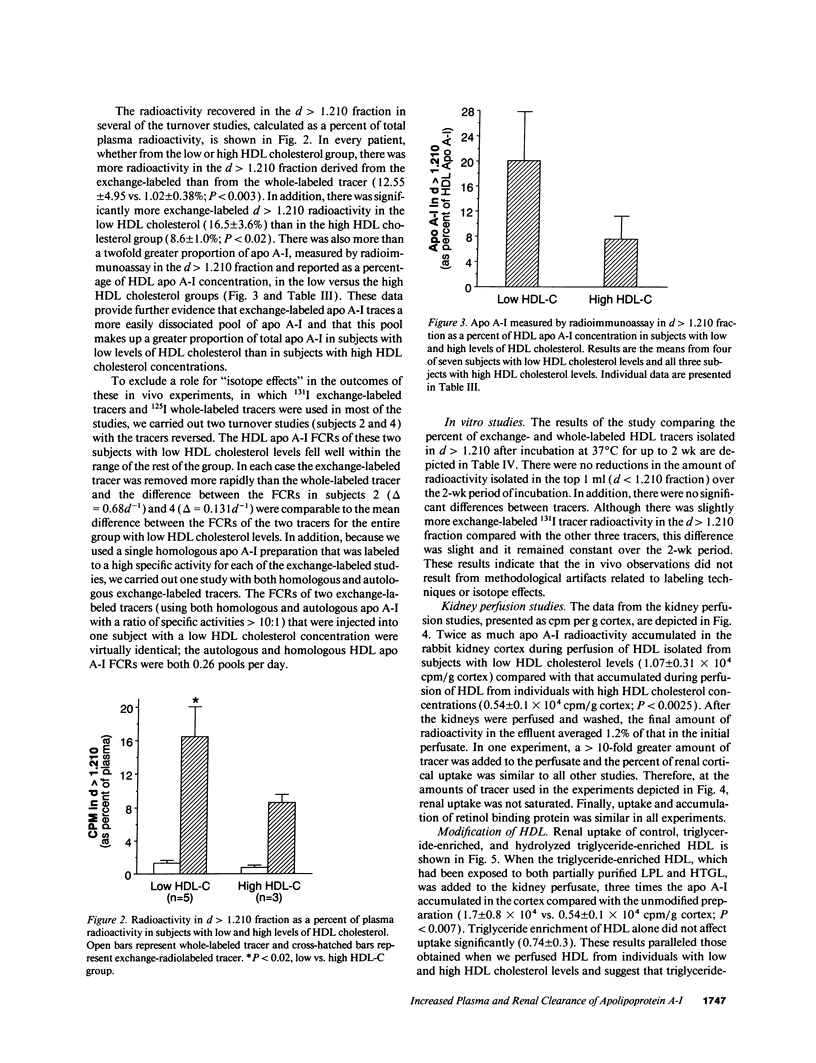
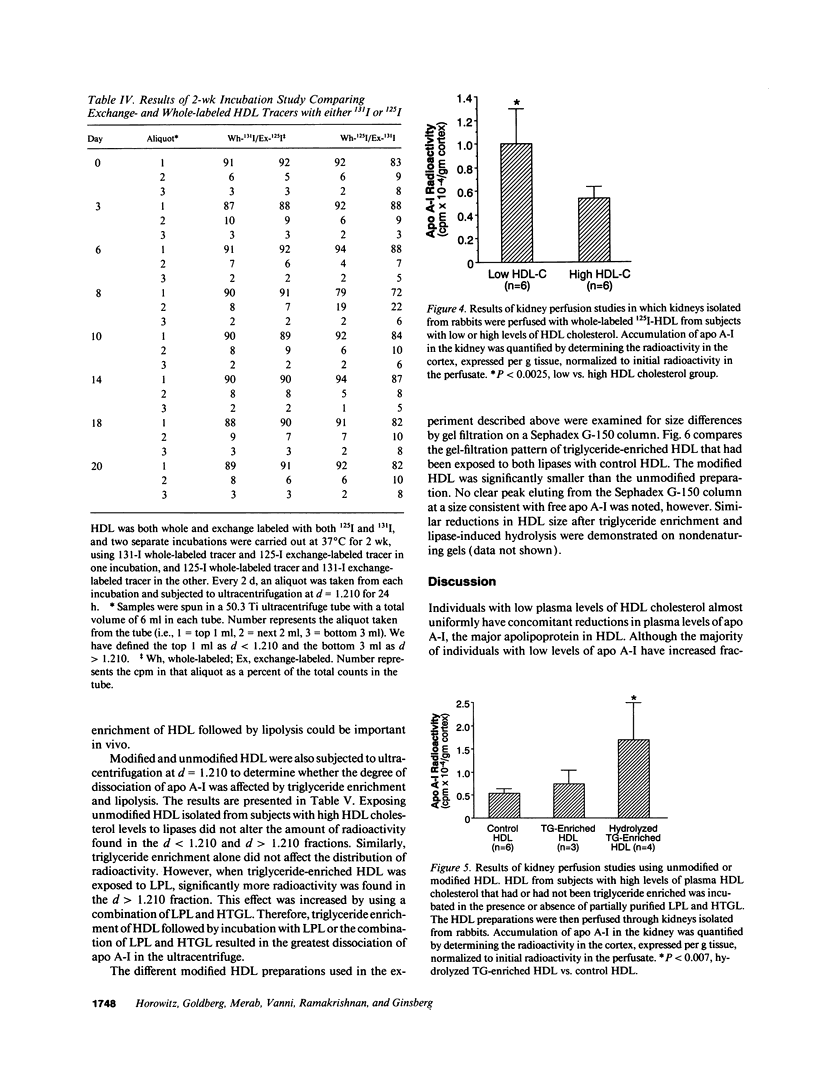
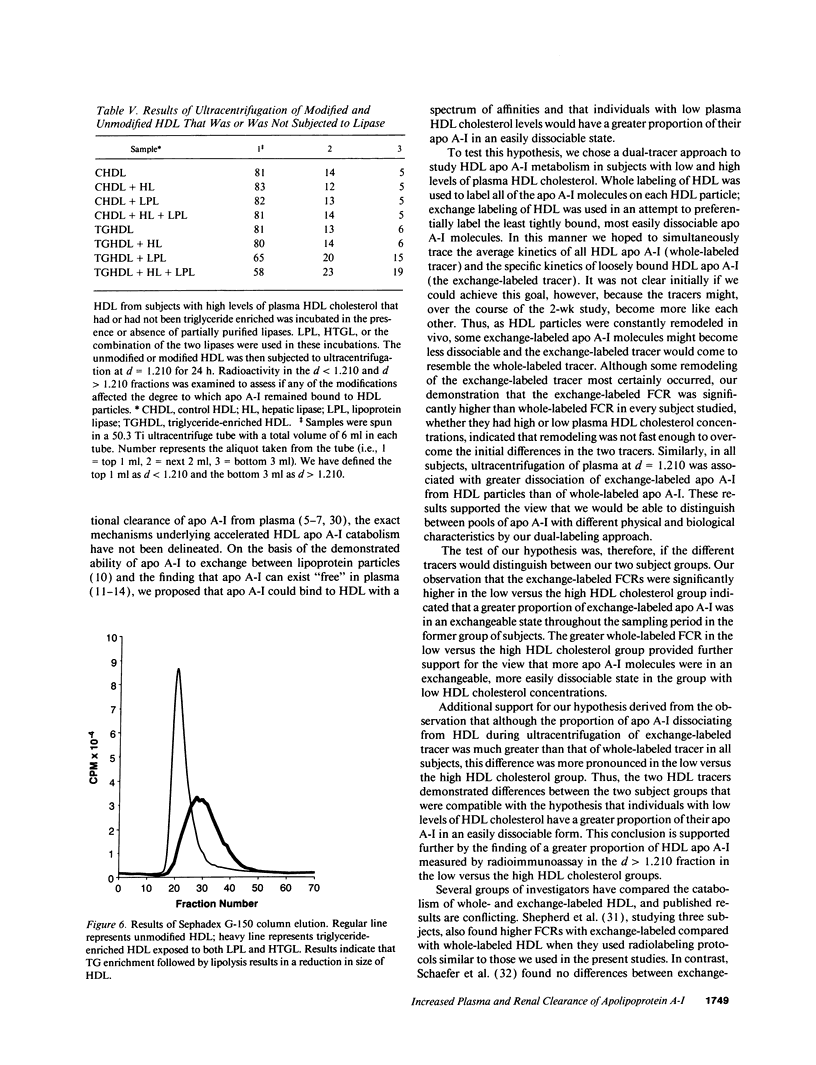
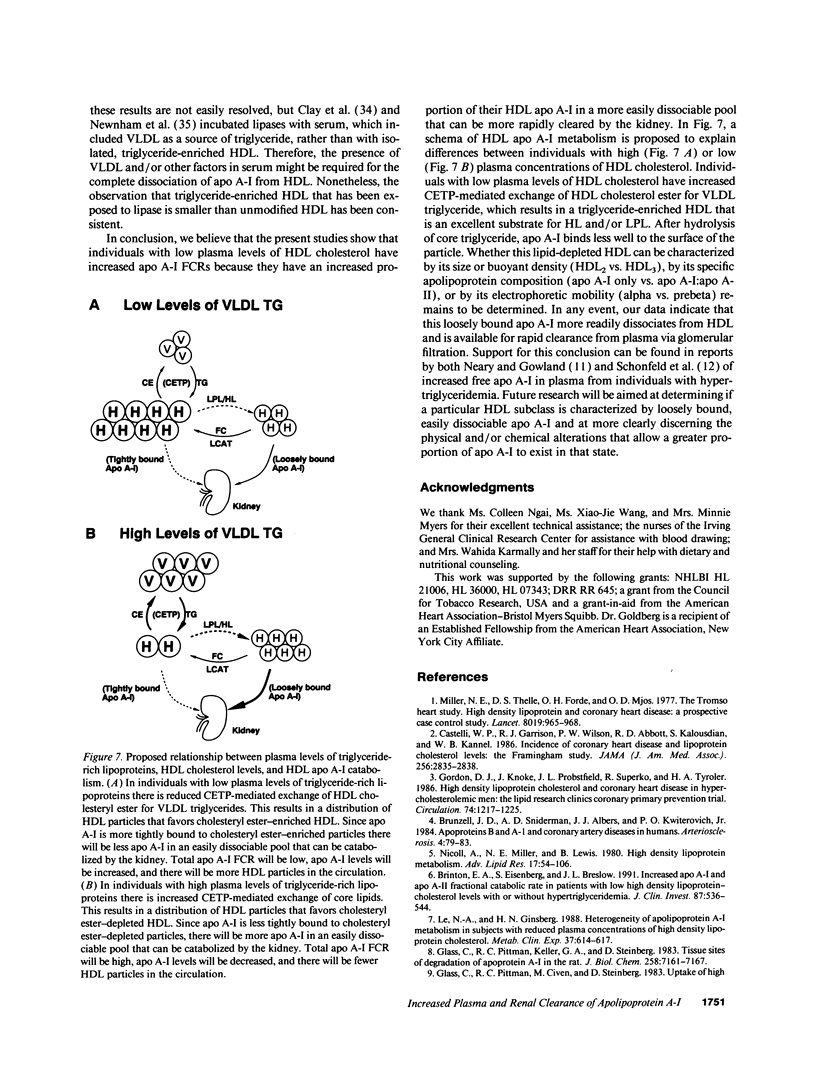
Plasma levels of HDL apo A-I are reduced in individuals with low HDL cholesterol (HDL-C) concentrations as a result of increased fractional catabolic rates (FCRs). To determine the basis for the high apo A-I FCRs, seven subjects with low HDL-C levels (31.0 +/- 4.3 mg/dl) were compared with three subjects with high HDL-C levels (72.0 +/- 4.5 mg/dl). Each subject received autologous HDL that was labeled directly by the iodine-monochloride method (whole-labeled) and autologous HDL that was labeled by exchange with homologous radiolabeled apo A-I (exchange-labeled). Blood was obtained for 2 wk, specific activities determined, and FCRs (d-1 +/- SD) estimated. In every subject, whether in the low or high HDL-C group, the exchange-labeled FCR was greater than the whole-labeled FCR. The exchange-labeled FCR was higher in the low HDL-C group (0.339 +/- 0.043) versus the high HDL-C group (0.234 +/- 0.047; P < 0.009). The whole-labeled FCR was also greater in the low HDL-C group (0.239 +/- 0.023) versus the high HDL-C group (0.161 +/- 0.064; P < 0.02). In addition, in both low and high HDL groups ultracentrifugation resulted in more radioactivity in d > 1.210 (as percentage of total plasma counts per minute) with the exchange-labeled tracer than with the whole-labeled tracer (12.55 +/- 4.95% vs. 1.02 +/- 0.38%; P < 0.003). With both HDL tracers, more radioactivity was found in d > 1.210 in the low versus the high HDL-C groups. When apo A-I catabolism was studied by perfusing isolated rabbit kidneys with whole-labeled HDL, there was twice as much accumulation (cpm/g cortex) of HDL apo A-I isolated from subjects with low HDL-C than from subjects with high HDL-C (P < 0.0025). Finally, HDL that had been isolated from subjects with high levels of HDL-C was triglyceride enriched and exposed to partially purified lipases before perfusion through kidneys. Threefold more apo A-I from modified HDL accumulated in the cortex compared with the unmodified preparation (P < 0.007). The results of these in vivo and ex vivo studies indicate that individuals with low HDL-C levels have more loosely bound, easily exchanged apo A-I and that this exchangeable apo A-I is more readily cleared by the kidney.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinton E. A., Eisenberg S., Breslow J. L. Elevated high density lipoprotein cholesterol levels correlate with decreased apolipoprotein A-I and A-II fractional catabolic rate in women. J Clin Invest. 1989 Jul;84(1):262–269. doi: 10.1172/JCI114149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton E. A., Eisenberg S., Breslow J. L. Increased apo A-I and apo A-II fractional catabolic rate in patients with low high density lipoprotein-cholesterol levels with or without hypertriglyceridemia. J Clin Invest. 1991 Feb;87(2):536–544. doi: 10.1172/JCI115028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunzell J. D., Sniderman A. D., Albers J. J., Kwiterovich P. O., Jr Apoproteins B and A-I and coronary artery disease in humans. Arteriosclerosis. 1984 Mar-Apr;4(2):79–83. doi: 10.1161/01.atv.4.2.79. [DOI] [PubMed] [Google Scholar]
- Castelli W. P., Garrison R. J., Wilson P. W., Abbott R. D., Kalousdian S., Kannel W. B. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA. 1986 Nov 28;256(20):2835–2838. [PubMed] [Google Scholar]
- Clay M. A., Newnham H. H., Barter P. J. Hepatic lipase promotes a loss of apolipoprotein A-I from triglyceride-enriched human high density lipoproteins during incubation in vitro. Arterioscler Thromb. 1991 Mar-Apr;11(2):415–422. doi: 10.1161/01.atv.11.2.415. [DOI] [PubMed] [Google Scholar]
- Daerr W. H., Minzlaff U., Greten H. Quantitative determination of apolipoprotein A-I in high-density lipoproteins and 'free' apolipoprotein A-I by two-dimensional agarose gel lipoprotein-'rocket' immunoelectrophoresis of human serum. Biochim Biophys Acta. 1986 Nov 14;879(2):134–139. doi: 10.1016/0005-2760(86)90095-0. [DOI] [PubMed] [Google Scholar]
- Ehnholm C., Greten H., Brown W. V. A comparative study of post-heparin lipolytic activity and a purified human plasma triacylglycerol lipase. Biochim Biophys Acta. 1974 Jul 26;360(1):68–77. doi: 10.1016/0005-2760(74)90180-5. [DOI] [PubMed] [Google Scholar]
- Gebhardt D. O., Schicht I. M., Paul L. C. The immunochemical determination of apolipoprotein A, total apolipoprotein A-I and 'free' apolipoprotein A-I in serum of patients on chronic haemodialysis. Ann Clin Biochem. 1984 Jul;21(Pt 4):301–305. doi: 10.1177/000456328402100412. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Pittman R. C., Keller G. A., Steinberg D. Tissue sites of degradation of apoprotein A-I in the rat. J Biol Chem. 1983 Jun 10;258(11):7161–7167. [PubMed] [Google Scholar]
- Glass C., Pittman R. C., Civen M., Steinberg D. Uptake of high-density lipoprotein-associated apoprotein A-I and cholesterol esters by 16 tissues of the rat in vivo and by adrenal cells and hepatocytes in vitro. J Biol Chem. 1985 Jan 25;260(2):744–750. [PubMed] [Google Scholar]
- Goldberg I. J., Blaner W. S., Vanni T. M., Moukides M., Ramakrishnan R. Role of lipoprotein lipase in the regulation of high density lipoprotein apolipoprotein metabolism. Studies in normal and lipoprotein lipase-inhibited monkeys. J Clin Invest. 1990 Aug;86(2):463–473. doi: 10.1172/JCI114732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomo Z. A., Henderson L. O. High-density lipoprotein apolipoproteins in urine: II. Enzyme-linked immunoassay of apolipoprotein A-I. Clin Chem. 1988 Sep;34(9):1781–1786. [PubMed] [Google Scholar]
- Gordon D. J., Knoke J., Probstfield J. L., Superko R., Tyroler H. A. High-density lipoprotein cholesterol and coronary heart disease in hypercholesterolemic men: the Lipid Research Clinics Coronary Primary Prevention Trial. Circulation. 1986 Dec;74(6):1217–1225. doi: 10.1161/01.cir.74.6.1217. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Le N. A., Ginsberg H. N. Heterogeneity of apolipoprotein A-I turnover in subjects with reduced concentrations of plasma high density lipoprotein cholesterol. Metabolism. 1988 Jul;37(7):614–617. doi: 10.1016/0026-0495(88)90077-7. [DOI] [PubMed] [Google Scholar]
- Maack T. Physiological evaluation of the isolated perfused rat kidney. Am J Physiol. 1980 Feb;238(2):F71–F78. doi: 10.1152/ajprenal.1980.238.2.F71. [DOI] [PubMed] [Google Scholar]
- Miller N. E., Thelle D. S., Forde O. H., Mjos O. D. The Tromsø heart-study. High-density lipoprotein and coronary heart-disease: a prospective case-control study. Lancet. 1977 May 7;1(8019):965–968. doi: 10.1016/s0140-6736(77)92274-7. [DOI] [PubMed] [Google Scholar]
- Neary R. H., Gowland E. Stability of free apolipoprotein A-1 concentration in serum, and its measurement in normal and hyperlipidemic subjects. Clin Chem. 1987 Jul;33(7):1163–1169. [PubMed] [Google Scholar]
- Neary R. H., Gowland E. The effect of renal failure and haemodialysis on the concentration of free apolipoprotein A-1 in serum and the implications for the catabolism of high-density lipoproteins. Clin Chim Acta. 1988 Feb 15;171(2-3):239–245. doi: 10.1016/0009-8981(88)90149-0. [DOI] [PubMed] [Google Scholar]
- Newnham H. H., Hopkins G. J., Devlin S., Barter P. J. Lipoprotein lipase prevents the hepatic lipase-induced reduction in particle size of high density lipoproteins during incubation of human plasma. Atherosclerosis. 1990 Jun;82(3):167–176. doi: 10.1016/0021-9150(90)90037-j. [DOI] [PubMed] [Google Scholar]
- Nicoll A., Miller N. E., Lewis B. High-density lipoprotein metabolism. Adv Lipid Res. 1980;17:53–106. doi: 10.1016/b978-0-12-024917-6.50008-2. [DOI] [PubMed] [Google Scholar]
- Polacek D., Edelstein C., Scanu A. M. Rapid fractionation of human high density apolipoproteins by high performance liquid chromatography. Lipids. 1981 Dec;16(12):927–929. doi: 10.1007/BF02534999. [DOI] [PubMed] [Google Scholar]
- Saku K., Reddy G. S., Hynd B. A., Kashyap M. L. Renal handling of high-density lipoproteins by isolated perfused kidneys. Metabolism. 1984 May;33(5):432–438. doi: 10.1016/0026-0495(84)90143-4. [DOI] [PubMed] [Google Scholar]
- Schaefer E. J., Foster D. M., Jenkins L. L., Lindgren F. T., Berman M., Levy R. I., Brewer H. B., Jr The composition and metabolism of high density lipoprotein subfractions. Lipids. 1979 May;14(5):511–522. doi: 10.1007/BF02533471. [DOI] [PubMed] [Google Scholar]
- Schonfeld G., Bailey A., Steelman R. Plasma, apolipoprotein, A-I and A-II levels in hyperlipidemia. Lipids. 1978 Dec;13(12):951–959. doi: 10.1007/BF02533855. [DOI] [PubMed] [Google Scholar]
- Shepherd J., Gotto A. M., Jr, Taunton O. D., Caslake M. J., Farish E. The in vitro interaction of human apolipoprotein A-I and high density lipoproteins. Biochim Biophys Acta. 1977 Dec 21;489(3):486–501. doi: 10.1016/0005-2760(77)90169-2. [DOI] [PubMed] [Google Scholar]
- Shepherd J., Packard C. J., Gotto A. M., Jr, Taunton O. D. A comparison of two methods to investigate the metabolism of human apolipoproteins A-I and and A-II. J Lipid Res. 1978 Jul;19(5):656–661. [PubMed] [Google Scholar]
- Smith S. J., Cooper G. R., Henderson L. O., Hannon W. H. An international collaborative study on standardization of apolipoproteins A-I and B. Part I. Evaluation of a lyophilized candidate reference and calibration material. Clin Chem. 1987 Dec;33(12):2240–2249. [PubMed] [Google Scholar]
- Socorro L., Green C. C., Jackson R. L. Preparation of a homogeneous and stable form of bovine milk lipoprotein lipase. Prep Biochem. 1985;15(3):133–143. doi: 10.1080/10826068508062267. [DOI] [PubMed] [Google Scholar]
- Vega G. L., Gylling H., Nichols A. V., Grundy S. M. Evaluation of a method for study of kinetics of autologous apolipoprotein A-I. J Lipid Res. 1991 May;32(5):867–875. [PubMed] [Google Scholar]
- de Mendoza S. G., Kashyap M. L., Chen C. Y., Lutmer R. F. High density lipoproteinuria in nephrotic syndrome. Metabolism. 1976 Oct;25(10):1143–1149. doi: 10.1016/0026-0495(76)90022-6. [DOI] [PubMed] [Google Scholar]


