Abstract
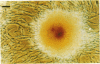
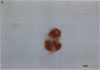
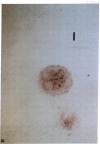
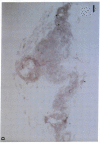
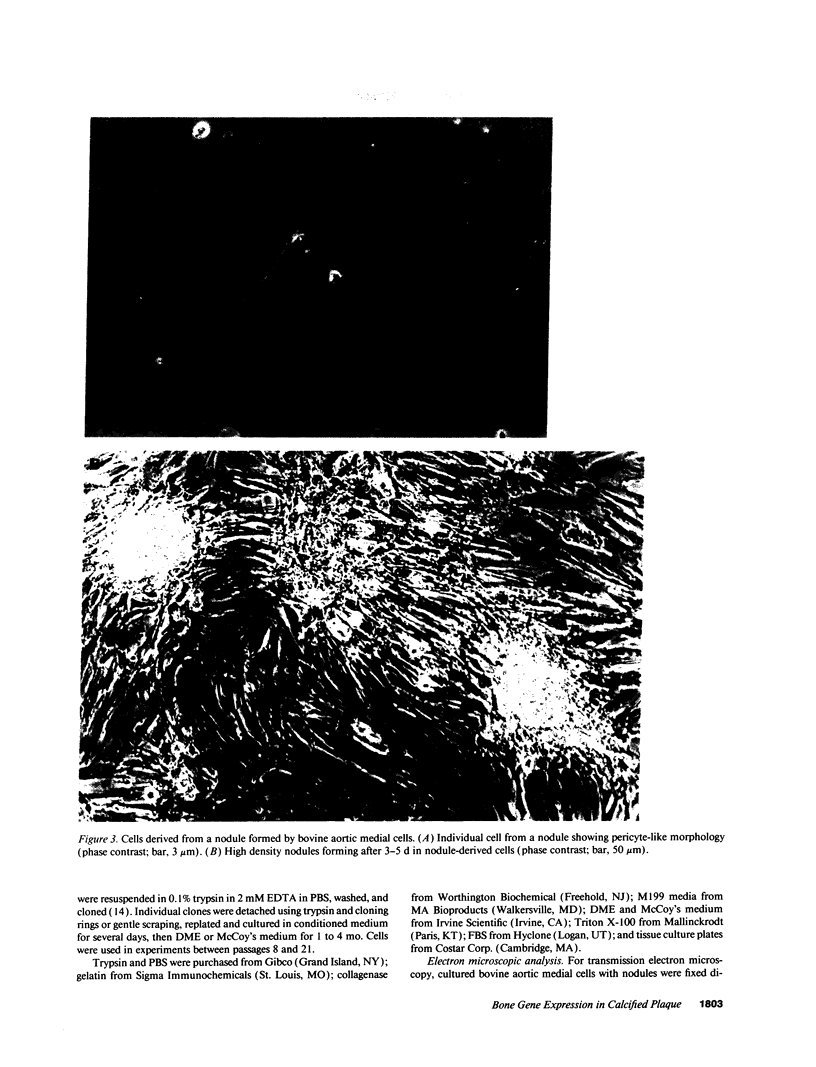
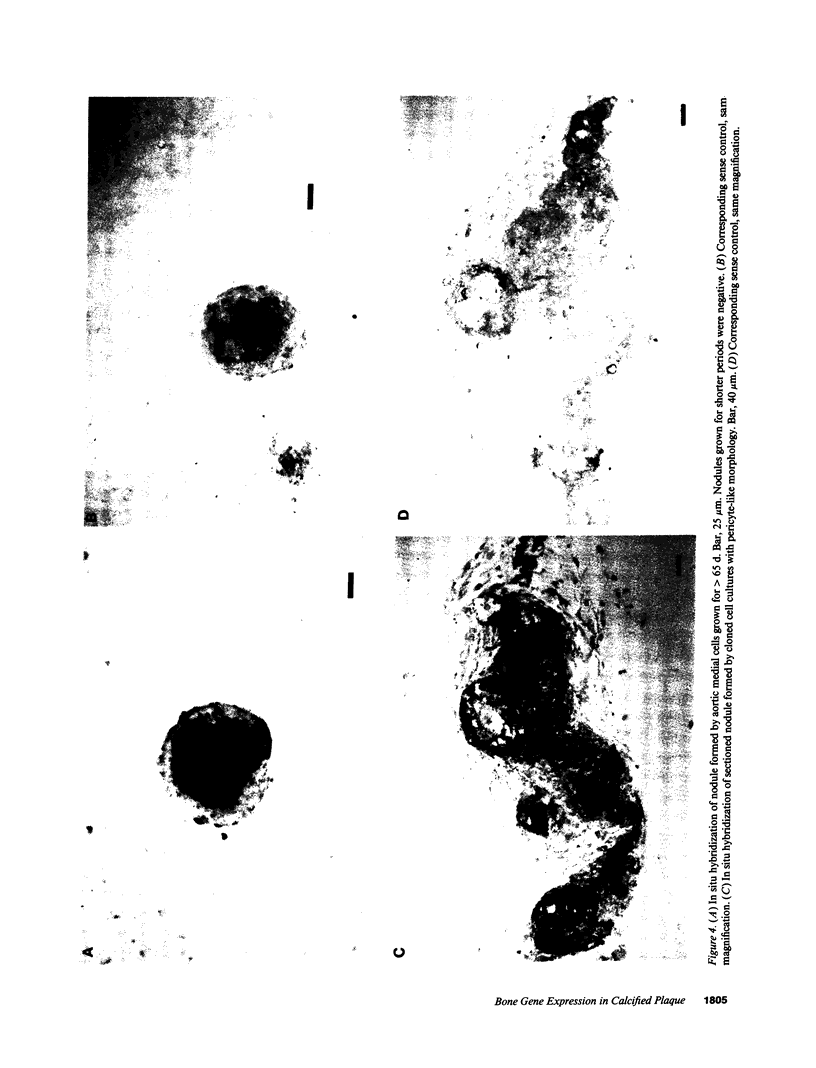
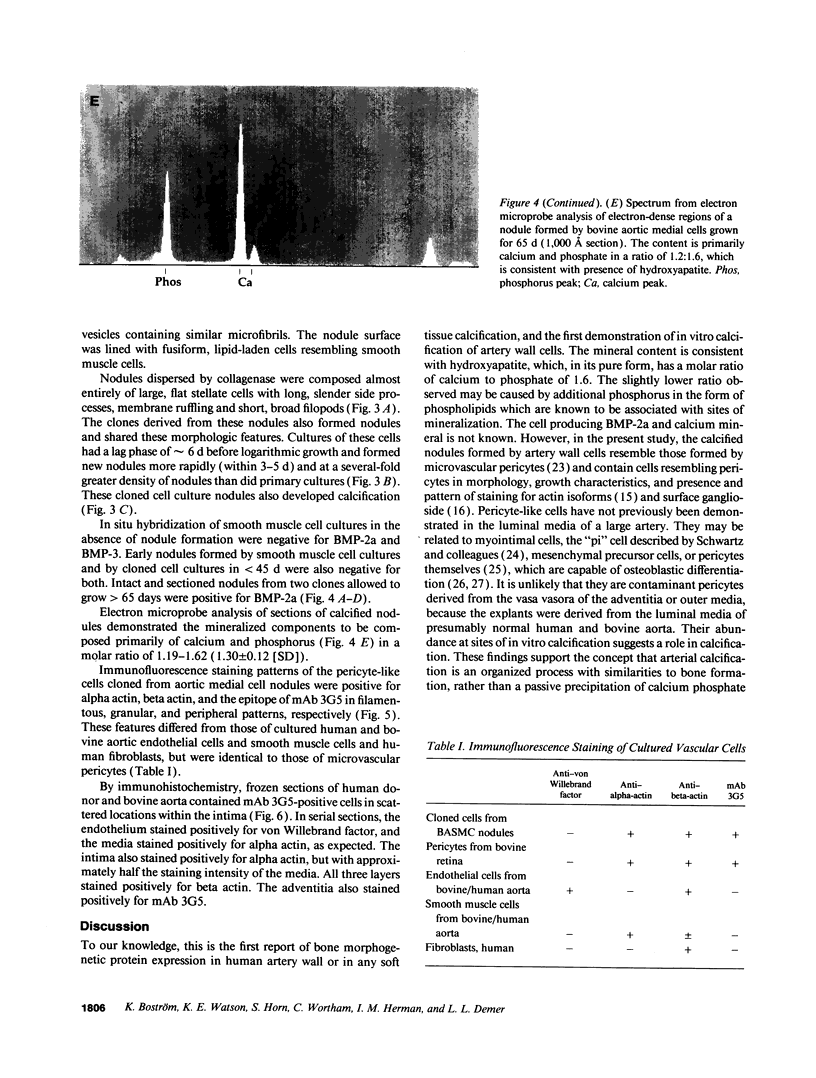
Artery wall calcification associated with atherosclerosis frequently contains fully formed bone tissue including marrow. The cellular origin is not known. In this study, bone morphogenetic protein-2a, a potent factor for osteoblastic differentiation, was found to be expressed in calcified human atherosclerotic plaque. In addition, cells cultured from the aortic wall formed calcified nodules similar to those found in bone cell cultures and expressed bone morphogenetic protein-2a with prolonged culture. The predominant cells in these nodules had immunocytochemical features characteristic of microvascular pericytes that are capable of osteoblastic differentiation. Pericyte-like cells were also found by immunohistochemistry in the intima of bovine and human aorta. These findings suggest that arterial calcification is a regulated process similar to bone formation, possibly mediated by pericyte-like cells.
Full text
PDF

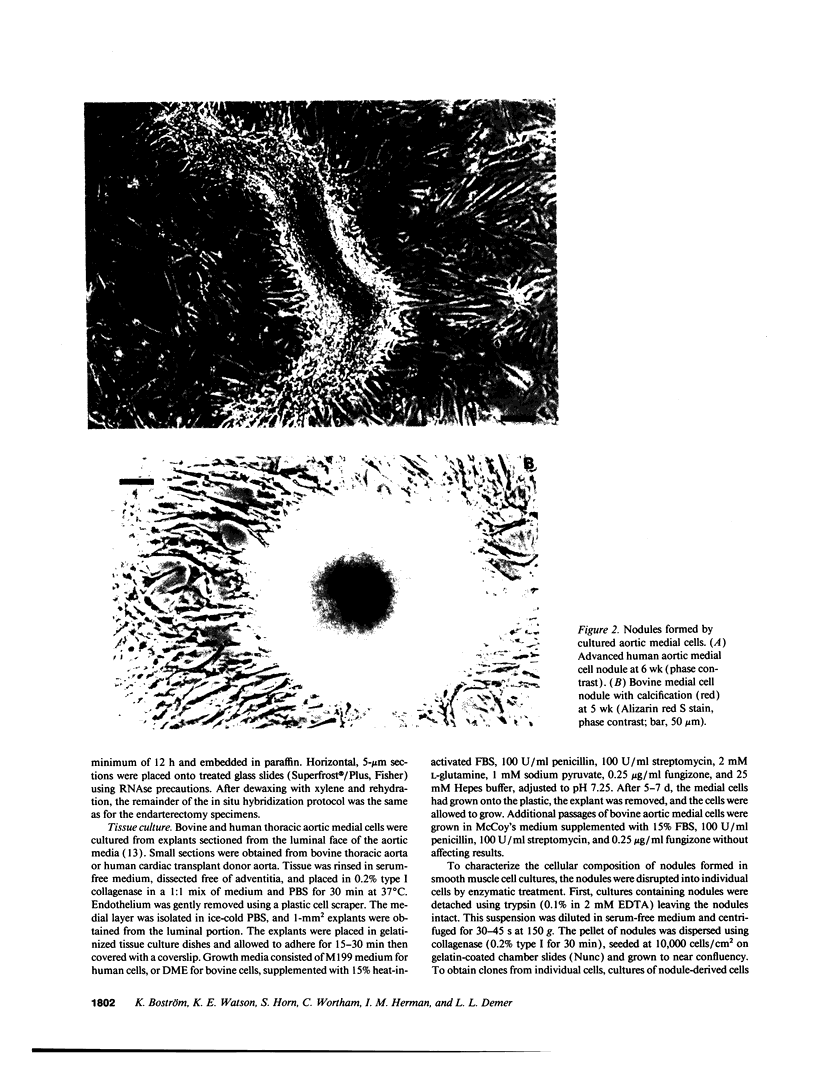




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson H. C. Calcific diseases. A concept. Arch Pathol Lab Med. 1983 Jul;107(7):341–348. [PubMed] [Google Scholar]
- BEADENKOPF W. G., DAOUD A. S., LOVE B. M. CALCIFICATION IN THE CORONARY ARTERIES AND ITS RELATIONSHIP TO ARTERIOSCLEROSIS AND MYOCARDIAL INFARCTION. Am J Roentgenol Radium Ther Nucl Med. 1964 Oct;92:865–871. [PubMed] [Google Scholar]
- Brighton C. T., Lorich D. G., Kupcha R., Reilly T. M., Jones A. R., Woodbury R. A., 2nd The pericyte as a possible osteoblast progenitor cell. Clin Orthop Relat Res. 1992 Feb;(275):287–299. [PubMed] [Google Scholar]
- Cox K. H., DeLeon D. V., Angerer L. M., Angerer R. C. Detection of mrnas in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984 Feb;101(2):485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- Diaz-Flores L., Gutierrez R., Lopez-Alonso A., Gonzalez R., Varela H. Pericytes as a supplementary source of osteoblasts in periosteal osteogenesis. Clin Orthop Relat Res. 1992 Feb;(275):280–286. [PubMed] [Google Scholar]
- Glagov S., Weisenberg E., Zarins C. K., Stankunavicius R., Kolettis G. J. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987 May 28;316(22):1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- Herman I. M., D'Amore P. A. Microvascular pericytes contain muscle and nonmuscle actins. J Cell Biol. 1985 Jul;101(1):43–52. doi: 10.1083/jcb.101.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honye J., Mahon D. J., Jain A., White C. J., Ramee S. R., Wallis J. B., al-Zarka A., Tobis J. M. Morphological effects of coronary balloon angioplasty in vivo assessed by intravascular ultrasound imaging. Circulation. 1992 Mar;85(3):1012–1025. doi: 10.1161/01.cir.85.3.1012. [DOI] [PubMed] [Google Scholar]
- Khouri R. K., Koudsi B., Reddi H. Tissue transformation into bone in vivo. A potential practical application. JAMA. 1991 Oct 9;266(14):1953–1955. [PubMed] [Google Scholar]
- Lee R. T., Grodzinsky A. J., Frank E. H., Kamm R. D., Schoen F. J. Structure-dependent dynamic mechanical behavior of fibrous caps from human atherosclerotic plaques. Circulation. 1991 May;83(5):1764–1770. doi: 10.1161/01.cir.83.5.1764. [DOI] [PubMed] [Google Scholar]
- Navab M., Hough G. P., Stevenson L. W., Drinkwater D. C., Laks H., Fogelman A. M. Monocyte migration into the subendothelial space of a coculture of adult human aortic endothelial and smooth muscle cells. J Clin Invest. 1988 Dec;82(6):1853–1863. doi: 10.1172/JCI113802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak R. C., Berman A. B., George K. L., Eisenbarth G. S., King G. L. A monoclonal antibody (3G5)-defined ganglioside antigen is expressed on the cell surface of microvascular pericytes. J Exp Med. 1988 Mar 1;167(3):1003–1015. doi: 10.1084/jem.167.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlidge A., D'Amore P. A. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol. 1987 Sep;105(3):1455–1462. doi: 10.1083/jcb.105.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodin J. A. Ultrastructure of mammalian venous capillaries, venules, and small collecting veins. J Ultrastruct Res. 1968 Dec;25(5):452–500. doi: 10.1016/s0022-5320(68)80098-x. [DOI] [PubMed] [Google Scholar]
- Schmid K., McSharry W. O., Pameijer C. H., Binette J. P. Chemical and physicochemical studies on the mineral deposits of the human atherosclerotic aorta. Atherosclerosis. 1980 Oct;37(2):199–210. doi: 10.1016/0021-9150(80)90005-2. [DOI] [PubMed] [Google Scholar]
- Schor A. M., Allen T. D., Canfield A. E., Sloan P., Schor S. L. Pericytes derived from the retinal microvasculature undergo calcification in vitro. J Cell Sci. 1990 Nov;97(Pt 3):449–461. doi: 10.1242/jcs.97.3.449. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Heimark R. L., Majesky M. W. Developmental mechanisms underlying pathology of arteries. Physiol Rev. 1990 Oct;70(4):1177–1209. doi: 10.1152/physrev.1990.70.4.1177. [DOI] [PubMed] [Google Scholar]
- Schönfeld H. J., Pöschl B., Wessner B., Kistler A. Altered differentiation of limb bud cells by transforming growth factors-beta isolated from bone matrix and from platelets. Bone Miner. 1991 Jun;13(3):171–189. doi: 10.1016/0169-6009(91)90067-a. [DOI] [PubMed] [Google Scholar]
- Takeo S., Anan M., Fujioka K., Kajihara T., Hiraga S., Miyake K., Tanonaka K., Minematsu R., Mori H., Taniguchi Y. Functional changes of aorta with massive accumulation of calcium. Atherosclerosis. 1989 Jun;77(2-3):175–181. doi: 10.1016/0021-9150(89)90079-8. [DOI] [PubMed] [Google Scholar]
- Wozney J. M., Rosen V., Celeste A. J., Mitsock L. M., Whitters M. J., Kriz R. W., Hewick R. M., Wang E. A. Novel regulators of bone formation: molecular clones and activities. Science. 1988 Dec 16;242(4885):1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]