Abstract
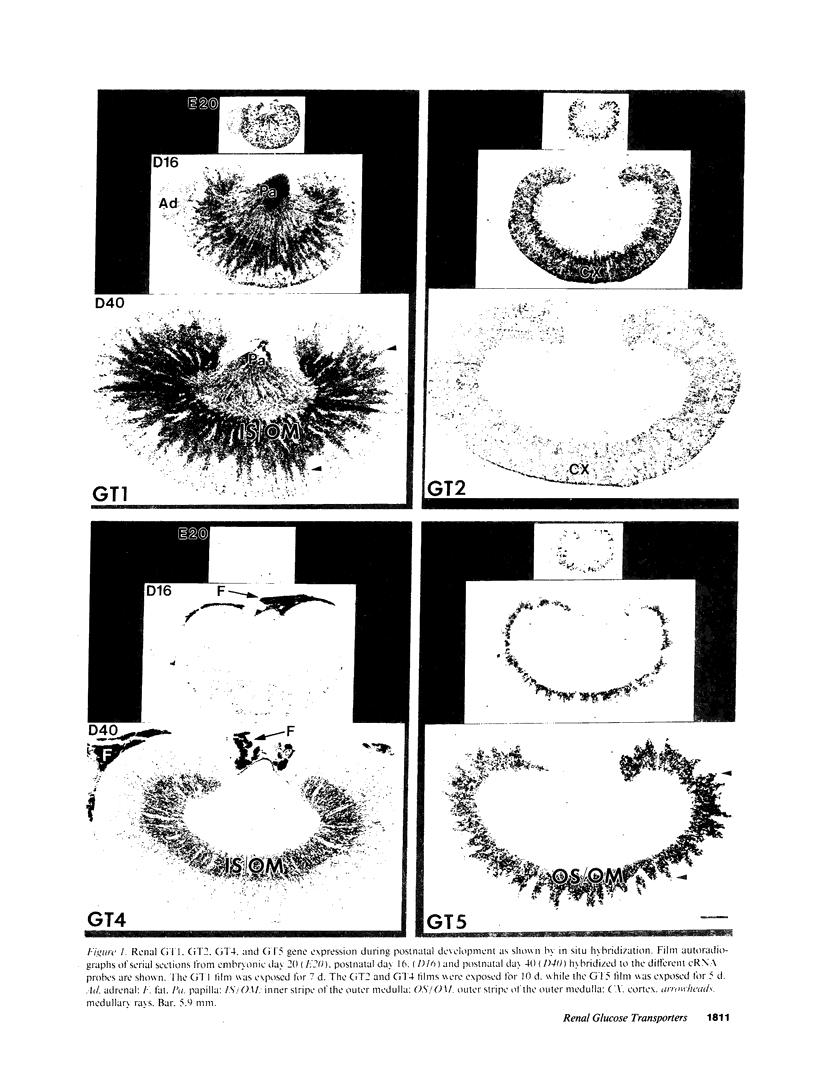
In situ hybridization was used to map cellular patterns of gene expression for facilitative glucose transporters (GTs) 1-5 in the developing and adult rat kidney. GT3 was not detected. GT1 mRNA was present in the proximal straight tubule (PST), distal nephron and collecting duct. GT2 mRNA was localized in both proximal convoluted and PST, while GT5 mRNA was detected only in the PST. GT4 mRNA and immunoreactivity were focally localized in the thick ascending limb of Henle's loop and were coexpressed with IGF-I. Thus, each of the four different isoforms demonstrated a distinct renal distribution, with GTs 1, 2, and 5 coexpressed in the PST. Renal GT1 and GT5 gene expression were unchanged throughout development, while GT2 was most abundant before weaning and GT4 was first detected after weaning. Only GT4 appeared to be hormonally regulated: It was decreased after hypophysectomy and increased after vasopressin treatment, but was not affected by 1 or 4 d of insulinopenic diabetes mellitus. The coexpression of GT4 and IGF-I in the thick ascending limb segment of the nephron suggests a novel autocrine/paracrine mechanism by which cells may control local fuel economy independently from that of the larger structure to which they belong and from the systemic hormonal milieu.
Full text
PDF
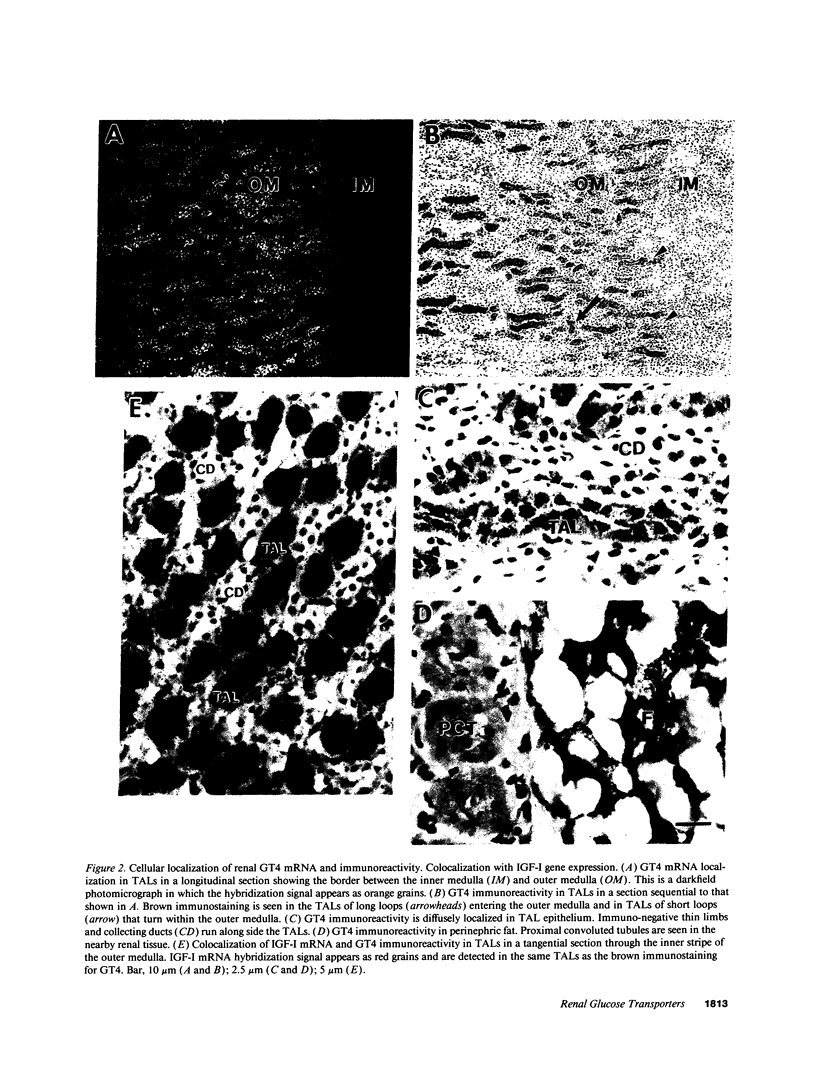
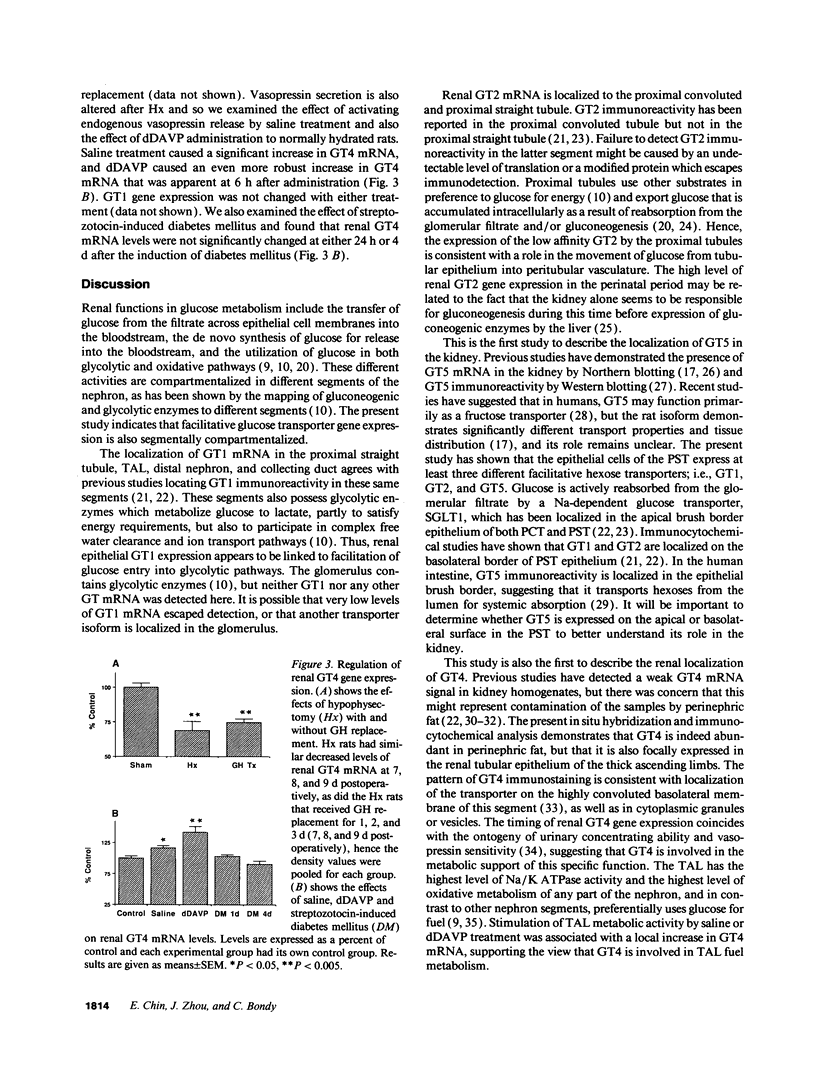

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaum M. J. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989 Apr 21;57(2):305–315. doi: 10.1016/0092-8674(89)90968-9. [DOI] [PubMed] [Google Scholar]
- Bondy C. A., Lightman S. L., Lightman S. L. Developmental and physiological regulation of aldose reductase mRNA expression in renal medulla. Mol Endocrinol. 1989 Sep;3(9):1409–1416. doi: 10.1210/mend-3-9-1409. [DOI] [PubMed] [Google Scholar]
- Bondy C. A., Werner H., Roberts C. T., Jr, LeRoith D. Cellular pattern of insulin-like growth factor-I (IGF-I) and type I IGF receptor gene expression in early organogenesis: comparison with IGF-II gene expression. Mol Endocrinol. 1990 Sep;4(9):1386–1398. doi: 10.1210/mend-4-9-1386. [DOI] [PubMed] [Google Scholar]
- Bondy C., Werner H., Roberts C. T., Jr, LeRoith D. Cellular pattern of type-I insulin-like growth factor receptor gene expression during maturation of the rat brain: comparison with insulin-like growth factors I and II. Neuroscience. 1992;46(4):909–923. doi: 10.1016/0306-4522(92)90193-6. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Takeda J., Brot-Laroche E., Bell G. I., Davidson N. O. Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem. 1992 Jul 25;267(21):14523–14526. [PubMed] [Google Scholar]
- Charron M. J., Brosius F. C., 3rd, Alper S. L., Lodish H. F. A glucose transport protein expressed predominately in insulin-responsive tissues. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2535–2539. doi: 10.1073/pnas.86.8.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin E., Zhou J., Bondy C. Anatomical relationships in the patterns of insulin-like growth factor (IGF)-I, IGF binding protein-1, and IGF-I receptor gene expression in the rat kidney. Endocrinology. 1992 Jun;130(6):3237–3245. doi: 10.1210/endo.130.6.1375897. [DOI] [PubMed] [Google Scholar]
- Cramer S. C., Pardridge W. M., Hirayama B. A., Wright E. M. Colocalization of GLUT2 glucose transporter, sodium/glucose cotransporter, and gamma-glutamyl transpeptidase in rat kidney with double-peroxidase immunocytochemistry. Diabetes. 1992 Jun;41(6):766–770. doi: 10.2337/diab.41.6.766. [DOI] [PubMed] [Google Scholar]
- Davidson N. O., Hausman A. M., Ifkovits C. A., Buse J. B., Gould G. W., Burant C. F., Bell G. I. Human intestinal glucose transporter expression and localization of GLUT5. Am J Physiol. 1992 Mar;262(3 Pt 1):C795–C800. doi: 10.1152/ajpcell.1992.262.3.C795. [DOI] [PubMed] [Google Scholar]
- Fukumoto H., Kayano T., Buse J. B., Edwards Y., Pilch P. F., Bell G. I., Seino S. Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J Biol Chem. 1989 May 15;264(14):7776–7779. [PubMed] [Google Scholar]
- Gould G. W., Bell G. I. Facilitative glucose transporters: an expanding family. Trends Biochem Sci. 1990 Jan;15(1):18–23. doi: 10.1016/0968-0004(90)90125-u. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- James D. E., Strube M., Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989 Mar 2;338(6210):83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- Kasanicki M. A., Pilch P. F. Regulation of glucose-transporter function. Diabetes Care. 1990 Mar;13(3):219–227. doi: 10.2337/diacare.13.3.219. [DOI] [PubMed] [Google Scholar]
- Kasanicki M. A., Pilch P. F. Regulation of glucose-transporter function. Diabetes Care. 1990 Mar;13(3):219–227. doi: 10.2337/diacare.13.3.219. [DOI] [PubMed] [Google Scholar]
- Kayano T., Burant C. F., Fukumoto H., Gould G. W., Fan Y. S., Eddy R. L., Byers M. G., Shows T. B., Seino S., Bell G. I. Human facilitative glucose transporters. Isolation, functional characterization, and gene localization of cDNAs encoding an isoform (GLUT5) expressed in small intestine, kidney, muscle, and adipose tissue and an unusual glucose transporter pseudogene-like sequence (GLUT6). J Biol Chem. 1990 Aug 5;265(22):13276–13282. [PubMed] [Google Scholar]
- Maher F., Harrison L. C. Stabilization of glucose transporter mRNA by insulin/IGF-1 and glucose deprivation. Biochem Biophys Res Commun. 1990 Aug 31;171(1):210–215. doi: 10.1016/0006-291x(90)91378-6. [DOI] [PubMed] [Google Scholar]
- Masters B. A., Werner H., Roberts C. T., Jr, LeRoith D., Raizada M. K. Developmental regulation of insulin-like growth factor-I-stimulated glucose transporter in rat brain astrocytes. Endocrinology. 1991 May;128(5):2548–2557. doi: 10.1210/endo-128-5-2548. [DOI] [PubMed] [Google Scholar]
- Mueckler M. Family of glucose-transporter genes. Implications for glucose homeostasis and diabetes. Diabetes. 1990 Jan;39(1):6–11. doi: 10.2337/diacare.39.1.6. [DOI] [PubMed] [Google Scholar]
- Nagamatsu S., Kornhauser J. M., Burant C. F., Seino S., Mayo K. E., Bell G. I. Glucose transporter expression in brain. cDNA sequence of mouse GLUT3, the brain facilitative glucose transporter isoform, and identification of sites of expression by in situ hybridization. J Biol Chem. 1992 Jan 5;267(1):467–472. [PubMed] [Google Scholar]
- Rajerison R. M., Butlen D., Jard S. Ontogenic development of antidiuretic hormone receptors in rat kidney: comparison of hormonal binding and adenylate cyclase activation. Mol Cell Endocrinol. 1976 Mar;4(4):271–285. doi: 10.1016/0303-7207(76)90061-7. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Morrison E. D., Usher P., Flier J. S. Platelet-derived growth factor regulates glucose transporter expression. J Biol Chem. 1988 Nov 15;263(32):16523–16526. [PubMed] [Google Scholar]
- Ross B. D., Espinal J., Silva P. Glucose metabolism in renal tubular function. Kidney Int. 1986 Jan;29(1):54–67. doi: 10.1038/ki.1986.8. [DOI] [PubMed] [Google Scholar]
- Shepherd P. R., Gibbs E. M., Wesslau C., Gould G. W., Kahn B. B. Human small intestine facilitative fructose/glucose transporter (GLUT5) is also present in insulin-responsive tissues and brain. Investigation of biochemical characteristics and translocation. Diabetes. 1992 Oct;41(10):1360–1365. doi: 10.2337/diab.41.10.1360. [DOI] [PubMed] [Google Scholar]
- Silva P. Energy and fuel substrate metabolism in the kidney. Semin Nephrol. 1990 Sep;10(5):432–444. [PubMed] [Google Scholar]
- Takata K., Kasahara T., Kasahara M., Ezaki O., Hirano H. Localization of Na(+)-dependent active type and erythrocyte/HepG2-type glucose transporters in rat kidney: immunofluorescence and immunogold study. J Histochem Cytochem. 1991 Mar;39(3):287–298. doi: 10.1177/39.3.1993828. [DOI] [PubMed] [Google Scholar]
- Thorens B., Lodish H. F., Brown D. Differential localization of two glucose transporter isoforms in rat kidney. Am J Physiol. 1990 Dec;259(6 Pt 1):C286–C294. doi: 10.1152/ajpcell.1990.259.2.C286. [DOI] [PubMed] [Google Scholar]
- Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Werner H., Adamo M., Lowe W. L., Jr, Roberts C. T., Jr, LeRoith D. Developmental regulation of rat brain/Hep G2 glucose transporter gene expression. Mol Endocrinol. 1989 Feb;3(2):273–279. doi: 10.1210/mend-3-2-273. [DOI] [PubMed] [Google Scholar]
- Zorzoli A., Turkenkopf I. J., Mueller V. L. Gluconeogenesis in developing rat kidney cortex. Biochem J. 1969 Jan;111(2):181–185. doi: 10.1042/bj1110181. [DOI] [PMC free article] [PubMed] [Google Scholar]