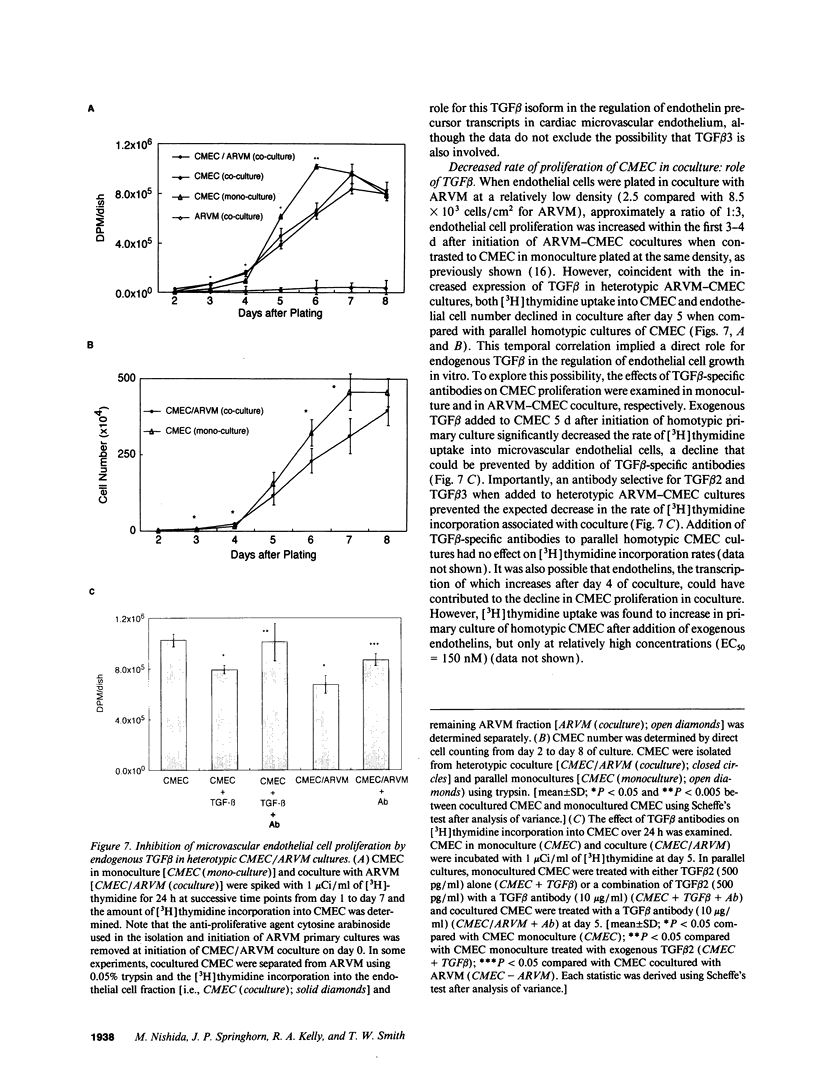
Abstract
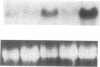
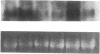
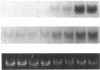
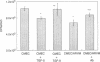
It is unclear whether signaling between endothelial cells and muscle cells within ventricular myocardium, known to be important during cardiac development, remains physiologically relevant in the adult heart. Also, the mechanisms regulating the synthesis and activation of locally acting autacoids such as endothelins, cytokines known to have potent effects on contractile function and gene expression in cardiac myocytes, are unknown, as their cells of origin within ventricular muscle. Microvascular endothelial cells isolated from ventricular tissue of adult rats do not express endothelins constitutively. However, the appearance of preproendothelin mRNA can be increased in cardiac microvascular endothelial cells by heterotypic primary culture with adult rat ventricular myocytes. Cell-cell contact, or at least close apposition, appears to be necessary to increase preproendothelin mRNA, as medium conditioned by ventricular myocytes alone was ineffective when applied to monocultures of microvascular endothelial cells. The level of TGF beta precursor mRNA is also markedly increased in microvascular endothelial cells in coculture and precedes the appearance of endothelin precursor transcripts. In coculture, TGF beta acts as an autocrine cytokine, increasing endothelin precursor mRNA and inhibiting the rate of microvascular endothelial cell proliferation. This regulation of endothelial cell phenotype in heterotypic primary cultures suggests that dynamic, reciprocal cell-cell signaling may also be occurring between microvascular endothelium and ventricular myocytes in vivo.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonelli-Orlidge A., Saunders K. B., Smith S. R., D'Amore P. A. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4544–4548. doi: 10.1073/pnas.86.12.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battegay E. J., Raines E. W., Seifert R. A., Bowen-Pope D. F., Ross R. TGF-beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell. 1990 Nov 2;63(3):515–524. doi: 10.1016/0092-8674(90)90448-n. [DOI] [PubMed] [Google Scholar]
- Bouvet P., Paris J., Phillippe M., Osborne H. B. Degradation of a developmentally regulated mRNA in Xenopus embryos is controlled by the 3' region and requires the translation of another maternal mRNA. Mol Cell Biol. 1991 Jun;11(6):3115–3124. doi: 10.1128/mcb.11.6.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutsaert D. L., Andries L. J. The endocardial endothelium. Am J Physiol. 1992 Oct;263(4 Pt 2):H985–1002. doi: 10.1152/ajpheart.1992.263.4.H985. [DOI] [PubMed] [Google Scholar]
- Bugaisky L. B., Zak R. Differentiation of adult rat cardiac myocytes in cell culture. Circ Res. 1989 Mar;64(3):493–500. doi: 10.1161/01.res.64.3.493. [DOI] [PubMed] [Google Scholar]
- Casscells W., Speir E., Sasse J., Klagsbrun M., Allen P., Lee M., Calvo B., Chiba M., Haggroth L., Folkman J. Isolation, characterization, and localization of heparin-binding growth factors in the heart. J Clin Invest. 1990 Feb;85(2):433–441. doi: 10.1172/JCI114456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claycomb W. C. Atrial-natriuretic-factor mRNA is developmentally regulated in heart ventricles and actively expressed in cultured ventricular cardiac muscle cells of rat and human. Biochem J. 1988 Oct 15;255(2):617–620. [PMC free article] [PubMed] [Google Scholar]
- Dasch J. R., Pace D. R., Waegell W., Inenaga D., Ellingsworth L. Monoclonal antibodies recognizing transforming growth factor-beta. Bioactivity neutralization and transforming growth factor beta 2 affinity purification. J Immunol. 1989 Mar 1;142(5):1536–1541. [PubMed] [Google Scholar]
- Dennis P. A., Rifkin D. B. Cellular activation of latent transforming growth factor beta requires binding to the cation-independent mannose 6-phosphate/insulin-like growth factor type II receptor. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):580–584. doi: 10.1073/pnas.88.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid H., Larson D. M., Springhorn J. P., Attawia M. A., Nayak R. C., Smith T. W., Kelly R. A. Role of epicardial mesothelial cells in the modification of phenotype and function of adult rat ventricular myocytes in primary coculture. Circ Res. 1992 Jul;71(1):40–50. doi: 10.1161/01.res.71.1.40. [DOI] [PubMed] [Google Scholar]
- Good D. J., Polverini P. J., Rastinejad F., Le Beau M. M., Lemons R. S., Frazier W. A., Bouck N. P. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iruela-Arispe M. L., Bornstein P., Sage H. Thrombospondin exerts an antiangiogenic effect on cord formation by endothelial cells in vitro. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):5026–5030. doi: 10.1073/pnas.88.11.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S. L., Piper H. M. Cell cultures of adult cardiomyocytes as models of the myocardium. J Mol Cell Cardiol. 1986 Jul;18(7):661–678. doi: 10.1016/s0022-2828(86)80939-7. [DOI] [PubMed] [Google Scholar]
- Jennings J. C., Mohan S., Linkhart T. A., Widstrom R., Baylink D. J. Comparison of the biological actions of TGF beta-1 and TGF beta-2: differential activity in endothelial cells. J Cell Physiol. 1988 Oct;137(1):167–172. doi: 10.1002/jcp.1041370120. [DOI] [PubMed] [Google Scholar]
- Kelly R. A., Eid H., Krämer B. K., O'Neill M., Liang B. T., Reers M., Smith T. W. Endothelin enhances the contractile responsiveness of adult rat ventricular myocytes to calcium by a pertussis toxin-sensitive pathway. J Clin Invest. 1990 Oct;86(4):1164–1171. doi: 10.1172/JCI114822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer B. K., Nishida M., Kelly R. A., Smith T. W. Endothelins. Myocardial actions of a new class of cytokines. Circulation. 1992 Jan;85(1):350–356. doi: 10.1161/01.cir.85.1.350. [DOI] [PubMed] [Google Scholar]
- Krämer B. K., Smith T. W., Kelly R. A. Endothelin and increased contractility in adult rat ventricular myocytes. Role of intracellular alkalosis induced by activation of the protein kinase C-dependent Na(+)-H+ exchanger. Circ Res. 1991 Jan;68(1):269–279. doi: 10.1161/01.res.68.1.269. [DOI] [PubMed] [Google Scholar]
- Kumar S., West D. C., Ager A. Heterogeneity in endothelial cells from large vessels and microvessels. Differentiation. 1987;36(1):57–70. doi: 10.1111/j.1432-0436.1987.tb00181.x. [DOI] [PubMed] [Google Scholar]
- Li K., Stewart D. J., Rouleau J. L. Myocardial contractile actions of endothelin-1 in rat and rabbit papillary muscles. Role of endocardial endothelium. Circ Res. 1991 Aug;69(2):301–312. doi: 10.1161/01.res.69.2.301. [DOI] [PubMed] [Google Scholar]
- Masaki T., Kimura S., Yanagisawa M., Goto K. Molecular and cellular mechanism of endothelin regulation. Implications for vascular function. Circulation. 1991 Oct;84(4):1457–1468. doi: 10.1161/01.cir.84.4.1457. [DOI] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- McClellan G., Weisberg A., Kato N. S., Ramaciotti C., Sharkey A., Winegrad S. Contractile proteins in myocardial cells are regulated by factor(s) released by blood vessels. Circ Res. 1992 Apr;70(4):787–803. doi: 10.1161/01.res.70.4.787. [DOI] [PubMed] [Google Scholar]
- Merwin J. R., Newman W., Beall L. D., Tucker A., Madri J. Vascular cells respond differentially to transforming growth factors beta 1 and beta 2 in vitro. Am J Pathol. 1991 Jan;138(1):37–51. [PMC free article] [PubMed] [Google Scholar]
- Mignatti P., Mazzieri R., Rifkin D. B. Expression of the urokinase receptor in vascular endothelial cells is stimulated by basic fibroblast growth factor. J Cell Biol. 1991 Jun;113(5):1193–1201. doi: 10.1083/jcb.113.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan F. A., Denhez F., Kondaiah P., Akhurst R. J. Embryonic gene expression patterns of TGF beta 1, beta 2 and beta 3 suggest different developmental functions in vivo. Development. 1991 Jan;111(1):131–143. doi: 10.1242/dev.111.1.131. [DOI] [PubMed] [Google Scholar]
- Mooradian D. L., Diglio C. A. Effects of epidermal growth factor and transforming growth factor-beta 1 on rat heart endothelial cell anchorage-dependent and -independent growth. Exp Cell Res. 1990 Jan;186(1):122–129. doi: 10.1016/0014-4827(90)90218-y. [DOI] [PubMed] [Google Scholar]
- Moses H. L., Yang E. Y., Pietenpol J. A. TGF-beta stimulation and inhibition of cell proliferation: new mechanistic insights. Cell. 1990 Oct 19;63(2):245–247. doi: 10.1016/0092-8674(90)90155-8. [DOI] [PubMed] [Google Scholar]
- Moses R. L., Claycomb W. C. Disorganization and reestablishment of cardiac muscle cell ultrastructure in cultured adult rat ventricular muscle cells. J Ultrastruct Res. 1982 Dec;81(3):358–374. doi: 10.1016/s0022-5320(82)90064-8. [DOI] [PubMed] [Google Scholar]
- Nathan C., Sporn M. Cytokines in context. J Cell Biol. 1991 Jun;113(5):981–986. doi: 10.1083/jcb.113.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelton R. W., Saxena B., Jones M., Moses H. L., Gold L. I. Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol. 1991 Nov;115(4):1091–1105. doi: 10.1083/jcb.115.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietenpol J. A., Stein R. W., Moran E., Yaciuk P., Schlegel R., Lyons R. M., Pittelkow M. R., Münger K., Howley P. M., Moses H. L. TGF-beta 1 inhibition of c-myc transcription and growth in keratinocytes is abrogated by viral transforming proteins with pRB binding domains. Cell. 1990 Jun 1;61(5):777–785. doi: 10.1016/0092-8674(90)90188-k. [DOI] [PubMed] [Google Scholar]
- Potts J. D., Dagle J. M., Walder J. A., Weeks D. L., Runyan R. B. Epithelial-mesenchymal transformation of embryonic cardiac endothelial cells is inhibited by a modified antisense oligodeoxynucleotide to transforming growth factor beta 3. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1516–1520. doi: 10.1073/pnas.88.4.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaciotti C., Sharkey A., McClellan G., Winegrad S. Endothelial cells regulate cardiac contractility. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4033–4036. doi: 10.1073/pnas.89.9.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RayChaudhury A., D'Amore P. A. Endothelial cell regulation by transforming growth factor-beta. J Cell Biochem. 1991 Nov;47(3):224–229. doi: 10.1002/jcb.240470307. [DOI] [PubMed] [Google Scholar]
- Runyan R. B., Potts J. D., Weeks D. L. TGF-beta 3-mediated tissue interaction during embryonic heart development. Mol Reprod Dev. 1992 Jun;32(2):152–159. doi: 10.1002/mrd.1080320211. [DOI] [PubMed] [Google Scholar]
- Sato Y., Tsuboi R., Lyons R., Moses H., Rifkin D. B. Characterization of the activation of latent TGF-beta by co-cultures of endothelial cells and pericytes or smooth muscle cells: a self-regulating system. J Cell Biol. 1990 Aug;111(2):757–763. doi: 10.1083/jcb.111.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubeita H. E., McDonough P. M., Harris A. N., Knowlton K. U., Glembotski C. C., Brown J. H., Chien K. R. Endothelin induction of inositol phospholipid hydrolysis, sarcomere assembly, and cardiac gene expression in ventricular myocytes. A paracrine mechanism for myocardial cell hypertrophy. J Biol Chem. 1990 Nov 25;265(33):20555–20562. [PubMed] [Google Scholar]
- Smith J. A., Shah A. M., Lewis M. J. Factors released from endocardium of the ferret and pig modulate myocardial contraction. J Physiol. 1991 Aug;439:1–14. doi: 10.1113/jphysiol.1991.sp018653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speir E., Tanner V., Gonzalez A. M., Farris J., Baird A., Casscells W. Acidic and basic fibroblast growth factors in adult rat heart myocytes. Localization, regulation in culture, and effects on DNA synthesis. Circ Res. 1992 Aug;71(2):251–259. doi: 10.1161/01.res.71.2.251. [DOI] [PubMed] [Google Scholar]
- Springhorn J. P., Ellingsen O., Berger H. J., Kelly R. A., Smith T. W. Transcriptional regulation in cardiac muscle. Coordinate expression of Id with a neonatal phenotype during development and following a hypertrophic stimulus in adult rat ventricular myocytes in vitro. J Biol Chem. 1992 Jul 15;267(20):14360–14365. [PubMed] [Google Scholar]
- Thompson N. L., Bazoberry F., Speir E. H., Casscells W., Ferrans V. J., Flanders K. C., Kondaiah P., Geiser A. G., Sporn M. B. Transforming growth factor beta-1 in acute myocardial infarction in rats. Growth Factors. 1988;1(1):91–99. doi: 10.3109/08977198809000251. [DOI] [PubMed] [Google Scholar]
- Vakalopoulou E., Schaack J., Shenk T. A 32-kilodalton protein binds to AU-rich domains in the 3' untranslated regions of rapidly degraded mRNAs. Mol Cell Biol. 1991 Jun;11(6):3355–3364. doi: 10.1128/mcb.11.6.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Swain J. L. Acidic fibroblast growth factor mRNA is expressed by cardiac myocytes in culture and the protein is localized to the extracellular matrix. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2683–2687. doi: 10.1073/pnas.86.8.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman M., Melton D. A. Growth factors in early embryogenesis. Annu Rev Cell Biol. 1989;5:93–117. doi: 10.1146/annurev.cb.05.110189.000521. [DOI] [PubMed] [Google Scholar]