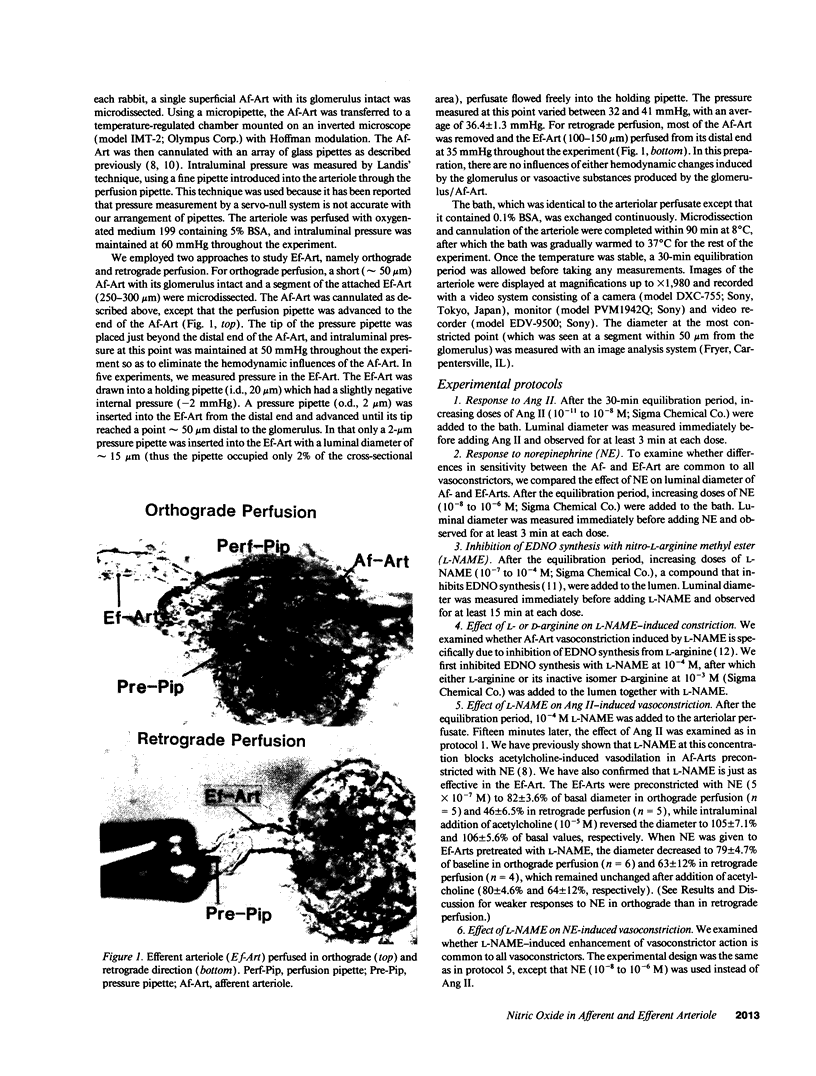
Abstract
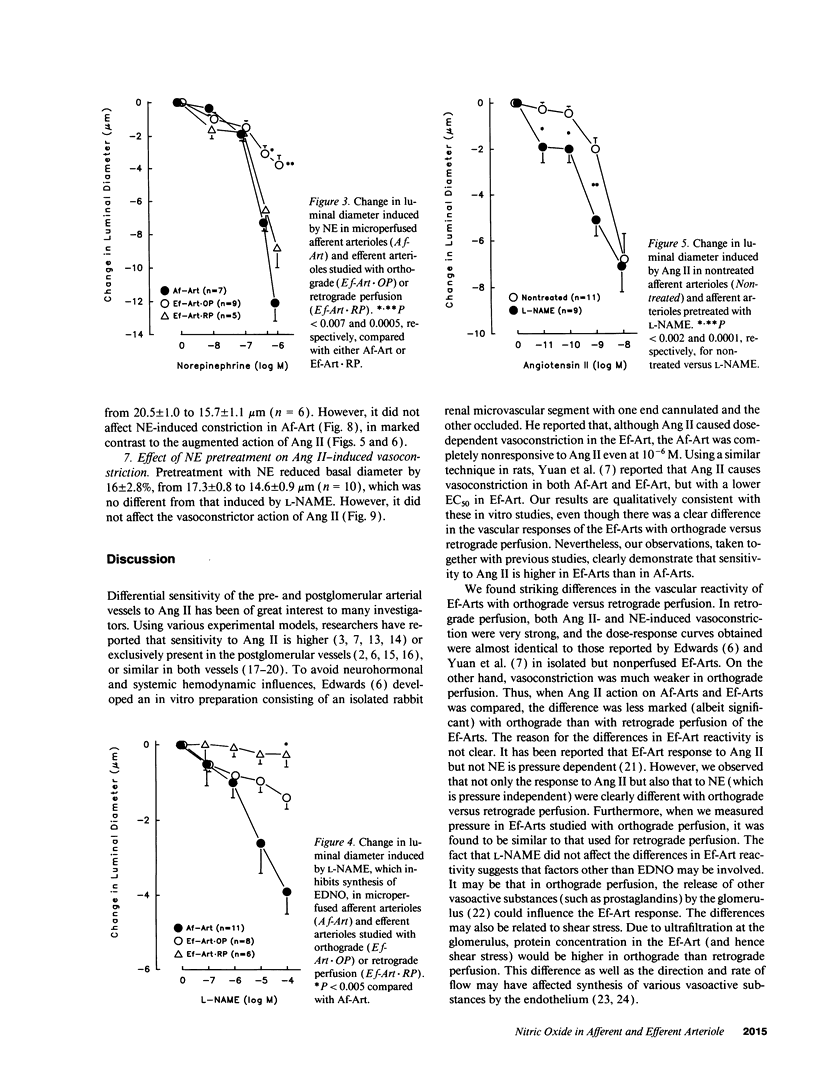
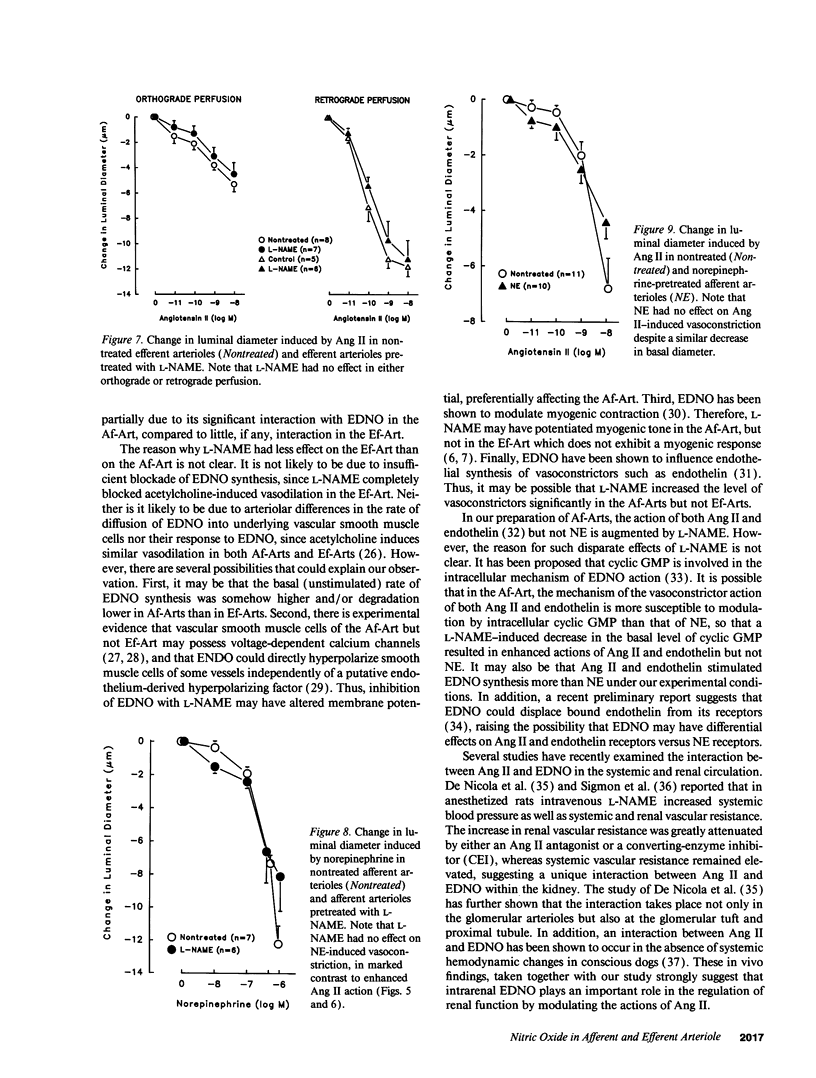
It has been reported that sensitivity to angiotensin II (Ang II) is higher in efferent (Ef) than afferent (Af) arterioles (Arts). We tested the hypothesis that this is due to arteriolar differences in the interaction between Ang II and endothelium-derived relaxing factor/nitric oxide (EDNO). Rabbit Af-Arts with glomerulus intact were microperfused in vitro at a constant pressure. Ef-Arts were perfused from the distal end of either the Af-Art (orthograde perfusion) or the Ef-Art (retrograde perfusion) to eliminate influences of the Af-Art or glomerulus, respectively. Ang II did not alter Af-Art luminal diameter until the concentration reached 10(-9) M, which decreased the diameter by 11 +/- 2.6% (n = 11; P < 0.002). In contrast, Ef-Arts became significantly constricted at concentrations as low as 10(-11) M with either perfusion. Surprisingly, the decrease in Ef-Art diameter at 10(-10), 10(-9), and 10(-8) M was significantly greater with retrograde perfusion (44 +/- 6.9%, 70 +/- 5.6%, and 74 +/- 4.1%, respectively; n = 5) than with orthograde perfusion (16 +/- 4.2%, 25 +/- 2.9%, and 35 +/- 3.5%; n = 9). ENDO synthesis inhibition with 10(-4) M nitro-L-arginine methyl ester (L-NAME) decreased the diameter to a greater extent in Af-Arts (22 +/- 3.0%; n = 11) compared to Ef-Arts with either orthograde (9.5 +/- 2.3%; n = 8) or retrograde perfusion (1.2 +/- 2.1%; n = 6). With L-NAME pretreatment, Af-Art constriction induced by 10(-10) M (14 +/- 4.0%, n = 9) and 10(-9) M Ang II (38 +/- 3.9%) was significantly greater compared to nontreated Af-Arts. In contrast, L-NAME pretreatment had no effect on Ang II-induced constriction in Ef-Arts with either perfusion. In conclusion, this study demonstrates higher sensitivity of Ef-Arts to Ang II, particularly with retrograde perfusion. Our results suggest that EDNO significantly modulates the vasoconstrictor action of Ang II in Af-Arts II but not Ef-Arts, contributing to the differential sensitivity to Ang II.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylis C., Harton P., Engels K. Endothelial derived relaxing factor controls renal hemodynamics in the normal rat kidney. J Am Soc Nephrol. 1990 Dec;1(6):875–881. doi: 10.1681/ASN.V16875. [DOI] [PubMed] [Google Scholar]
- Boulanger C. M., Lüscher T. F. Differential effect of cyclic GMP on the release of endothelin-1 from cultured endothelial cells and intact porcine aorta. J Cardiovasc Pharmacol. 1991;17 (Suppl 7):S264–S266. doi: 10.1097/00005344-199100177-00075. [DOI] [PubMed] [Google Scholar]
- Carmines P. K., Morrison T. K., Navar L. G. Angiotensin II effects on microvascular diameters of in vitro blood-perfused juxtamedullary nephrons. Am J Physiol. 1986 Oct;251(4 Pt 2):F610–F618. doi: 10.1152/ajprenal.1986.251.4.F610. [DOI] [PubMed] [Google Scholar]
- Carmines P. K., Navar L. G. Disparate effects of Ca channel blockade on afferent and efferent arteriolar responses to ANG II. Am J Physiol. 1989 Jun;256(6 Pt 2):F1015–F1020. doi: 10.1152/ajprenal.1989.256.6.F1015. [DOI] [PubMed] [Google Scholar]
- Casellas D., Carmines P. K., Navar L. G. Microvascular reactivity of in vitro blood perfused juxtamedullary nephrons from rats. Kidney Int. 1985 Nov;28(5):752–759. doi: 10.1038/ki.1985.194. [DOI] [PubMed] [Google Scholar]
- Click R. L., Joyner W. L., Gilmore J. P. Reactivity of gomerular afferent and efferent arterioles in renal hypertension. Kidney Int. 1979 Feb;15(2):109–115. doi: 10.1038/ki.1979.16. [DOI] [PubMed] [Google Scholar]
- Conger J. D., Robinette J. B., Schrier R. W. Smooth muscle calcium and endothelium-derived relaxing factor in the abnormal vascular responses of acute renal failure. J Clin Invest. 1988 Aug;82(2):532–537. doi: 10.1172/JCI113628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nicola L., Blantz R. C., Gabbai F. B. Nitric oxide and angiotensin II. Glomerular and tubular interaction in the rat. J Clin Invest. 1992 Apr;89(4):1248–1256. doi: 10.1172/JCI115709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dávalos M., Frega N. S., Saker B., Leaf A. Effect of exogenous and endogenous angiotensin II in the isolated perfused rat kidney. Am J Physiol. 1978 Dec;235(6):F605–F610. doi: 10.1152/ajprenal.1978.235.6.F605. [DOI] [PubMed] [Google Scholar]
- Edwards R. M. Response of isolated renal arterioles to acetylcholine, dopamine, and bradykinin. Am J Physiol. 1985 Feb;248(2 Pt 2):F183–F189. doi: 10.1152/ajprenal.1985.248.2.F183. [DOI] [PubMed] [Google Scholar]
- Edwards R. M. Segmental effects of norepinephrine and angiotensin II on isolated renal microvessels. Am J Physiol. 1983 May;244(5):F526–F534. doi: 10.1152/ajprenal.1983.244.5.F526. [DOI] [PubMed] [Google Scholar]
- Fleming J. T., Zhang C., Chen J., Porter J. P. Selective preglomerular constriction to nerve stimulation in rat hydronephrotic kidneys. Am J Physiol. 1992 Mar;262(3 Pt 2):F348–F353. doi: 10.1152/ajprenal.1992.262.3.F348. [DOI] [PubMed] [Google Scholar]
- Frangos J. A., Eskin S. G., McIntire L. V., Ives C. L. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985 Mar 22;227(4693):1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- Frega N. S., Davalos M., Leaf A. Effect of endogenous angiotensin on the efferent glomerular arteriole of rat kidney. Kidney Int. 1980 Sep;18(3):323–327. doi: 10.1038/ki.1980.142. [DOI] [PubMed] [Google Scholar]
- Hall J. E., Guyton A. C., Jackson T. E., Coleman T. G., Lohmeier T. E., Trippodo N. C. Control of glomerular filtration rate by renin-angiotensin system. Am J Physiol. 1977 Nov;233(5):F366–F372. doi: 10.1152/ajprenal.1977.233.5.F366. [DOI] [PubMed] [Google Scholar]
- Ichikawa I., Miele J. F., Brenner B. M. Reversal of renal cortical actions of angiotensin II by verapamil and manganese. Kidney Int. 1979 Aug;16(2):137–147. doi: 10.1038/ki.1979.115. [DOI] [PubMed] [Google Scholar]
- Ichikawa I., Pfeffer J. M., Pfeffer M. A., Hostetter T. H., Brenner B. M. Role of angiotensin II in the altered renal function of congestive heart failure. Circ Res. 1984 Nov;55(5):669–675. doi: 10.1161/01.res.55.5.669. [DOI] [PubMed] [Google Scholar]
- Ichikawi I., Harris R. C. Angiotensin actions in the kidney: renewed insight into the old hormone. Kidney Int. 1991 Oct;40(4):583–596. doi: 10.1038/ki.1991.249. [DOI] [PubMed] [Google Scholar]
- Ito S., Carretero O. A. An in vitro approach to the study of macula densa-mediated glomerular hemodynamics. Kidney Int. 1990 Dec;38(6):1206–1210. doi: 10.1038/ki.1990.335. [DOI] [PubMed] [Google Scholar]
- Ito S., Johnson C. S., Carretero O. A. Modulation of angiotensin II-induced vasoconstriction by endothelium-derived relaxing factor in the isolated microperfused rabbit afferent arteriole. J Clin Invest. 1991 May;87(5):1656–1663. doi: 10.1172/JCI115181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Juncos L. A., Nushiro N., Johnson C. S., Carretero O. A. Endothelium-derived relaxing factor modulates endothelin action in afferent arterioles. Hypertension. 1991 Jun;17(6 Pt 2):1052–1056. doi: 10.1161/01.hyp.17.6.1052. [DOI] [PubMed] [Google Scholar]
- Kastner P. R., Hall J. E., Guyton A. C. Control of glomerular filtration rate: role of intrarenally formed angiotensin II. Am J Physiol. 1984 Jun;246(6 Pt 2):F897–F906. doi: 10.1152/ajprenal.1984.246.6.F897. [DOI] [PubMed] [Google Scholar]
- Kato T., Iwama Y., Okumura K., Hashimoto H., Ito T., Satake T. Prostaglandin H2 may be the endothelium-derived contracting factor released by acetylcholine in the aorta of the rat. Hypertension. 1990 May;15(5):475–481. doi: 10.1161/01.hyp.15.5.475. [DOI] [PubMed] [Google Scholar]
- Lacolley P. J., Lewis S. J., Brody M. J. L-NG-nitro arginine produces an exaggerated hypertension in anesthetized SHR. Eur J Pharmacol. 1991 May 17;197(2-3):239–240. doi: 10.1016/0014-2999(91)90533-v. [DOI] [PubMed] [Google Scholar]
- Lahera V., Salom M. G., Miranda-Guardiola F., Moncada S., Romero J. C. Effects of NG-nitro-L-arginine methyl ester on renal function and blood pressure. Am J Physiol. 1991 Dec;261(6 Pt 2):F1033–F1037. doi: 10.1152/ajprenal.1991.261.6.F1033. [DOI] [PubMed] [Google Scholar]
- Levinsky N. G. Pathophysiology of acute renal failure. N Engl J Med. 1977 Jun 23;296(25):1453–1458. doi: 10.1056/NEJM197706232962509. [DOI] [PubMed] [Google Scholar]
- Loutzenhiser R., Hayashi K., Epstein M. Divergent effects of KCl-induced depolarization on afferent and efferent arterioles. Am J Physiol. 1989 Oct;257(4 Pt 2):F561–F564. doi: 10.1152/ajprenal.1989.257.4.F561. [DOI] [PubMed] [Google Scholar]
- Lüscher T. F., Bock H. A., Yang Z. H., Diederich D. Endothelium-derived relaxing and contracting factors: perspectives in nephrology. Kidney Int. 1991 Apr;39(4):575–590. doi: 10.1038/ki.1991.68. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B. D., Deen W. M., Brenner B. M. Effects of norepinephrine and angiotensin II on the determinants of glomerular ultrafiltration and proximal tubule fluid reabsorption in the rat. Circ Res. 1975 Jul;37(1):101–110. doi: 10.1161/01.res.37.1.101. [DOI] [PubMed] [Google Scholar]
- Navar L. G., Gilmore J. P., Joyner W. L., Steinhausen M., Edwards R. M., Casellas D., Carmines P. K., Zimmerhackl L. B., Yokota S. D. Direct assessment of renal microcirculatory dynamics. Fed Proc. 1986 Dec;45(13):2851–2861. [PubMed] [Google Scholar]
- Nollert M. U., Hall E. R., Eskin S. G., McIntire L. V. The effect of shear stress on the uptake and metabolism of arachidonic acid by human endothelial cells. Biochim Biophys Acta. 1989 Sep 11;1005(1):72–78. doi: 10.1016/0005-2760(89)90033-7. [DOI] [PubMed] [Google Scholar]
- Ohishi K., Carmines P. K., Inscho E. W., Navar L. G. EDRF-angiotensin II interactions in rat juxtamedullary afferent and efferent arterioles. Am J Physiol. 1992 Nov;263(5 Pt 2):F900–F906. doi: 10.1152/ajprenal.1992.263.5.F900. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Radermacher J., Klanke B., Schurek H. J., Stolte H. F., Frölich J. C. Importance of NO/EDRF for glomerular and tubular function: studies in the isolated perfused rat kidney. Kidney Int. 1992 Jun;41(6):1549–1559. doi: 10.1038/ki.1992.225. [DOI] [PubMed] [Google Scholar]
- Romero J. C., Lahera V., Salom M. G., Biondi M. L. Role of the endothelium-dependent relaxing factor nitric oxide on renal function. J Am Soc Nephrol. 1992 Mar;2(9):1371–1387. doi: 10.1681/ASN.V291371. [DOI] [PubMed] [Google Scholar]
- Sigmon D. H., Carretero O. A., Beierwaltes W. H. Angiotensin dependence of endothelium-mediated renal hemodynamics. Hypertension. 1992 Nov;20(5):643–650. doi: 10.1161/01.hyp.20.5.643. [DOI] [PubMed] [Google Scholar]
- Steinhausen M., Sterzel R. B., Fleming J. T., Kühn R., Weis S. Acute and chronic effects of angiotensin II on the vessels of the split hydronephrotic kidney. Kidney Int Suppl. 1987 May;20:S64–S73. [PubMed] [Google Scholar]
- Tare M., Parkington H. C., Coleman H. A., Neild T. O., Dusting G. J. Hyperpolarization and relaxation of arterial smooth muscle caused by nitric oxide derived from the endothelium. Nature. 1990 Jul 5;346(6279):69–71. doi: 10.1038/346069a0. [DOI] [PubMed] [Google Scholar]
- Trippodo N. C., Frohlich E. D. Similarities of genetic (spontaneous) hypertension. Man and rat. Circ Res. 1981 Mar;48(3):309–319. doi: 10.1161/01.res.48.3.309. [DOI] [PubMed] [Google Scholar]
- Yuan B. H., Robinette J. B., Conger J. D. Effect of angiotensin II and norepinephrine on isolated rat afferent and efferent arterioles. Am J Physiol. 1990 Mar;258(3 Pt 2):F741–F750. doi: 10.1152/ajprenal.1990.258.3.F741. [DOI] [PubMed] [Google Scholar]
- Zatz R., de Nucci G. Effects of acute nitric oxide inhibition on rat glomerular microcirculation. Am J Physiol. 1991 Aug;261(2 Pt 2):F360–F363. doi: 10.1152/ajprenal.1991.261.2.F360. [DOI] [PubMed] [Google Scholar]