OVERVIEW
Human ehrlichiosis and anaplasmosis are acute febrile tick-borne diseases caused by various members from the genera Ehrlichia and Anaplasma (Anaplasmataceae). Human monocytotropic ehrlichiosis was first reported in the United States in 1987, but during the ensuing 20 years it has become the most prevalent life-threatening tick borne disease in the US. The emergence of ehrlichiosis and anaplasmosis is become more frequently diagnosed as the cause of human infections, as animal reservoirs and tick vectors have increased in numbers and humans have inhabited areas where reservoir and tick populations are high.
Ehrlichia chaffeensis, the etiologic agent of human monocytotropic ehrlichiosis (HME) is an emerging zoonosis that causes clinical manifestations ranging from a mild febrile illness to a fulminant disease characterized by multi-organ system failure. The primary tick vector of HME is the Lone Star tick (Amblyomma americanum). A. phagocytophilum causes human granulocytotropic anaplasmosis (HGA), previously known as human granulocytotropic ehrlichiosis (HGE). Anaplasma phagocytophilum is transmitted by Ixodes scapularis, which also transmits agents that cause Lyme disease and babesiosis.
HME and HGA have similar clinical presentations including fever, headache, leukopenia, thrombocytopenia and elevated liver enzymes. Symptoms typically begin a median of 9 days following tick bite, with the majority of patients seeking medical attention within the first 4 days of illness. Neurologic manifestations are most frequently reported with HME. In this article, we review recent advances in the understanding of ehrlichial diseases related to microbiology, epidemiology, diagnosis, pathogenesis, immunity, and treatment of the two prevalent tick-borne diseases found in the United States, HME and HGA.
MICROBIOLOGY
The agents of human tick-borne ehrlichiosis include Anaplasma (formerly Ehrlichia) phagocytophilum, Ehrlichia chaffeensis, Ehrlichia ewingii, and recently reported E. canis (Table 1). These pathogens are members of the family Anaplasmataceae, in the order Rickettsiales, and they are classified as α-proteobacteria (1–4). The evolutionary relationships determined by 16S ribosomal RNA gene (rrs) and groESL comparisons indicate that Ehrlichia and Anaplasma spp., share a common ancestor with other obligate intracellular pathogens such as Wolbachia, Neorickettsia, Orientia, and Rickettsia (3–7). In addition to causing human disease, Ehrlichia species are important veterinary pathogens. Canine ehrlichiosis, first described in 1935 in Africa, is caused by Ehrlichia canis and is transmitted by the Brown Dog tick, Rhipicephalus sanguineus (6, 7). Ehrlichia ewingii, an agent that is transmitted by Amblyomma americanum, infects granulocytes and causes human ehrlichiosis ewingii (HEE) (8–10). Recent phylogenetic studies have concluded that the economically important veterinary pathogen Ehrlichia (formerly Cowdria) ruminantium (described in 1925) belongs to the genus Ehrlichia (10–16) (Figure 1).
Table 1.
Ehrlichiae and Anaplasmae Species Causing Medical and Veterinary Diseases
| Genus | Human or animal disease | Target Cells | Geographic Distribution | Vector | Reservoir |
|---|---|---|---|---|---|
| 1-Ehrlichia | |||||
| Ehrlichia chaffeensis | Human monocytic ehrlichiosis (HME) | Monocytes/Macrophages | southeast, south central, and Midwest states | Lone star tick Amblyomma | Deer |
| Ehrlichia canis | Canine ehrlichiosis, HME | Monocytes/Macrophages | southeast, south central, and Midwest states | Rhipicephalus | Dogs |
| Ehrlichia ewingii | Human ewingii ehrlichiosis (HEE) | Neutrophils | Southeast, south central, and Midwest states | Lone star tick Amblyomma Dermacenter variabilis | Dogs, Deer |
| Ehrlichia muris | Murine monocytic ehrlichiosis, possibly HME | Monocytes/Macrophages | Unknown | Ticks (Ixodes persulcatus, Haemaphysalis flava) | Apodemus mice, vole |
| Ehrlichia ruminantium | Heartwater in Cattle | Monocytes/Macrophages Endothelium | Africa | Lone star tick Amblyomma | Cattle, sheep, goats |
| 2-Anaplasma | |||||
| Anaplasma marginale | Bovine Anaplasmosis | Erythrocytes | Unknown | Cattle, wild ruminants | |
| Anaplasma phagocytophilum | Human granulocytic anaplasmosis (HGA) | Neutrophils | Northeastern and north central states and northern California | Ixodes | white-footed mice, wood rats, mice, horses, dogs, cats, sheep, cattle, white- tailed deer |
| 3-Neorickettsia | |||||
| Neorickettsia helminthoeca | No human infection | Monocytes/Macrophages | Unknown | Fish | Dogs |
| Neorickettsia sennetsu | Human infection Infectious mononucleosis like syndrome | Monocytes/Macrophages | Unknown | Ticks (Boophilus, Rhipicephalus, and others) | No animal reservoir |
| Neorickettsia risticii | No human infection Cause rickettsial disease in horses |
Macrophages, enterocytes, Ticks (Ixodes persulcatus, Haemophysalis flava )mast cells | Unknown | Trematode larvae | Horse |
Figure 1.
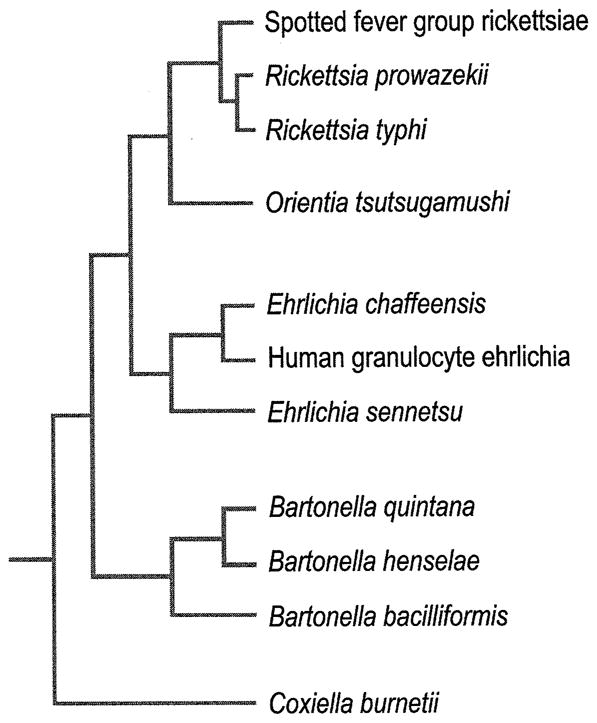
Phylogenetic relationships between rickettsias based on 16S rRNA gene sequences. (From Mason PR, Kelly PJ. Ch. 235: Rickettsia and Rickettsia-Like Organisms. In: Cohen & Powderly, editors. Infectious Diseases, 2nd ed. Mosby; 2004. Permission requested from Elsevier.)
Agents of human tick-borne ehrlichioses are small (approximately 0.4–1.5 μm), obligately intracellular Gram negative bacteria that replicate in membrane-bound compartments inside host granulocytes (A. phagocytophilum and E. ewingii) or mononuclear phagocytes (E. chaffeensis and E. canis) (17, 18) (Figure 2). Ehrlichiae replicate within the host vacuoles forming microcolonies called morulae, derived from the Latin word “morus” for mulberry (18–20). All Ehrlichia species pathogenic for humans can be cultivated in cell culture except E. ewingii (Figure 3A–C).
Figure 2.
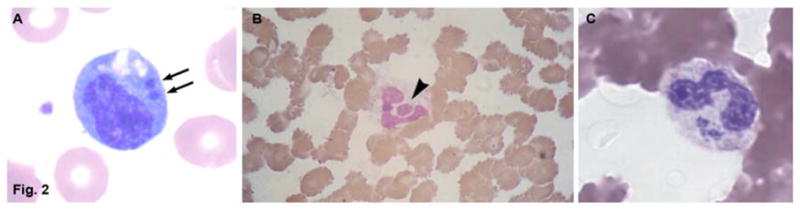
Peripheral blood leukocytes containing ehrlichial morula in patients with human monocytic ehrlichiosis (A) and human granulocytic anaplasmosis (B and C). A and B, a morula (arrow) containing Ehrlichia chaffeensis in a monocyte in patient with HME. B and C; a morula (arrowhead) containing Anaplasma phagocytophilum in a neutrophil in patient with HGA. Wright stains, original magnifications ×1,200. (A From Walker DH, Dumler JS. Ch. 190: Ehrlichia chaffeensis (Human Monocytotropic Ehrlichiosis), Anaplasma phagocytophilum (Human Granulocytotropic Anaplasmosis), and Other Ehrlichiae. In: Mandell, Bennett, & Dolin, editors. Principles and Practice of Infectious Diseases, 6th ed. Church Livingstone; 2005. Permission requested from Elsevier. B From Siberry GK, Dumler JS. Ch. 228: Ehrlichiosis and Anaplasmosis. In: Kliegman, editor. Nelson Textbook of Pediatrics, 18th ed. Saunders; 2007. Permission requested from Elsevier. C From Walker DH, Paddock CD, Dumler JS. Emerging and Re-emerging Tick-Transmitted Rickettsial and Ehrlichial Infections. Medical Clinics of North America, 2008; 92(6). Permission requested from Elsevier.)
Figure 3.
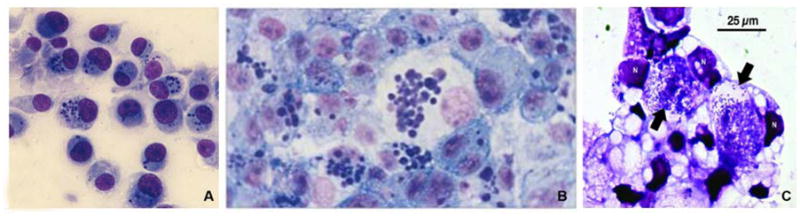
Light microscopic picture of canine monocytes (DH82) are heavily infected in vitro with E. chaffeenesis (A) and E. canis (B). Typical ehrlichial inclusions (morulae) are present inside the cytoplasm of infected cells (Giemsa staining. Orginal magnification × 200. (C) Giemsa-stained ISE6 cells infected with A. phagocytophilum strain isolated from a female I. scapularis tick. Anaplasma organisms exist in large intracellular vacuoles (arrow). N, host cell nucleus. Giemsa stain. (A Courtesy of Ismail N. B From Mason PR, Kelly PJ. Ch. 235: Rickettsia and Rickettsia-Like Organisms. Cohen & Powderly: Infectious Diseases, 2nd ed. 2004; permission requested from Elsevier. C From Massung RF, Levin ML, Munderloh UG et al. Isolation and Propagation of the Ap-Variant 1 Strain of Anaplasma phagocytophilum in a Tick Cell Line. J of Clin Micro, 2007; 2138–2143. Permission requested from JCM.)
Ehrlichia and Anaplasma exist intracellularly in two morphologically distinct ultrastructural forms dense-cored cells (DC) (0.4–0.6 μm) and reticulate cells (RC) (0.4–0.6 μm by 0.7–1.9) (Figure 4) (20). DCs are smaller and have an electron dense chromatin while the larger RCs have uniformly dispersed nucleoid filaments and ribosomes. In vitro kinetic analyses have shown that DC ehrlichiae predominate during the first 24 hour post-infection, suggesting that dense core forms are critical for bacterial adhesion and internalization. By 48 h post-infection, RC forms of ehrlichiae that divide by binary fission predominate. At 72h after infection, the RC ehrlichiae mature into dense-cored cell forms, correlating with the time when DC ehrlichiae are released to begin a new cycle (21, 22) (Figure 4). Consistent with their life cycle, DC and RC forms of ehrlichiae differentially express two tandem repeat containing proteins (TRP); TRP120 and TRP47. The TRP47 is a secreted effector protein that interacts with numerous host cell proteins involved in cell signaling, transcriptional regulation and vesicle trafficking. (23–26).
Figure 4. Electron micrographs of E. chaffeensis interaction with DH82 cells.
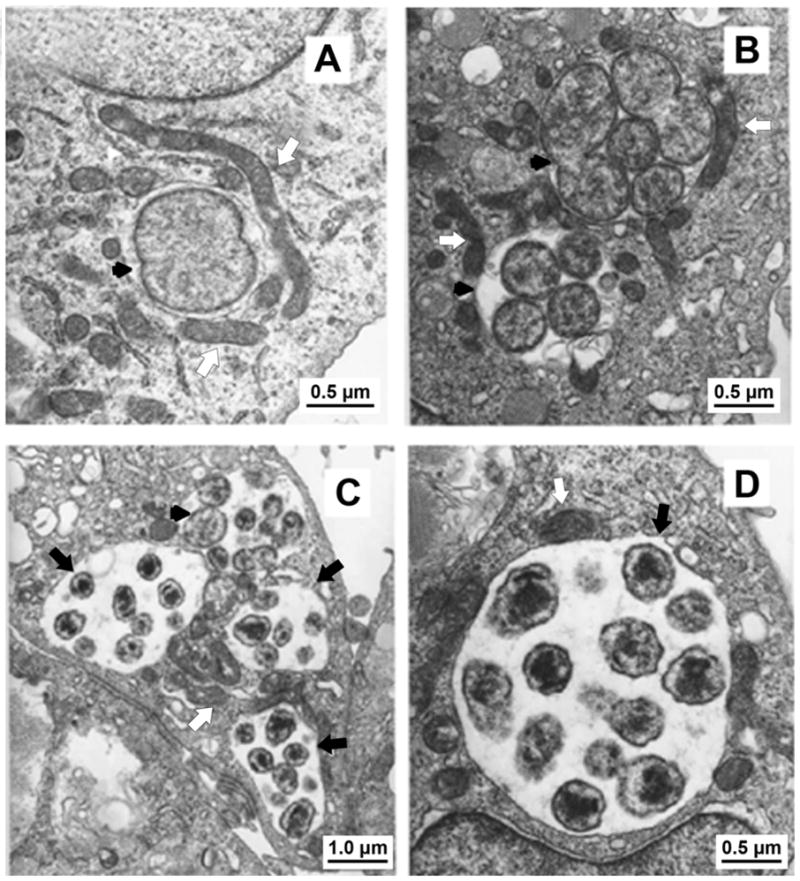
(A) At 24h post-infection, a single reticulate cell (RC) divide by binary fission (black arrowhead). (B) At 48h post-infection, two morulae (black arrowheads) contain RC. (C) At 72 h post infection, Ehrlichia have matured into dense core (DC). Three morulae contain DC (black arrows), and one morula contains RC (black arrowhead). (D) High power of a morula containing DC at 72 h post infection. Mitochondria surrounding morulae were indicated by white arrows. (From Zhang J-Z, Popov VL, Gao S, et al. The developmental cycle of Ehrlichia chaffeensis in vertebrate cells. Cellular Microbiology, 2006; 9(3):610–618. Permission requested from Blackwell Publishing Ltd.)
Ehrlichia and Anaplasma species have relatively small genomes (0.8–1.5Mb) that have undergone several types of reductive evolutionary processes as they have lost redundant genes and developed dependence on the host cell for necessary functions (27). Ehrlichia have a small subsets of genes associated with host-pathogen interactions including tandem repeat containing proteins and ankyrin repeat proteins. Other common features of the Ehrlichia genomes include low GC content and high proportion of non-coding sequences. E. chaffeensis and A. phagocytophilum also have genes for synthesis of all nucleotides, vitamins and cofactors (27).
Ehrlichia and Anaplasma have the characteristic Gram negative cell wall structure, but lack important cell membrane components including lipopolysaccharide and peptidoglycan (28). However, the ehrlichial cell wall is rich in cholesterol, which is derived from the host cell and appears to be important for ehrlichial survival and entry into mammalian cells. Recent study has demonstrated that A. phagocytophilum exploits host cell cholesterol derived from the low density lipoprotein receptor (LDLR)- mediated uptake pathway and LDLR regulatory system, to accumulate cholesterol in their inclusions to facilitate replication (29). In addition to the possible role of cholesterol in supporting bacterial cell wall and promoting internalization, it is possible that cholesterol rich cell walls may also function as ligands for stimulation of innate and acquired immune responses. In support of this conclusion, a recent report showed that heat-killed Ehrlichia muris are recognized by mouse CD1d-restricted natural killer T-cells, which recognize lipids and glycolipid (30).
E. chaffeensis, E. ewingii, E. canis, and A. phagocytophilum have immunodominant outer membrane proteins, which are members of Pfam PF01617 and constitute the OMP-1/MSP2/P44 families (31–39). Evaluation of different E. chaffeensis isolates demonstrated a differentially expression of p28/30-Omp proteins in infected macrophages and tick cell cultures. In infected macrophages, the dominant E. chaffeensis expressed proteins are the products of the p28-Omp 19 and 20 genes (40, 41). In cultured tick cells derived from E. chaffeensis vector, Amblyomma americanum and non-vector (Ixodes scapularis) ticks, E. chaffeenesis expression consists only of the p28-Omp 14 protein (40, 41). It is postulated that this differential expression of proteins within the p28/p30-Omp locus may be critical for the adaptation of Ehrlichia species to their different hosts (mammals and ticks). These observations support the long evolutionary relationship between the bacterium, its vector and its niche within host monocytes. How differential expression of these proteins provides an advantage to the organism within these extremely different environments is not known. Thus, in vivo investigations of ehrlichial infection in animal models and natural tick vectors will be necessary to determine in vivo the biological significance of these in vitro observations. To this end, a recent study by Ganta et al. (42) determined that C57BL/6 mice have different responses to infection depending on the source of the inoculum. ISE6 tick cell-derived Ehrlichia inoculated into mice results in a more persistent infection, which includes relapses of increasing bacterial load On the other hand, the A. phagocytophilum genome has three omp-1, one msp2, two msp2 homologs, one msp4, and 113 p44 loci belonging to the OMP-1/MSP2/P44 superfamily (37–40). P44 plays a role in the binding of A. phagocytophilum to surfaces of neutrophils and in antigenic variation and evasion of host immune responses. The P44s are diverse, include several paralogs (p44-1 to p44-65) expressed in mammals and ticks and confer antigenic environmental adaptation, especially during tick transmission (37–40).
Ehrlichiae also express several secreted ankyrin, tandem repeat (23–25, 43–46) and putative lipoptoteins (28, 29, 35) that are major targets of the humoral immune response. Several genes code for major immunoreactive proteins of E. chaffeensis including ankyrin (200 kDa) and tandem repeat containing proteins (120-, 47- and 32-kDa proteins) (23–25). These proteins contain major antibody eptiopes that are molecularly distinct and elicit strong species-specific host immune responses. The TRP47 has recently linked with host-pathogen interactions that suggest a complex network of interactions between TRP47 and the host (25) (Figure 5). Another major immunoreactive protein includes TRP32 (variable-length PCR target protein) E. chaffeensis, which has one conformational and one continuous epitope within the 90-bp TRs (24). TRP32 repeats vary in number among E. chaffeensis isolates; hence, it has been utilized as a molecular target for differentiation of E. chaffeensis isolates (24).
Figure 5.
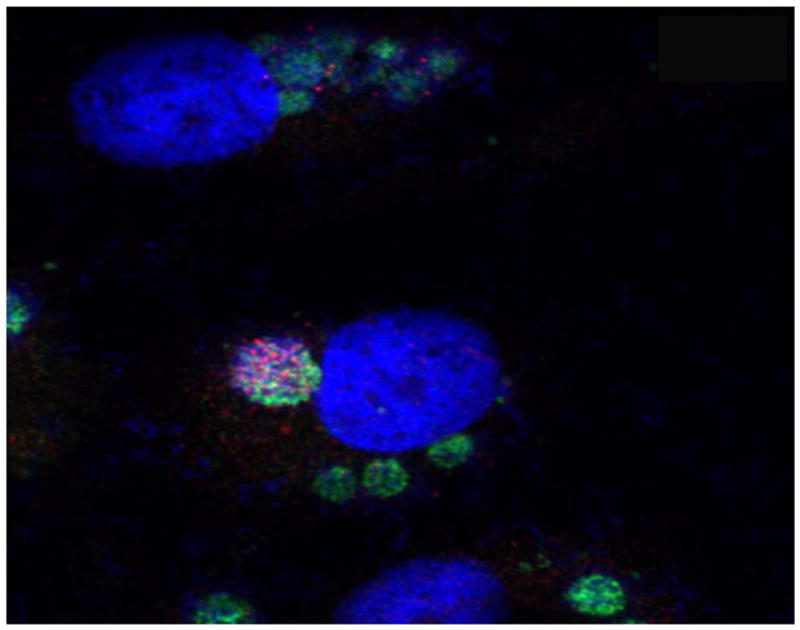
Ehrlichia TR47: ultrastructure and confocal microscopy. Differential expression of TRP47 (red) on dense-cored E. chaffeensis cultured in vitro (DH82 cells) as visualized using three color scanning laser confocal fluorescent microscopy. E. chaffeensis infected cells were dually stained with rabbit anti-Ehrlichia disulfide bond formation protein (Dsb) (green-Alexa Fluor 488), and mouse anti-E. chaffeensis TRP47 (red-Alexa Fluor 568). Host cell nuclei were counterstained with 4′, 6′-diamidino-2-phenylindole, dihydrochloride (DAPI) and images merged. (Courtesy of McBride JW.)
Ehrlichia and Anaplsama also contain genes for type IV secretion systems, which are structures known to use a complex of transmembrane proteins and a pilus to mediate the translocation of macromolecules across the cell envelopes of both gram negative and gram positive bacteria (47–49, 27). Genes for Type IV secretion are cotranscribed with genes for enzymes, such as superoxide dismutase (sodB), that catabolize reactive oxygen species. However, whether these are secreted by type IV secretion complexes needs to be investigated. Interestingly, among the genes that encode the classic type IV secretion system, VirB is common to all Ehrlichia species and has been associated with secretion of toxins (47–49). One substrate, Ank A of A. phagocytophilum, of the type IV secretion system has been identified. AnkA is secreted into host cell cytoplasm where it interacts with host cell tyrosine kinase Abl and phosphotase SHP-1 and eventually transported into the host cell nucleus. There it interacts with nuclear chromatin and appears to target gene regulatory regions (44–46). Other virulence genes, such as those that encode two-component regulatory systems have also been described and studied, and appear to be involved in intracellular survival by inhibiting lysosomal fusion. Further comparison of ehrlichial genomes will provide insight and facilitate investigations of bacterial virulence factors, disease pathogenesis, and mechanisms of immune modulation, and will provide targets for vaccines or new antimicrobial therapies.
EPIDEMIOLOGY
HME Epidemiology
HME was first described in 1986, and now more than 2,300 cases have been reported to the CDC in the past 19 years (50–52). While the average incidence of HME in the United States is estimated at 0.7 cases/million population, this incidence is based on passive surveillance and is likely a significant underestimation of the actual disease incidence (Figure 6A). Active surveillance in endemic areas has suggested rates of HME of 100–200 cases per a population of 100,000 (51, 52). The true incidence of human infection with E. chaffeensis is likely to be much higher, as two-thirds of the infections are either asymptomatic or minimally symptomatic (53–59). A sero-prevalence study found that 20% of the children residing in endemic areas had detectable antibody to E. chaffeensis, without prior history of clinical disease (58, 59).
Figure 6.
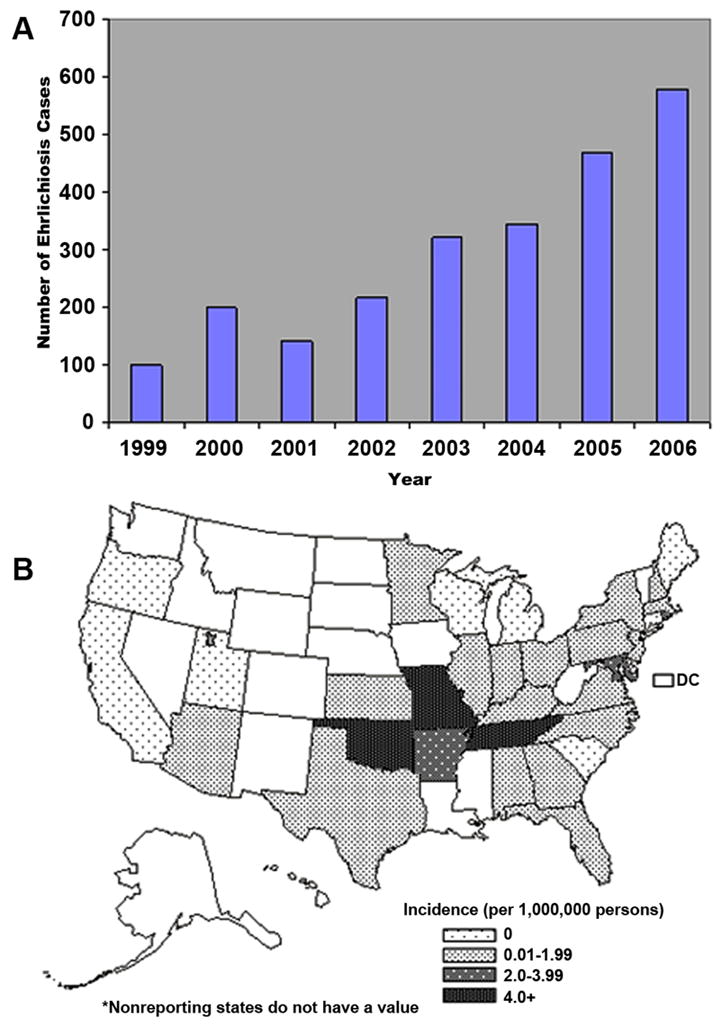
A) Number of ehrlichiosis cases caused by E. chaffeensis reported to CDC by State Health Department from 1999–2006. B) Average reported annual incidence of human monocytic ehrlichiosis by state –United States, 2001–2002 and nationwide. (Open access: published by the Centers for Disease Control and Prevention, a U.S. Government agency and are in the public domain and can be used without permission.)
Similar to other tick borne diseases, the distribution of the arthropod vectors and vertebrate reservoirs correlates with the human disease incidence (60, 61). States with the highest reported rates of HME include Mississippi, Oklahoma, Tennessee, Arkansas, and Maryland (62) (Figure 6B). The dominant zoonotic cycle of E. chaffeensis involves a reservoir of many persistently infected white-tailed deer (Odocoileus virginianus) and the tick vector, Amblyomma americanum, prevalent throughout the southeast and southcentral United States (63–66) (Figure 7A). Other reservoirs such as dogs and coyotes and other tick vectors including Ixodes pacificus (67), Ixodes ricinus (68), Haemophysalis yeni (69), Amblyomma testudinarium, Amblyomma maculatum and Dermacentor variabilis (69) may also have a limited role in human transmission. Similar to other ticks, Amblyomma ticks have three feeding stages (larval, nymph, and adult); each developmental stage feeds only once. Trans-stadial (i.e. larva-nymph-adults) transmission of Ehrlichia occurs during nymph and adult feeding stages because larvae are uninfected. In contrast to Rickettsia spp., Ehrlichia are not maintained by trans-ovarial transmission (Figure 7B).
Figure 7.
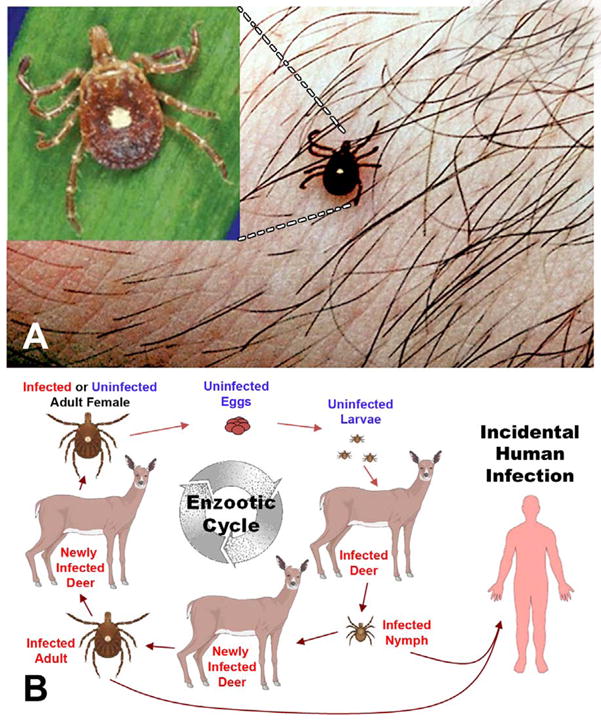
A) Lone Star tick; Amblyomma americanum that transmit the agent of human monocytic ehrlichiosis B) Life Cycle of monocytotropic E. chaffeensis. (Courtesy of Bloch KC, and open access: published by the Centers for Disease Control and Prevention, a U.S. Government agency and are in the public domain and can be used without permission)
There have been case reports of patients co-infected with both E. chaffeensis and R. rickettsii, which, although spread by different tick vectors, share a common geographic distribution (70). Among cases of HME reported to the CDC between 2001 and 2002, 61% were male, and 95% of cases self-identified as Caucasian (62). The median age for infection was 53 years; however the age-specific incidence was highest in the group aged 70 years and above. While cases were reported year-round, the greatest number of cases occurred during the period of May through August, corresponding to periods of abundant tick populations and human outdoor recreation.
Epidemiology of HGA
In the early 1990s, patients from Michigan and Wisconsin with a tick bite history experiencing a febrile illness similar to HME were described (71, 72). These cases were distinguishable by the presence of inclusion bodies in granulocytes rather than monocytes, causing this syndrome initially to be termed human granulocytic ehrlichiosis (HGE). The disease has recently been renamed human granulocytic anaplasmosis, or HGA, after phylogenetic analysis reclassified Ehrlichia phagocytophilum as a member of the genus Anaplasma (72, 73).
More than 2,900 cases of HGA have been reported to the CDC between 1994 and 2005, with the annual number of cases of HGA exceeding that of HME at an estimated annual incidence of 1.6 cases per million in the US. The highest annual incidence rates of HGA in the United States have been reported in Connecticut (14–16 cases per 100,000), Wisconsin (24 to 58 cases per 100,000 population), and New York state (2.7/100,000) (64) (Figures 8A and B). Active surveillance in endemic areas has identified incidence rates of >50 cases per 100,000 population (73–75). As with E. chaffeensis, serosurveillence studies suggest that asymptomatic disease is common (74–75).
Figure 8.
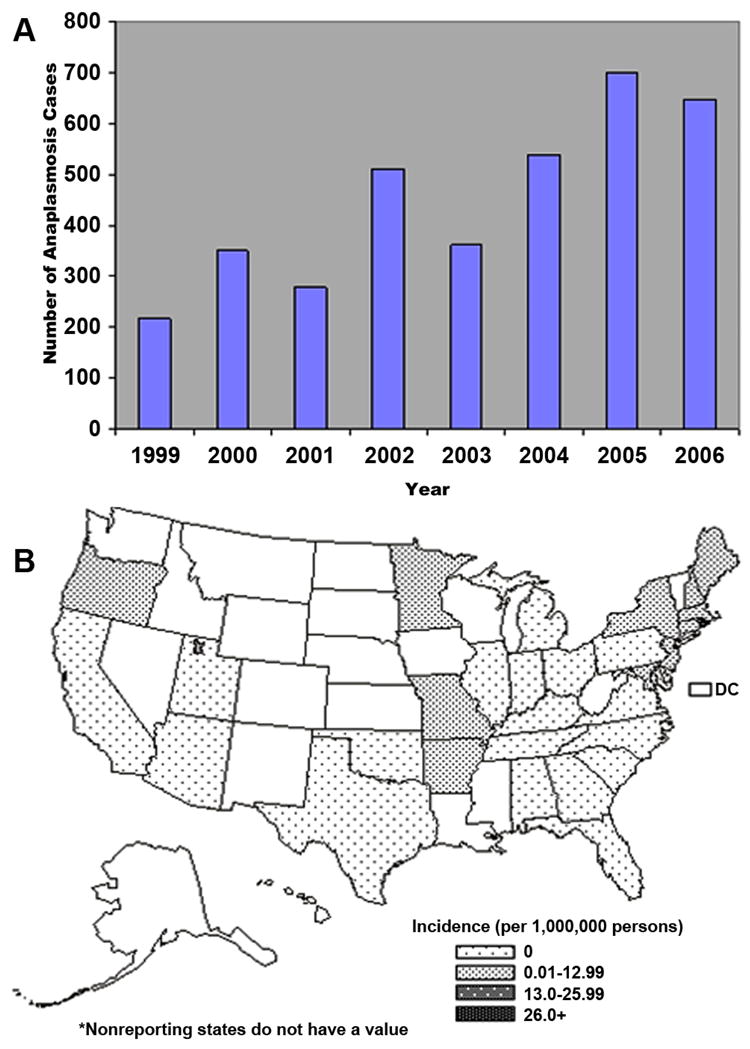
A) Number of ehrlichiosis cases caused by A. phagocytophilum reported to CDC by State Health Department from 1999–2006. B) Average reported annual incidence of human monocytic ehrlichiosis by state –United States, 2001–2002 and nationwide. (Open access: published by the Centers for Disease Control and Prevention, a U.S. Government agency and are in the public domain and can be used without permission.)
A. phagocytophilum is transmitted by Ixodes scapularis (Figure 9A) in New England and North Central United States, Ixodes pacificus (Figure 9B) in the western United States, I. ricinus in Europe, and I. persulcatus in Asia. Ixodes scapulais is also the tick vector for Borrelia burgdorferi, Babesia microti, and tickborne encephalitis viruses, and therefore ~10% of patients with HGA have serologic evidence of coinfection with Lyme disease, or babesiosis (76). The reservoir for A. phagocytophilum is primarily small mammals such as the White-footed mouse (Peromyscus Leucopus); Dusky-footed Wood rats (Neotoma fuscipes) or others such as such as Apodemus, Microtus or Clethrionymus species, with humans serving as dead-end hosts (72). Transmission of A. phagocytophilum from tick-mammalian reservoir to humans is similar to that of E. chaffeensis.
Figure 9.

A) Blacklegged tick; Ixodes Scapularis that transmit the agent of human granulocytic ehrlichiosis and Lyme disease. B) Western black-legged tick; Ixodes Scapularis that transmit the agent of human granulocytic ehrlichiosis (A & B Open access: published by the Centers for Disease Control and Prevention, a U.S. Government agency and are in the public domain and can be used without permission)
Demographic characteristics of HGA patients are similar to HME patients. The median age is 51, with more than 95% of cases are reported in Caucasians, with a slight male predominance (62). Cases occur year-round, with a peak incidence during June and July, perhaps reflecting the shorter arthropod season in these northern states or the relative importance of the nymphal stage of Ixodes ticks in disease transmission. Given the ubiquity of the tick vector, it is not surprising that cases of HGA have been confirmed world-wide, including Europe and Asia (China, Siberian Russia, and Korea) (Figure 10A and B).
Figure 10.
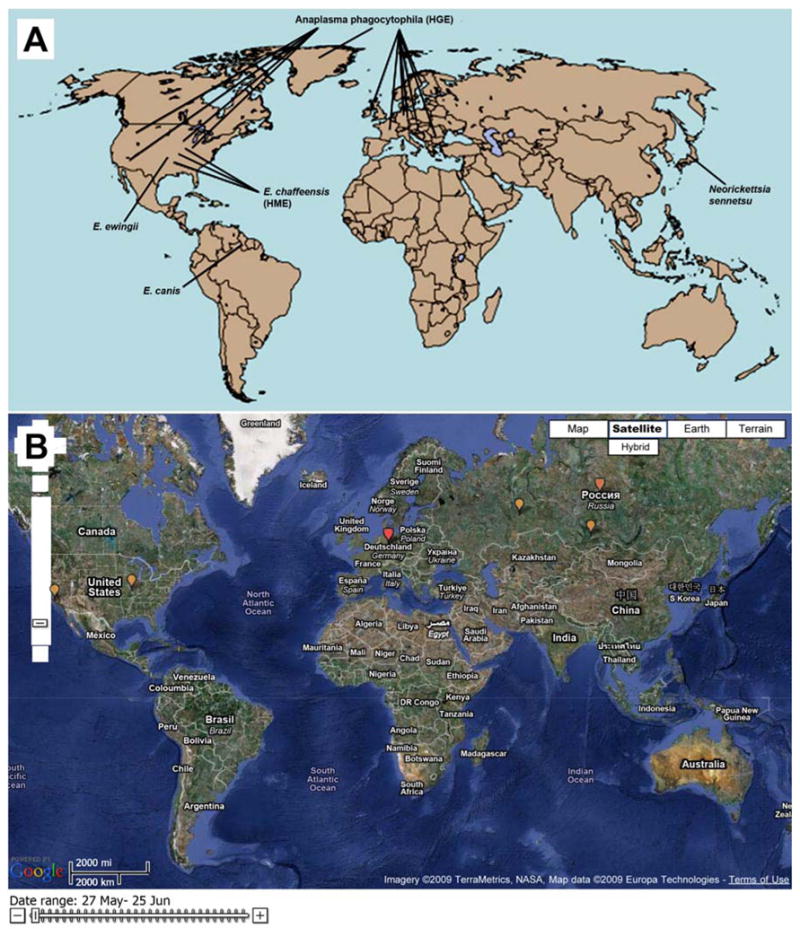
A) Worldwide distribution of Ehrlichia and Anaplasma. HGA: human granulocytic anaplasmosis; HME: human monocytic ehrlichiosis. B) Global health map shows worldwide distribution of HGA and HME marked by yellow dots. (A From Raoult D. Ch. 348: Rickettsioses. In Goldman, editor. Cecil Medicine, 23rd ed. Saunders, 2007. Permission requested from Elsevier. B From “Worldwide Outbreak of Ehrilichia” tracking map from http://www.healthmap.com [accessed June 15, 2009]. Permission granted.)
Epidemiology of HEE
Ehrlichia ewingii was exclusively a canine pathogen until a series of four human cases of E. ewingii infection were described in 1999 (5, 8, 9). The epidemiology of HEE remains poorly defined due to the lack of a specific serologic assay for this organism and the absence of a dedicated reporting system for this infection. Most infections reported to date have occurred in patients with HIV (5, 8, 9), or who were immunosuppressed following organ transplantation (77). Amblyomma americanum, the primary vector for E. chaffeensis, is also the primary vector for E. ewingii. Most cases of HEE have been reported in Tennessee, Missouri, and Oklahoma. However, E. ewingii infection in deer, dogs and ticks have been described throughout the range of the Lone Star tick, suggesting that human infection with this pathogen might be more widespread than is currently documented (72).
CLINICAL PRESENTATION
HME General Clinical Features
Human monocytotropic ehrlichiosis is a more severe disease than HGA or HEE, with 42% of cases requiring hospitalization, and a case-fatality rate of 3% (1, 3, 51–54, 65). The median age of patients with either infection is ~50 years, and slightly more males (57% – 61%) are infected than females. Up to 17% of patients develop life-threatening complications, although severe disease and death are more common in immunocompromised patients (5, 19, 62). HME can be fatal in immunocompetent patients and manifested as a multisystem disease resembling toxic or septic shock syndrome, or Rocky Mountain spotted fever, except for the infrequent occurrence of rash (78–80). Other life-threatening manifestations include cardiovascular failure, aseptic meningitis, hemorrhages, hepatic insufficiency or failure, interstitial pneumonia, and adult respiratory distress syndrome (79–82). The severity of the disease is greater in elderly and immunocompromised patients; however, HME can be fatal even in immunocompetent patients. Several studies have reported an association between the use of sulfonamide antibiotics and severe manifestations of Ehrlichia (53, 83). Whether this represents a causal relationship is unknown. A study of HME among transplant patients found no difference in severity of illness among patients taking prophylactic sulfa-antibiotics (53, 77).
Fever is an almost universal symptom (97%), followed by headaches (80%), myalgias (57%), and arthralgias (41%) (49–51, 74). A skin eruption is relatively common among children with HME, occurring in 66% of pediatric cases compared to 21% of adults (51, 52). A rash is present in 10% of cases of HME and can be maculopapular, petechial, or be characterized by diffuse erythroderma (80), but typically spares the face, palms, and soles of the feet. Nausea, vomiting, abdominal pain and cough are variably present. Gastrointestinal symptoms such as nausea, vomiting, diarrhea and anorexia are sometimes reported in the course of HME, mainly in children. Other frequently observed signs and symptoms in children and pregnant women with HME are altered mental status and abdominal pain that can be severe mimicing acute appendicitis..
Although the clinical manifestations of E. chaffeensis infection are nonspecific, laboratory abnormalities provide important diagnostic clues. A prospective cohort study of patients in an endemic area presenting with a febrile illness following a tick-bite, found a significantly lower WBC (mean 4.6 × 109 cells/L), neutrophil (mean 2.6 × 109 cells/L), and platelet count (mean 172 ×109 cells/L) among patients with HME than non-infected patients, and elevated transaminase levels were present in 83% of cases (72). In pediatric patients, mild hyponatremia has been reported in 50% of the cases (51, 52, 72), but this finding has been less frequently noted among infected adults.
HME Neurologic Features
The most frequent neurologic manifestation of HME is meningitis or meningoencephalitis. Central nervous system involvement is identified in approximately 20% of patients with HME (82), and in some cases may be associated with seizures and coma. Uncommon complications include cranial nerve palsy, with onset after initiation of effective antimicrobial therapy being reported (79–82). Long-term neurologic sequelae in children are uncommon, but include cognitive delays, fine motor impairment, and persistent foot drop. Subjective neurocognitive deficits following meningoencephalitis have also been reported in adults (72, 82–87).
Among patients with HME who undergo lumbar puncture, CSF pleocytosis is identified in approximately 60% (82). While most samples have a lymphocytic predominance, a neutrophilic or mixed picture is found in a third of cases (82). The CSF WBC count is typically < 100 cells/mm3, and protein may be mildly elevated. Morulae are rarely identified in CSF monocytes by Giemsa stain (1, 80–82). Radiographic imaging may be normal, or may show leptomeningeal enhancement. Bilateral medial temporal lobe enhancement has been reported for a case with PCR evidence of E. chaffeensis and A. phagocytophilum coinfection (88). Electroencephalogram may show nonspecific slowing (82). Although pathologic review of brain tissue from patients with HME neurologic dysfunction is limited, one study reported atypical lymphoid infiltration of the leptomeninges and Virchow-Robin space, with sparing of the brain parenchyma, while others have not shown CNS pathology (82).
HGA General Clinical Features
HGA resembles HME with respect to the frequency of fever, headache, and myalgias, but rash is uncommon, noted in less than 10% of patients (44, 50, 75, 81). As with HME, leukopenia, thrombocytopenia, and elevations in transaminases are important clues to the diagnosis. HGA tends to be a less severe illness than HME, although life-threatening complications including acute respiratory distress syndrome, acute renal failure, and hemodynamic collapse have been reported.
HGA Neurologic Features
Central nervous system involvement is uncommon in HGA, with meningoencephalitis reported in only approximately 1% of cases (88, 90). In contrast, a number of different peripheral nervous system manifestations have been described, including brachial plexopathy, cranial nerve palsies, and demyelinating polyneuropathy (1, 88, 90), and bilateral facial nerve palsy (88, 90), where recovery of neurologic function may be delayed over several months. As the geographic distribution of B. burgdorforii is similar to A. phagocytophila, patients should be tested for co-infection since Lyme disease has similar neurologic manifestations. Although the cause of neurologic dysfunction in HGA is not yet known, it is thought to be due to complicating opportunistic infections, or concomitant co-infection with B. burgdorforii. Lumber puncture is performed less frequently for HGA than HME. Reported CSF abnormalities include lymphoctyic pleocytosis and moderate elevation in proteins (87, 88, 90).
HEE General Clinical Features
Little is known of the clinical spectrum of HEE due to the paucity of reported cases. Symptoms appear to be similar to those described for HME and HGA. Despite the fact that the majority of HEE infections have been in immunocompromised hosts, the clinical manifestations appear to be milder (77, 84). Findings of leukopenia, thrombocytopenia, and abnormal liver function tests are variably present (77, 84).
HEE Neurologic Features
Headache is a frequent symptom in HEE, and may be associated with meningitis, but the frequency of this finding and the spectrum of neurologic manifestations are unknown. One instance of neutrophilic pleocytosis in a patient with HEE has been reported (8).
Pathogenesis
Following tick bite, Ehrlichia and Anaplasma enter circulation where they multiply within their target cells monocytes/macrophages and polymorphonucelar leukocytes, respectively. Ehrlichia and Anaplasma enter through receptor-mediated endocytosis via a glycophosphoinositol anchored receptor within caveolae- or lipid rafts. Ehrlichiae were found to localize exclusively within endosomes that avoid phagolysosomal pathways (91). Ehrlichia and Anaplasma multiply within endosomes ultimately reprograming host cell defense mechanisms and processes in order to facilitate their survival.
Pathogenesis of HME
Following entry into mononuclear phagocytes, E. chaffeensis inhibits phagolysosome fusion involving genes controlled by a two-component regulatory system. E. chaffeensis also suppresses and induces host genes to facilitate their intracellular survival (91–93). Microarray analysis of THP-1 cells infected with E. chaffeensis revealed downregulation of Th1 cytokines such as IL-12 and IL-18, which are important inducers of adaptive Th1 mediated immune responses, and genes such as SNAP 23 (synaptosomal-associated protein, 23 kDa), Rab5A (member of RAS oncogene family), and STX16 (syntaxin 16), which are involved in membrane trafficking, while upregulating apoptosis inhibitors and cell cyclins (93). Recently an immunoreactive, secreted 200 kDa ankyrin protein (Ank200) of E. chaffeensis has been shown to be translocated to the host cell nucleus where it binds gene regulatory regions within Alu elements and is thought to play a role in host cell gene regulation (25). E. chaffeensis also circumvents host defenses by inhibiting the signal transduction pathway (Jak/Stat) of interferon-γ–mediated anti-ehrlichial activity, and increasing transferrin receptor delivery of iron to the ehrlichial vacuole. In vitro studies have shown that E. chaffeensis down-regulates surface expression of toll-like receptors (TLR) 2 and 4 and CD14 on infected target cells, and inhibits activation of several transcription factors that are involved in the induction of proinflammatory innate immune responses (92, 93). The mechanism by which down-regulation of TLR 2 and 4 benefits survival within the macrophage is not understood, as pathogen-associated molecular patterns (PAMPs) have not been identified in ehrlichiae, and as mentioned above, the traditional ligands for these receptors, peptidoglycan and LPS, that activate TLR-2 and -4, respectively, are not present in the bacterium (28). However, it is postulated that loss of traditional PAMPs in Ehrlichia genome may enable them to persist within their tick vectors, without inducing strong innate defenses that are normally elicited by these patterns (94).
Studies with Ehrlichia muris have demonstrated that activation of innate lymphocytes such as natural killer T cells (NKT) occurs in a manner that is independent of TLRs, but dependent upon CD1d expression on antigen presenting cells such as the dendritic cells. Although NKT stimulation by Ehrlichia results in the production of IFN-γ and elimination of intracellular ehrlichiae (30), they also contribute to the development of Ehrlichia induced toxic shock-like syndrome in murine models of fatal monocytic ehrlichiosis (95).
Frequent pathologic findings of HME include granuloma formation, myeloid hyperplasia, and megakaryocytosis in the bone marrow (79–81, 88). Some patients develop erythrophagocytosis and plasmacytosis, suggesting a compensatory response. Other pathologic findings in patients with severe HME include focal hepatocellular necrosis; hepatic granulomas; cholestasis; splenic and lymph node necrosis; diffuse mononuclear phagocyte hyperplasia of the spleen, liver, lymph node, and bone marrow; perivascular lymphohistiocytic infiltrates of various organs including kidney, heart, liver, meninges and brain; and interstitial mononuclear cell pneumonitis (96–98). The severe pathology and multi-organ involvement in severe and fatal HME in immunocompetent patients is thought to be related to dysregulation of the host immune response that leads to tissue damage and eventually multi-system organ failure (99). This conclusion is based on the finding that in fatal disease in the form of toxic shock-like syndrome, uninfected hepatocytes undergo apoptosis without evidence of ehrlichial infection (96–98). Similarly, analysis of hepatic tissues from autopsy cases in immunocompetent patients with HME showed lymphohistiocytic foci, centrilobular and/or coagulation necrosis, Kupffer cell hyperplasia, and marked monocytic infiltration, while Ehrlichia-infected cells are rarely identified (96–98, 100). In contrast, an overwhelming ehrlichial burden in the organs was observed in HME patients who are immunocompromised due to other infections such as HIV or chemotherapy (96, 98). The hypothesis that severe and fatal Ehrlichia-induced toxic shock like syndrome is due to an immunopathologic mechanism and is supported by studies in murine models of fatal ehrlichiosis, where lethal ehrlichial infection with virulent Ehrlichia species named Ixodes Ovatus Ehrlichia (IOE) (101, 102) results in a progressive, fatal ehrlichiosis that mimics toxic shock-like syndrome (103). Characteristic of this disease in murine model of fatal monocytotropic ehrlichiosis include focal hepatic necrosis and apoptosis compared to formation of granuloma in animal model of mild monocytotropic ehrlichiosis (Figure 11), liver dysfunction marked by elevated liver enzymes (AST &ALT), significant leucopenia and lymphopenia, and CD4+T cell apoptosis (103–106). Further analysis indicated that severe and fatal primary HME in animals is due to the early overproduction of pro-inflammatory cytokines (e.g. TNF-α), and anti-inflammatory IL-10 cytokines. Interestingly, CD8+T cells appear to play a pathogenic role in HME where fatal disease is correlated with significant expansion of cytotoxic CD8+T cell producing TNF-α and IFN-γ. Mice that lack CD8+T cells and infected with virulent Ehrlichia species are protected against fatal disease, showing decreased tissue injury, normal CD4+T cell populations and increased protective CD4+Th1 responses, which suggest that CD8+T cell mediate lymphopenia and tissue damage in fatal HME in immuocompetent host (105). Consistent with pathogenic role of CD8+T cells in fatal murine ehrlichiosis, Dierberg and Dumler (107) reported a significantly greater amount of hemophagocytosis (macrophage activation) and an increased number of CD8+ cells associated with low bacterial burden in the lymph nodes of patients who died of HME (Figure 12A-C). Thus, both human and animal model data suggest that organ pathology and fatal disease is indeed due to immune mediated pathology.
Figure 11.
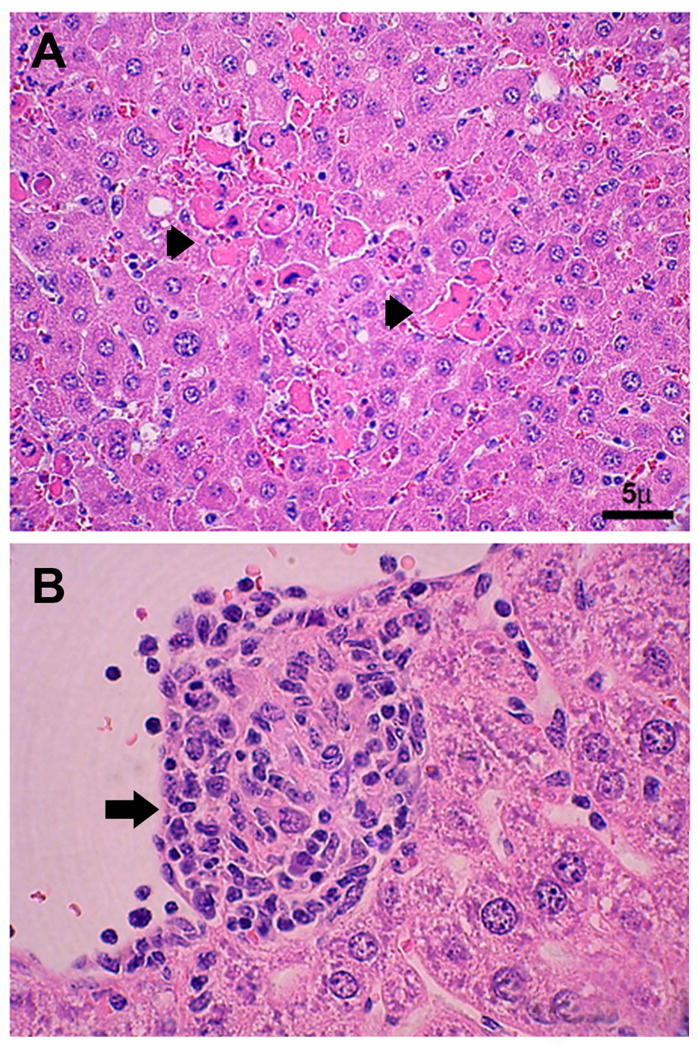
A) Hepatic histopathology in the murine model of fatal monocytic ehrlichiosis caused by systemic infection with virulent moncytic Ehrlichia (IOE). H&E staining shows extensive focal necrosis and apoptosis of hepatocytes (arrows). B) Hepatic histopathology in the murine model of mild monocytic ehrlichiosis caused by infection with mildly virulent Ehrlichia muris. H&E staining shows formation of well formed granuloma (arrowhead). (From Ismail N, Soong L, McBride JW, et al. Overproduction of TNF-alpha by CD8+ type 1 cells and down-regulation of IFN-gamma production by CD4+ Th1 cells contribute to toxic shock-like syndrome in an animal model of fatal monocytotropic ehrlichiosis. J Immunol, 2004; 172(3):1786–800. Permission granted.)
Figure 12.
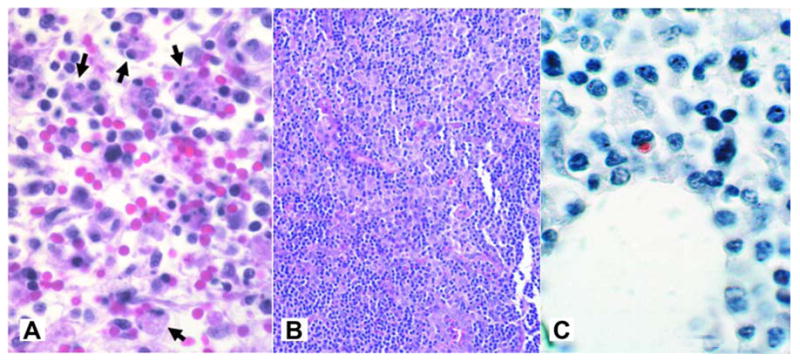
A) Hemophagocytosis (arrows) in lymph nodes from a patient with HME (H&E; original magnification 240×). B) High cellularity in lymph node from patient with HME (magnificationX 64). C) Immunohistochemical staining of lymph nodes from patient with HME shows typical low bacterial burden (immunoperoxidase with hematoxylin counterstain; original magnification 240×). (From Dierberg KL, Dumler JS. Lymph node hemophagocytosis in rickettsial diseases: a pathogenetic role for CD8 T lymphocytes in human monocytic ehrlichiosis (HME)? In: BMC Infect Dis. 2006; 6:121. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.)
Pathogenesis of HGA
A. phagocytophilum is observed predominantly in neutrophils in the peripheral blood and tissues from infected individuals. A. phagocytophilum has the unique ability to selectively survive and multiply within cytoplasmic vacuoles of PMN cells by delaying their apoptosis through upregulation of anti-apoptotic bcl-2 family member bfl-1 (A1), and blocking anti-FAS (CD95/Apo-1)-induced programmed cell death of human neutrophils (108, 109). A. phagocytophilum uses multiple evasion strategies to inhibit neutrophil anti-microbial functions (110, 111). Some studies have suggested that one of the mechanisms by which A. phagocytophilum avoids the toxic effects of neutrophils is its ability to inhibit the fusion of the lysomes with the cytoplasmic vacuoles and by arresting or inhibiting other signaling pathways related to respiratory burst (108–113). The mechanism by which A. phagocytophilum inhibits phagosome–lysosome fusion remains to be elucidated. A. phagocytophilum appears to modulate host cell gene transcription through AnkA, a secreted protein which is transported to the infected host cell nucleus through binding to protein: DNA complexes in neutrophil nuclei (114). Further studies are required to establish the effects of AnkA binding on neutrophil functions. The molecular basis by which A. phagocytophilum affects respiratory burst of granulocytes shown to be due to the down-regulation of gp91phox and rac2, two important components of NADPH oxidase (111, 113). Paradoxically, another study showed that infection with A. phagocytophilum induced protracted degranulation in human neutrophils (115), which was attributed to inflammatory tissue injury. In addition, A. phagocytophilum upregulates the production of chemokine IL-8 as well as pro-inflammatory cytokines. This increased proinflammatory activity and chemokines may facilitate the recruitment of additional host neutrophil cells and localized tissue injury when neutrophils are unable to generate effective antimicrobial responses (116).
Pathologic findings in patients with HGA and animal models include normocellular or hypercellular bone marrow, erythrophagocytosis, hepatic apoptosis and periportal lymphohistiocytic infiltrates, focal splenic necrosis, and mild interstitial pneumonitis and pulmonary hemorrhage (96–98). Similar to HME, HGA hematologic abnormalities including marked leukopenia and thrombocytopenia. Although the immune mechanisms that account for severe and fatal HGA are not completely understood, there is some evidence of immunosuppression in patients with HGA (79, 87, 90, 92). This conclusion is supported by the high number of fatal cases due to secondary opportunistic infections and organ failures (81). The mechanism by which this immune suppression occurs in HGA is not yet completely defined.
RE-INFECTION AND IMMUNITY
HME
Immunity to primary E. chaffeensis infection in humans has not been investigated, but comparative murine models have provided some insight. A role for cell-mediated immunity has been suggested based on the severity of E. chaffeensis infection in HIV-infected patients and the lympho-proliferative responses observed in patients following recovery from HME (54, 56, 86, 117). However, the relative importance of cell-mediated and humoral immunity has not been firmly established (118). Studies using murine models have suggested that protective immunity during primary and secondary ehrlichial infection is mediated by cellular immunity, mainly CD4+ T cells producing IFN-γ (i.e. Th1 cells) (103–106, 119–122). IFN-γ production by CD4+Th1 cells likely leads to macrophage activation and induction of bactericidal mechanisms such as production of reactive oxygen species, which in turn lead to bacterial elimination (120–122). In addition to IFN-γ, murine studies have shown that proinflammatory cytokines such as IL-12p40 and TNF-α are also important factors in the clearance of Ehrlichia and protection. Humoral immunity appears to also play a role in protection against Ehrlichia infection as evidenced by significant seroconversion in patients who recover from disease. In murine studies, Ehrlichia-specific antibodies, mainly IgG2a (Th1-dependent Ig subclass), protect SCID mice from severe E. chaffeenesis infection (103, 122, 123)
It is unknown whether patients that recover from HME are immune or susceptible to reinfection. Such evidence is limited to a single report of re-infection with a genetically distinct E. chaffeensis strain in a liver transplant recipient receiving immunosuppressive therapy (124). In murine studies, the development of heterologous protection model of ehrlichiosis has provided a mechanism to investigate immunity, including memory immune responses (103, 125). These studies indicate that prior infection of immunocompetent mice with E. muris that cause persistent asymptomatic infection in mice and strong cell mediated immune responses (Figure 11B), protect against secondary infection with highly virulent Ehrlichia species (IOE) which cause fatal disease in uninfected host (103, 125). Heterologous protection against Ehrlichia is associated with minimal tissue injury, development of well-defined granulomas, expansion of IFN-γ producing effector memory CD4+ and CD8+type-1 cells and substantial production of Ehrlichia specific IgG2a antibodies. Vaccines are currently unavailable for HME.
HGA
Currently, little is known about immunity following an A. phagocytophilum infection. Although infection may result in long-term immunity, there have been rare reports of laboratory-confirmed reinjection. Thus, individuals who live in endemic areas and are at risk of exposure to infected ticks should be vigilant about avoiding tick bites and other tick-borne pathogens. Similar to immunity against E. chaffeensis, protective immunity against A. phagocytophilum is mediated by cellular and humoral immune mechanisms (43, 75). It is generally believed that individuals who develop high titer antibodies are protected against re-infection; patients previously infected with A. phagocytophilum develop high titer antibodies that may last for as long as 3 years. Whether this persistence of antibodies is due to persistent infection or re-infection is not clearly determined. Similarly, it is not known if previous infection of human leads to antigen specific memory T and B cell responses that protect the individual against reinfection. Vaccines are currently unavailable for HGA.
DIAGNOSIS
HME and HGA
Diagnosis of HME and HGA rests primarily on clinical suspicion due to the limited availability of rapid diagnostic tests such as PCR, and the absence of detectable serum antibodies at the time of clinical presentation (4 days after the onset of clinical illness) (19, 117). The prognosis worsens if treatment is not administered or delayed (5, 51, 52, 72, 117) and therefore, it is important that empiric therapy with doxycycline be started for any patient with compatible clinical and laboratory findings. Initial diagnosis of ehrlichiosis can be based on non specific biochemical and hemaotological findings. However, confirmatory tests should be performed at different intervals after the onset of illness.
Hematologic and Biochemical Abnormalities
Presumptive diagnosis of HME is based on clinical manifestation, clues from medical history such as history of tick bite and outdoor activities, as well as specific laboratory abnormalities. Pancytopenias are a hallmark laboratory feature of HME early in the course of the illness (126). Anemia occurs within 2 weeks of illness and influence 50% of patients. Mild to moderate leukopenia with largest decline in lymphocyte population is observed approximately in 60 to 70% of patients during the first week of illness (51, 52, 72). Interestingly, during convalescence, a significant increase in lymphocyte count, i.e. relative and absolute lymphocytosis, is seen in most patients and is characterized predominantly by the expansion of activated γδ T cells (118). Marked thrombocytopenia is one of the pathognomonic findings in HME, which is usually detected in 70 to 90% of patients during their illness. Mildly or moderately elevated hepatic transaminase levels are detected in ~ 90% of patients associated with increased levels of alkaline phosphatase and bilirubin in some patients. Mild to moderate hyponatremia has been reported in as many as 50% of adult patients and 70% of pediatric patients (52, 72, 117).
Other laboratory abnormalities that occur in severe disease are specific to the organ involved. Examples are increased serum creatinine, lactate dehydrogenase, creatine phosphokinase, amylase, and electrolyte abnormalities including hypocalcemia, hypomagnesemia, hypophosphatemia, prolonged prothrombin times, increased levels of fibrin degradation products, metabolic acidosis, profound hypotension, disseminated intravascular coagulopathy, hepatic and renal failure, adrenal insufficiency, and myocardial dysfunction (49, 50, 75).
Specific Laboratory Diagnostic Tests
A diagnosis of HME can be confirmed by several laboratory methods. These tests include serological detection of specific antibodies, detection of morulae in peripheral blood or in CSF leukocytes, detection of ehrlichial DNA by PCR of blood or CSF, direct detection of ehrlichiae in tissue samples by immunohistochemistry, and isolation of bacteria.
Serologic Testing
Serologic testing of IgM and IgG antibodies specific to E.chaffeensis using indirect immunofluorescence assay (IFA) is the “gold standard” and is most frequently utilized confirmatory tests for HME (72, 90, 127). Paired sera collected during a 3- to 6-week interval represent the preferred specimens for serologic evaluation of HME. A single IgG antibody titer of at least 256; seroconversion from negative to positive antibody status (with a minimum titer of 64), a 4-fold rise in titer during convalescence is indicative of HME when acute- and convalescent-phase samples are compared (127, 128). Although serology is one of major diagnostic criterion for ehrlichiosis, it has several limitations that should be considered: 1) IgG IFA test is negative in as many as 80% of patients during the first week of illness and the IgM titers may also be uninformative at this time. Thus a negative serologic result for the acute-phase sample does not exclude the diagnosis; 2) A high rate of false positive serology usually occurs due to cross reactive antigens shared by Ehrlichia and Anaplasma that induce cross reactive antibodies. Because of this cross-reactivity among ehrlichial species, sera should be tested against both E. chaffeensis and A. phagocytophilium antigens when ascribing a specific etiology; 3) Failure to seroconvert in some cases can be attributed to immune impairment; and 4) Early treatment with a tetracycline-class antibiotics occasionally reduces or abrogates the antibody response to E. chaffeensis (32, 79, 98, 129).
Blood Smear Staining
Diagnosis of HME can be accomplished by staining of blood smears from peripheral blood, bone marrow or CSF to detect morulae. Smears can be stained with Wright’s, Diff-Quik, or Giemsa stains (19, 28). Although this method is rapid, it is relatively insensitive compared to other confirmatory tests, especially in immunocompetent patients where severe disease is usually associated with very low bacterial burden in blood and peripheral organs. Morulae are detected within monocytes in only about 3% of patients with HME. In contrast, blood smear is more useful for HGA diagnosis where 25% – 75% of patients have morulae in peripheral blood examinations, and sensitivity is highest during the first week of infection (130, 131).
PCR Amplification
Due to its high specificity (60–85%) and sensitivity (60–85% for E. chaffeensis and 67–90% for A. phagocytophilum) as well as rapid turnaround time, diagnosis of ehrlichial infection by PCR has become the test of choice for confirming serology indicating HME and HGA (130–132). PCR is the only definitive diagnostic test for E. ewingii infection since the bacteria is unculturable, although the sensitivity and specificity of this approach is unknown. Multiplex or multicolour testing capable of detecting several related etiologic agents in a single test has been described (135). PCR of whole blood is commercially available, and allows rapid diagnosis of infection in up to 85% of cases (130). Blood samples should be collected in EDTA or sodium citrate anti-coagulants and obtained before or at the initiation of therapy to increase sensitivity. However, since doxycycline treatment is effective only at early stages of infection, treatment should start as soon as possible while waiting for laboratory result. PCR detection is particularly important for detection of ehrlichial infection at early stages when antibody levels are very low or undetectable. While PCR of CSF may be positive, the sensitivity is lower than for whole blood, likely due to the significantly lower volume of infected cells (2, 4, 130, 131). Several PCR targets have been employed to conserved genes among different Ehrlichia isolates including the rrs (16S rRNA) and groESL heat shock operon (133). Other genes have been utilized such as genus-specific disulfide bond formation protein gene (dsb), the E. chaffeensis-specific 120-kDa and TRP32 protein (VLPT) genes, and the 28-kDa outer membrane proteins (p28) (134).
Isolation
Similar to other infectious diseases, cultivation of Ehrlichia is the gold standard in diagnosis of HME and HGA however, primary isolation may take up to several weeks. The sensitivity of E. chaffeensis isolation compared with PCR amplification is very low (3, 11, 49). In contrast, the sensitivity of culture for detection of A. phagocytophilum can be equivalent to that of PCR and blood smear examination (69). Similar to PCR amplification and blood smear examination, prior doxycycline treatment diminishes the sensitivity of culture to a greater degree. Due to lower sensitivity of this method, therapeutic decisions must often be based on a high index of clinical suspicion and laboratory evidence of the infection, such as PCR assays and peripheral blood smears (69, 74).
Immunohistochemistry
Immunohistichemical staining of the formalin fixed biopsy or autopsy tissues is another confirmatory method for diagnosis of Ehrlichia and Anaplasma infection. The IHC method is most useful in documenting the presence of organisms in patients before the initiation of antibiotic therapy or within the first 48 hours after antibiotic therapy has been initiated. IHC techniques also are available for diagnosing cases of ehrlichiosis and anaplasmosis from bone marrow biopsies and tissue obtained at autopsy of fatal cases, including the spleen, lymph nodes, liver, and lung (51, 52, 79).
HEE Diagnosis
A diagnosis of HEE is suggested by visualization of intracytoplasmic morulae in neutrophils in a patient with residence or travel to an area of HME (rather than HGA) endemnicity. Morulae may be visualized in both blood and, rarely, CSF (8, 9, 11). While there is no specific serologic assay for E. ewingii, there is significant serologic cross-reactivity to E. chaffeenesis (8, 9). It is conceivable that in the absence of visualization of morulae in granulocytes or confirmatory PCR for E. ewingii, a proportion of cases meeting serologic criteria for HME actually represent HEE infection. A specific PCR for E. ewingii exists, but is limited to research laboratories. Similar to the PCR for E. chaffeensis and A. phagocytophilium, sensitivity is maximal early in the course of the illness, prior to antibiotic therapy.
Differential Diagnosis
The differential diagnosis of HME, HGA and HEE at early stages of the disease where the symptoms and signs of disease are non specific and patient presents with fever, headache, myalgia, and malaise, may include various viral syndromes, Rocky Mountain spotted fever, upper respiratory illness, urinary tract infection, and sepsis. If history of tick bite and outdoor activities exist with these symptoms, the physician should consider other tick-borne febrile illnesses such as Rocky Mountain spotted fever, relapsing fever, tularaemia, Lyme borreliosis, Colorado tick fever, and babesiosis (52, 127). CNS signs and symptoms with CSF pleocytosis suggest viral or bacterial meningoencephalitis. Other diseases that share clinical and laboratory findings of ehrlichial disease, particularly if patients presented with rash are meningococcemia, toxic shock syndrome, murine typhus, Q fever, typhoid fever, leptospirosis, hepatitis, enteroviral infection, influenza, bacterial sepsis, endocarditis, Kawasaki disease, collagen-vascular diseases, and immune thrombocytopenic purpura (72).
Treatment
Most patients with HME or HGA respond well to tetracyclines if administrated early in illness. In vitro antimicrobial susceptibilty testing has shown an excellent sensitivity of all Ehrlichia and Anaplasma species to doxycycline. In vivo, doxycycline is preferred over tetracycline because it has fewer side effects and better patient tolerance (90, 136). Doxycycline remains the treatment of choice in pediatric patients, despite the risk of dental discoloration in this age group (137). This drug is bacteriostatic in its activity against rickettsial organisms.
Pregnant patients with ehrlichial infection represent a particular challenge, as doxycycline is contraindicated. In this population, as well as in patients with a specific contraindication to doxycycline, rifampin (adults: 300 mg twice daily; children < 100lb 10 mg/kg twice daily) may be substituted (138–140). In vitro susceptibility testing has shown that E. chaffeensis is resistant to representatives of most classes of antibiotics including aminoglycosides (gentamicin), fluoroquinolones (ciprofloxacin), penicillins (penicillin), macrolides and ketolides (erythromycin and telithromycin), and sulfa-containing drugs (co-trimoxazole) (87). Chloramphenicol is an alternative drug that has been considered for treatment of HGA or HME. However, this drug is associated with various side effects and might require monitoring of blood indices, and therefore, it is no longer available in the oral form in the United States.
HME Treatment
Doxycycline is the recommended treatment for HME. Response to treatment is typically rapid, and fever persisting >72 hours after initiation of treatment strongly suggests an alternative diagnosis. The recommended dose is 100 mg per dose administered twice daily (orally or intravenously) for adults or 2.2 mg/kg body weight per dose administered twice daily (orally or intravenously) for children weighing <100 lbs. (45.4 kg). While no studies have specifically addressed duration of treatment, most authorities advocate continuing antibiotics for 3 to 5 days after defervescence (90, 137), and perhaps longer (e.g., total of 10–14 days) if there is CNS involvement (141).
HGA Treatment
Therapeutic considerations for HGA are similar to HME, with doxycycline remaining the drug of choice for both pediatric and adult cases. If coinfection with B. burgdorferii is suspected based on characteristic skin findings or elevated antibodies, doxycycline should be continued for at least 10 days for adults (89, 142). In B. burgdorferii coinfected children < 8 years of age, doxycycline should be continued until the patient is afebrile for three days, with the remainder of the 14 day course completed with an alternative agent active against B. burgdorferii (e.g., amoxicillin or cefuroxime axetil) to minimize the risk of dental discoloration (10, 11, 49, 72). Patients who fail to respond clinically to doxycycline monotherapy after 72 hours should be evaluated for an alternative diagnosis or the possibility of Babesia co-infection.
HEE Treatment
There are no prospective studies evaluating treatment of E. ewingii, however doxycycline is considered the treatment of choice in both adults and children. When therapy with this agent is started promptly, outcomes are uniformly excellent (8, 9, 11). Considerations in dosing and administration of doxycycline are discussed in the section on HME management.
Prevention
Preventive antibiotic therapy for ehrlichial infection is not indicated for patients who have had recent tick bites and are not ill. Avoidance of tick bites and immediate removal of ticks remains the ultimate prevention approach. Individuals who live in endemic areas should wear light colored clothes during outdoor activities, which allow the person to see crawling ticks (143). Adults who are at high risk of getting bitten by ticks should apply Chemo-prophylactic repellents such as DEET (n, n-diethyl-m-toluamide) to exposed skin that prevents tick attachment. Individuals should carefully inspect their bodies, hair, and clothes for ticks upon return from potentially tick-infested areas and should immediately remove any attached ones. Studies have shown that a period of 4–24 hours of infected ticks attached to the host may be required before effective transmission of Ehrlichia and Anaplasma occur (143–45). Therefore, immediate and complete removal of attached ticks is critical for prevention of transmission and infection.
Acknowledgments
We thank Dr. Veera Rajaratnam, Director of Scientific Publications and Grant Support at the Center for Women’s Health Research, Meharry Medical College, for the meticulous editing and expediting the chapter review, and Christina Nelson for her graphics assistance.
This work was supported by the NIH/NCRR-RCMI fund (5G12RR003032).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dumler JS, Barbet AF, Bekker CP, et al. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001;51:2145–2165. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- 2.Dawson JE, Anderson BE, Fishbein DB, et al. Isolation and characterization of an Ehrlichia sp. from a patient diagnosed with human ehrlichiosis. J Clin Microbiol. 1991;29:2741–2745. doi: 10.1128/jcm.29.12.2741-2745.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dumler JS, Bakken JS. Ehrlichial diseases of humans: emerging tick-borne infections. Clin Infect Dis. 1995;20:1102–1110. doi: 10.1093/clinids/20.5.1102. [DOI] [PubMed] [Google Scholar]
- 4.Anderson BE, Dawson JE, Jones DC, et al. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paddock CD, Liddell AM, Storch GA. Other causes of tick-borne ehrlichioses, including Ehrlichia ewingii. In: Goodman JL, Dennis DT, Sonenshine DE, editors. Tick-borne diseases of humans. ASM Press; Washington DC: 2005. pp. 258–267. [Google Scholar]
- 6.Perez M, Bodor M, Zhang C, et al. Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Ann NY Acad Sci. 2006;1078:110–117. doi: 10.1196/annals.1374.016. [DOI] [PubMed] [Google Scholar]
- 7.Maeda K, Markowitz N, Hawley RC, et al. Human infection with Ehrlichia canis, a leukocytic Rickettsia. N Engl J Med. 1987;316:853–856. doi: 10.1056/NEJM198704023161406. [DOI] [PubMed] [Google Scholar]
- 8.Buller RS, Arens M, Hmiel SP, et al. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Engl J Med. 1999;341:148–155. doi: 10.1056/NEJM199907153410303. [DOI] [PubMed] [Google Scholar]
- 9.Anderson BE, Greene CE, Jones DC, et al. Ehrlichia ewingii sp. nov. the etiologic agent of canine granulocytic ehrlichiosis. Int J Syst Bacteriol. 1992;42:299–302. doi: 10.1099/00207713-42-2-299. [DOI] [PubMed] [Google Scholar]
- 10.Bakken JS, Dumler JS, Chen SM, et al. Human granulocytic ehrlichiosis in the upper Midwest United States. A new species emerging? JAMA. 1994;272:212–218. [PubMed] [Google Scholar]
- 11.Chen S-M, Dumler JS, Bakken JS, et al. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pretzman C, Ralph D, Stothard DR, et al. 16S rRNA gene sequence of Neorickettsia helminthoeca and its phylogenetic alignment with members of the genus Ehrlichia. Int J Syst Bacteriol. 1995;45:207–211. doi: 10.1099/00207713-45-2-207. [DOI] [PubMed] [Google Scholar]
- 13.Wen B, Rikihisa Y, Yamamoto S, et al. Characterization of the SF agent, an Ehrlichia sp. isolated from the fluke Stellantchasmus falcatus, by 16S rRNA base sequence, serological, and morphological analyses. Int J Syst Bacteriol. 1996;46:149–154. doi: 10.1099/00207713-46-1-149. [DOI] [PubMed] [Google Scholar]
- 14.Taylor MJ. Wolbachia endosymbiotic bacteria of filarial nematodes. A new insight into disease pathogenesis and control. Arch Med Res. 2002;33:422–424. doi: 10.1016/s0188-4409(02)00377-6. [DOI] [PubMed] [Google Scholar]
- 15.Palmer GH, Brayton KA. Gene conversion is a convergent strategy for pathogen antigenic variation. Trends Parasitol. 2007;23:408–13. doi: 10.1016/j.pt.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Frutos R, Viari A, Vachiery N, Boyer F, Martinez D. Ehrlichia ruminantium: genomic and evolutionary features. Trends Parasitol. 2007;23:414–9. doi: 10.1016/j.pt.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Rikihisa Y. The tribe Ehrlichieae and ehrlichial diseases. Clin Microbiol Rev. 1991;4:286–308. doi: 10.1128/cmr.4.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rikihisa Y. Clinical and biological aspects of infection caused by Ehrlichia chaffeensis. Microbes Infect. 1999;1:367–376. doi: 10.1016/s1286-4579(99)80053-7. [DOI] [PubMed] [Google Scholar]
- 19.Paddock CD, Sumner JW, Shore GM, et al. Isolation and characterization of Ehrlichia chaffeensis strains from patients with fatal ehrlichiosis. J Clin Microbiol. 1997;35:2496–2502. doi: 10.1128/jcm.35.10.2496-2502.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popov VL, Chen SM, Feng HM, et al. Ultrastructural variation of cultured Ehrlichia chaffeensis. J Med Microbiol. 1995;43:411–421. doi: 10.1099/00222615-43-6-411. [DOI] [PubMed] [Google Scholar]
- 21.Ohashi N, Zhi N, Zhang Y, et al. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect Immun. 1998;66:132–139. doi: 10.1128/iai.66.1.132-139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang JZ, Popov VL, Gao S, et al. The developmental cycle of Ehrlichia chaffeensis in vertebrate cells. Cell Microbiol. 2007;9(3):610–8. doi: 10.1111/j.1462-5822.2006.00812.x. Epub 2006 Sep 20. [DOI] [PubMed] [Google Scholar]
- 23.Doyle CK, Nethery KA, Popov VL, et al. Differentially expressed and secreted major immunoreactive protein orthologs of Ehrlichia canis and E. chaffeensis elicit early antibody responses to epitopes on glycosylated tandem repeats. Infect Immun. 2006;74:711–720. doi: 10.1128/IAI.74.1.711-720.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo Tian, Zhang Xiaofeng, Wakeel Abdul, et al. A Variable-Length PCR Target Protein of Ehrlichia chaffeensis Contains Major Species-Specific Antibody Epitopes in Acidic Serine-Rich Tandem Repeats. Infect Immun. 2008;76(4):1572–1580. doi: 10.1128/IAI.01466-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wakeel A, Kuriakose JA, McBride JW. An Ehrlichia chaffeensis tandem repeat protein interacts with multiple host targets involved in cell signaling, transcriptional regulation, and vesicle trafficking. Infect Immun. 2009;77:1734–45. doi: 10.1128/IAI.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Popov VL, Yu XJ, Walker DH. The 120-kDa outer membrane protein of Ehrlichia chaffeensis: preferential expression on dense-core cells and gene expression in Escherichia coli associated with attachment and entry. Microb Pathog. 2000;28:71–80. doi: 10.1006/mpat.1999.0327. [DOI] [PubMed] [Google Scholar]
- 27.Hotopp JC, Lin M, Madupu R, et al. Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet. 2006;2:e21. doi: 10.1371/journal.pgen.0020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin M, Rikihisa Y. Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival. Infect Immun. 2003;71(9):5324–31. doi: 10.1128/IAI.71.9.5324-5331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiong Q, Lin M, Rikihisa Y. Cholesterol-dependent Anaplasma phagocytophilum exploits the low-density lipoprotein uptake pathway. PLoS Pathog. 2009;5(3):e1000329. doi: 10.1371/journal.ppat.1000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattner J, Debord KL, Ismail N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 31.Yu XJ, Crocquet-Valdes P, Walker DH. Cloning and sequencing of the gene for a 120-kDa immunodominant protein of Ehrlichia chaffeensis. Gene. 1997;184:149–154. doi: 10.1016/s0378-1119(96)00586-0. [DOI] [PubMed] [Google Scholar]
- 32.Yu XJ, McBride JW, Diaz CM, et al. Molecular cloning and characterization of the 120-kilodalton protein gene of Ehrlichia canis and application of the recombinant 120-kilodalton protein for serodiagnosis of canine ehrlichiosis. J Clin Microbiol. 2000;38:369–374. doi: 10.1128/jcm.38.1.369-374.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohashi N, Zhi N, Zhang Y, et al. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect Immun. 1998;66:132–139. doi: 10.1128/iai.66.1.132-139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu X, McBride JW, Zhang X, et al. Characterization of the complete transcriptionally active Ehrlichia chaffeensis 28 kDa outer membrane protein multigene family. Gene. 2000;248:59–68. doi: 10.1016/s0378-1119(00)00147-5. [DOI] [PubMed] [Google Scholar]
- 35.Huang H, Lin M, Wang X, et al. Proteomic analysis of and immune responses to Ehrlichia chaffeensis lipoproteins. Infect Immun. 2008;76:3405–14. doi: 10.1128/IAI.00056-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park J, Choi KS, Dumler JS. Major surface protein 2 of Anaplasma phagocytophilum facilitates adherence to granulocytes. Infect Immun. 2003;71:4018–4025. doi: 10.1128/IAI.71.7.4018-4025.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.IJdo JW, Wu C, Telford SR, et al. Differential expression of the p44 gene family in the agent of human granulocytic ehrlichiosis. Infect Immun. 2002;70:5295–5298. doi: 10.1128/IAI.70.9.5295-5298.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhi N, Ohashi N, Rikihisa Y. Multiple p44 genes encoding major outer membrane proteins are expressed in the human granulocytic ehrlichiosis agent. J Biol Chem. 1999;274:17828–17836. doi: 10.1074/jbc.274.25.17828. [DOI] [PubMed] [Google Scholar]
- 39.Caspersen K, Park JH, Patil S, et al. Genetic variability and stability of Anaplasma phagocytophila msp2(p44) Infect Immun. 2002;70:1230–1234. doi: 10.1128/IAI.70.3.1230-1234.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peddireddi L, Cheng C, Ganta RR. Promoter analysis of macrophage- and tick cell-specific differentially expressed Ehrlichia chaffeensis p28–Omp genes. BMC Microbiol. 2009;9:99. doi: 10.1186/1471-2180-9-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganta RR, Peddireddi L, Seo GM, et al. Molecular characterization of Ehrlichia interactions with tick cells and macrophages. Front Biosci. 2009;14:3259–73. doi: 10.2741/3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ganta RR, Cheng C, Miller EC, et al. Differential Clearance and Immune Responses to Tick Cell vs. Macrophage Culture-Derived Ehrlichia chaffeensis in Mice. Infect Immun. 2006 doi: 10.1128/IAI.01127–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunning Hotopp JC, Lin M, Madupu R, et al. Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet. 2006;2:e21. doi: 10.1371/journal.pgen.0020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin M, den Dulk-Ras A, Hooykaas PJ. Anaplasma phagocytophilum AnkA secreted by type IV secretion system is tyrosine phosphorylated by Abl-1 to facilitate infection. Cell Microbiol. 2007;9:2644–2657. doi: 10.1111/j.1462-5822.2007.00985.x. [DOI] [PubMed] [Google Scholar]
- 45.Caturegli P, Asanovich KM, Walls JJ, et al. an Ehrlichia phagocytophila group gene encoding a cytoplasmic protein antigen with ankyrin repeats. Infect Immun. 2000;68(9):5277–83. doi: 10.1128/iai.68.9.5277-5283.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park J, Kim KJ, Choi KS, et al. Anaplasma phagocytophilum AnkA binds to granulocyte DNA and nuclear proteins. Cell Microbiol. 2004;6(8):743–51. doi: 10.1111/j.1462-5822.2004.00400.x. [DOI] [PubMed] [Google Scholar]
- 47.Christie PJ, Atmakuri K, Krishnamoorthy V, et al. Biogenesis, architecture, and function of bacterial type IV secretion systems. Annu Rev Microbiol. 2005;59:451–485. doi: 10.1146/annurev.micro.58.030603.123630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohashi N, Zhi N, Lin Q, et al. Characterization and transcriptional analysis of gene clusters for a type IV secretion machinery in human granulocytic and monocytic ehrlichiosis agents. Infect Immun. 2002;70:2128–2138. doi: 10.1128/IAI.70.4.2128-2138.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dumler JS. Anaplasma and Ehrlichia infection. Ann NY Acad Sci. 2005;1063:361–373. doi: 10.1196/annals.1355.069. [DOI] [PubMed] [Google Scholar]
- 50.Dumler JS, Barat NC, Barat CE, et al. Human granulocytic anaplasmosis and macrophage activation. Clin Infect Dis. 2007;45:199–204. doi: 10.1086/518834. [DOI] [PubMed] [Google Scholar]
- 51.Olano JP, Hogrefe W, Seaton B, et al. Clinical manifestations, epidemiology, and laboratory diagnosis of human monocytotropic ehrlichiosis in a commercial laboratory setting. Clin Diagn Lab Immunol. 2003;10:891–896. doi: 10.1128/CDLI.10.5.891-896.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olano JP, Masters E, Hogrefe W, et al. Human monocytotropic ehrlichiosis, Missouri. Emerg Infect Dis. 2003;9:1579–1586. doi: 10.3201/eid0912.020733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peters TR, Edwards KM, Standaert SM. Severe ehrlichiosis in an adolescent taking trimethoprim-sulfamethoxazole. Pediatr Infect Dis J. 2000;19:170–2. doi: 10.1097/00006454-200002000-00021. [DOI] [PubMed] [Google Scholar]
- 54.Fishbein DB, Dawson JE, Robinson LE. Human ehrlichiosis in the United States, 1985–1990. Ann Intern Med. 1994;120:736–743. doi: 10.7326/0003-4819-120-9-199405010-00003. [DOI] [PubMed] [Google Scholar]
- 55.Harkess JR, Ewing SA, Crutcher JM, et al. Human ehrlichiosis in Oklahoma. J Infect Dis. 1989;159:576–579. doi: 10.1093/infdis/159.3.576. [DOI] [PubMed] [Google Scholar]
- 56.Fishbein DB, Kemp A, Dawson JE, et al. Human ehrlichiosis: Prospective active surveillance in febrile hospitalized patients. J Infect Dis. 1989;160:803–809. doi: 10.1093/infdis/160.5.803. [DOI] [PubMed] [Google Scholar]
- 57.Carpenter CF, Gandhi TK, Kong LK, et al. The incidence of ehrlichial and rickettsial infection in patients with unexplained fever and recent history of tick bite in central North Carolina. J Infect Dis. 1999;180:900–903. doi: 10.1086/314954. [DOI] [PubMed] [Google Scholar]
- 58.Yevich SJ, Sanchez JL, DeFraites RF, et al. Seroepidemiology of infections due to spotted fever group rickettsiae and Ehrlichia species in military personnel exposed in areas of the United States where such infections are endemic. J Infect Dis. 1995;171:1266–73. doi: 10.1093/infdis/171.5.1266. [DOI] [PubMed] [Google Scholar]
- 59.Marshall GS, Jacobs RF, Schutze GE, et al. Ehrlichia chaffeensis seroprevalence among children in the southeast and south-central regions of the United States. Arch Pediatr Adolesc Med. 2002;156:166–70. doi: 10.1001/archpedi.156.2.166. [DOI] [PubMed] [Google Scholar]
- 60.Parola P, Davoust B, Raoult D. Tick- and flea-borne rickettsial emerging zoonoses. Vet Res. 2005;36(3):469–92. doi: 10.1051/vetres:2005004. Review. [DOI] [PubMed] [Google Scholar]
- 61.Estrada-Peña A, Horak IG, Petney T. Climate changes and suitability for the ticks Amblyomma hebraeum and Amblyomma variegatum (Ixodidae) in Zimbabwe (1974–1999) Vet Parasitol. 2008;151(2–4):256–67. doi: 10.1016/j.vetpar.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 62.Demma LJ, Holman RC, McQuiston JH, et al. Human monocytic ehrlichiosis and human granulocytic anaplasmosis in the United States, 2001–2002. Ann N Y Acad Sci. 2006;1078:118–9. doi: 10.1196/annals.1374.017. [DOI] [PubMed] [Google Scholar]
- 63.Anderson BE, Sims KG, Olson JG, et al. Amblyomma americanum: A potential vector of human ehrlichiosis. Am J Trop Med Hyg. 1993;49:239–244. doi: 10.4269/ajtmh.1993.49.239. [DOI] [PubMed] [Google Scholar]
- 64.Ewing SA, Dawson JE, Kocan AA, et al. Experimental transmission of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) among white-tailed deer by Amblyomma americanum (Acari: Ixodidae) J Med Entomol. 1995;32:368–374. doi: 10.1093/jmedent/32.3.368. [DOI] [PubMed] [Google Scholar]
- 65.Lockhart JM, Davidson WR, Stallknecht DE, et al. Site-specific geographic association between Amblyomma americanum (Acari: Ixodidae) infestations and Ehrlichia chaffeensis–reactive (Rickettsiales: Ehrlichieae) antibodies in white-tailed deer. J Med Entomol. 1996;33:153–158. doi: 10.1093/jmedent/33.1.153. [DOI] [PubMed] [Google Scholar]
- 66.Lockhart JM, Davidson WR, Dawson JE, et al. Temporal association of Amblyomma americanum with the presence of Ehrlichia chaffeensis reactive antibodies in white-tailed deer. J Wildl Dis. 1995;31:119–124. doi: 10.7589/0090-3558-31.2.119. [DOI] [PubMed] [Google Scholar]
- 67.Belongia EA. Epidemiology and impact of coinfections acquired from Ixodes ticks. Vector Borne Zoonotic Dis. 2002;2:265–273. doi: 10.1089/153036602321653851. [DOI] [PubMed] [Google Scholar]
- 68.MacLeod JR, Gordon WS. Studies in tick-borne fever of sheep. I. Transmission by the tick, Ixodes ricinus, with a description of the disease produced. Parasitology. 1933;25:273–285. [Google Scholar]
- 69.Cao WC, Gao YM, Zhang PH, et al. Identification of Ehrlichia chaffeensis by nested PCR in ticks from Southern China. J Clin Microbiol. 2000;38(7):2778–80. doi: 10.1128/jcm.38.7.2778-2780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sexton DJ, Corey GR, Carpenter C, et al. Dual infection with Ehrlichia chaffeensis and a spotted fever group rickettsia: a case report. Emerg Infect Dis. 1998;4(2):311–6. doi: 10.3201/eid0402.980222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walls JJ, Greig B, Neitzel DF, et al. Natural infection of small mammal species in Minnesota with the agent of human granulocytic ehrlichiosis. J Clin Microbiol. 1997;35:853–855. doi: 10.1128/jcm.35.4.853-855.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chapman AS, Bakken JS, Folk SM, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis-United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55(RR-4):1–27. [PubMed] [Google Scholar]
- 73.Bakken JS, Dumler JS. Human granulocytic ehrlichiosis. Clin Infect Dis. 2000;31:554–560. doi: 10.1086/313948. [DOI] [PubMed] [Google Scholar]
- 74.Bakken JS, Krueth J, Wilson-Nordskog C, et al. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1996;275:199–205. [PubMed] [Google Scholar]
- 75.Bakken JS, Dumler JS. Ehrlichiosis and anaplasmosis. Infect Med. 2004;21:433–51. [Google Scholar]
- 76.Nadelman RB, Horowitz HW, Hsieh T-C, et al. Simultaneous human granulocytic ehrlichiosis and Lyme borreliosis. N Engl J Med. 1997;337:27–30. doi: 10.1056/NEJM199707033370105. [DOI] [PubMed] [Google Scholar]
- 77.Thomas LD, Hongo I, Bloch KC, et al. Human ehrlichiosis in transplant recipients. Am J Transplant. 2007;7(6):1641–7. doi: 10.1111/j.1600-6143.2007.01821.x. [DOI] [PubMed] [Google Scholar]
- 78.Fichtenbaum CJ, Peterson LR, Weil GJ. Ehrlichiosis presenting as a life-threatening illness with features of the toxic shock syndrome. Am J Med. 1993;95:351–357. doi: 10.1016/0002-9343(93)90302-6. [DOI] [PubMed] [Google Scholar]
- 79.Walker DH, Dumler JS. Human monocytic and granulocytic ehrlichioses discovery and diagnosis of emerging tick-borne infections and the critical role of the pathologist. Arch Pathol Lab Med. 1997;121:785–791. [PubMed] [Google Scholar]
- 80.Sehdev AE, Dumler JS. Hepatic pathology in human monocytic ehrlichiosis. Ehrlichia chaffeensis infection. Am J Clin Pathol. 2003;119:859–865. doi: 10.1309/F7EA-B5P7-3217-16LJ. [DOI] [PubMed] [Google Scholar]
- 81.Marty AM, Dumler JS, Imes G, et al. Ehrlichiosis mimicking thrombotic thrombocytopenic purpura. Case report and pathological correlation. Human Pathol. 1995;26:920–025. doi: 10.1016/0046-8177(95)90017-9. [DOI] [PubMed] [Google Scholar]
- 82.Ratnasamy N, Everett ED, Roland WE, et al. Central nervous system manifestations of human ehrlichiosis. Clin Infect Dis. 1996;23:314–319. doi: 10.1093/clinids/23.2.314. [DOI] [PubMed] [Google Scholar]
- 83.Brantley RK. Trimethoprim-sulfamethoxazole and fulminant ehrlichiosis [Letter] Pediatr Infect Dis J. 2001;20:231. doi: 10.1097/00006454-200102000-00028. [DOI] [PubMed] [Google Scholar]
- 84.Paddock CD, Folk SM, Shore GM, et al. Infections with Ehrlichia chaffeensis and Ehrlichia ewingii in persons coinfected with human immunodeficiency virus. Clin Infect Dis. 2001;33:1586–94. doi: 10.1086/323981. [DOI] [PubMed] [Google Scholar]
- 85.Everett ED, Evans KA, Henry RB, et al. Human ehrlichiosis in adults after tick exposure: diagnosis using polymerase chain reaction. Ann Intern Med. 1994;120:730–5. doi: 10.7326/0003-4819-120-9-199405010-00002. [DOI] [PubMed] [Google Scholar]
- 86.Harkess JR. Ehrlichiosis. Infect Dis Clin North Amer. 1991;5:37–51. [PubMed] [Google Scholar]
- 87.Walker DH, Raoult D. Rickettsia rickettsii and other spotted fever group rickettsiae (Rocky Mountain spotted fever and other spotted fevers) In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. 6. Philadelphia, PA: Churchill Livingstone; 2005. pp. 2287–95. [Google Scholar]
- 88.Horowitz HW, Marks SJ, Weintraub M, et al. Brachial plexopathy associated with human granulocytic ehrlichiosis. Neurology. 1996;46(4):1026–9. doi: 10.1212/wnl.46.4.1026. [DOI] [PubMed] [Google Scholar]
- 89.Klein MB, Nelson CM, Goodman JL. Antibiotic susceptibility of the newly cultivated agent of human granulocytic ehrlichiosis: promising activity of quinolones and rifamycins. Antimicrob Agents Chemother. 1997;41:76–9. doi: 10.1128/aac.41.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dumler JS, Madigan JE, Pusterla N, et al. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;4(1):S45–51. doi: 10.1086/518146. [DOI] [PubMed] [Google Scholar]
- 91.Rikihisa Y. Ehrlichia subversion of host innate responses. Curr Opin Microbiol. 2006;9:95–101. doi: 10.1016/j.mib.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 92.Lin M, Rikihisa Y. Ehrlichia chaffeensis downregulates surface toll-like receptors 2/4, CD14 and transcription factors PU.1 and inhibits lipopolysaccharide activation of NF-kB, ERK 1/2 and p38 MAPK in host monocytes. Cell Microbiol. 2004;6:175–186. doi: 10.1046/j.1462-5822.2003.00355.x. [DOI] [PubMed] [Google Scholar]
- 93.Zhang JZ, Sinha M, Luxon BA, et al. Survival strategy of obligately intracellular Ehrlichia chaffeensis: novel modulation of immune response and host cell cycles. Infect Immun. 2004;72:498–507. doi: 10.1128/IAI.72.1.498-507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mavromatis K, Doyle CK, Lykidis A, et al. The genome of the obligately intracellular bacterium Ehrlichia canis reveals themes of comples membrane structure and immune evasion strategies. J Bacteriol. 2006;188:4015–4023. doi: 10.1128/JB.01837-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stevenson H, Walker DH, Ismail N. Regulatory role of CD1d-restricted NKT cells in the induction of toxic shock-like syndrome in an animal model of fatal ehrlichiosis. Infection and Immunity. 2008;76:1434–1444. doi: 10.1128/IAI.01242-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dumler JS, Brouqui P, Aronson J, et al. Identification of Ehrlichia in human tissue. N Engl J Med. 1991;325:1109–1110. doi: 10.1056/NEJM199110103251517. [DOI] [PubMed] [Google Scholar]
- 97.Dumler JS, Sutker WL, Walker DH. Persistent infection with Ehrlichia chaffeensis. Clin Infect Dis. 1993;17:903–905. doi: 10.1093/clinids/17.5.903. [DOI] [PubMed] [Google Scholar]
- 98.Walker DH, Dumler JS. Human monocytic and granulocytic ehrlichioses. Discovery and diagnosis of emerging tick-borne infections and the critical role of the pathologist. Arch Pathol Lab Med. 1997;121:785–791. [PubMed] [Google Scholar]
- 99.Schutze GE, Buckingham SC, Marshall GS, et al. Human monocytic ehrlichiosis in children. Pediatr Infect Dis J. 2007;26:475–479. doi: 10.1097/INF.0b013e318042b66c. [DOI] [PubMed] [Google Scholar]
- 100.Sehdev AE, Dumler JS. Hepatic pathology in human monocytic ehrlichiosis. Ehrlichia chaffeensis infection. Am J Clin Pathol. 2003;119:859–865. doi: 10.1309/F7EA-B5P7-3217-16LJ. [DOI] [PubMed] [Google Scholar]
- 101.Shibata S, Kawahara M, Rikihisa Y, et al. New Ehrlichia species closely related to Ehrlichia chaffeensis isolated from Ixodes ovatus ticks in Japan. J Clin Microbiol. 2000;38:1331–1338. doi: 10.1128/jcm.38.4.1331-1338.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sotomayor E, Popov V, Feng H-M. Animal model of fatal human monocytotropic ehrlichiosis. American Journal of Pathology. 2001;158:757–769. doi: 10.1016/S0002-9440(10)64018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ismail N, Soong L, McBride JW, et al. Overproduction of TNF-a by CD8+ type 1 cells and down-regulation of IFN-g production by CD4+ Th1 cells contribute to toxic shock-like syndrome in an animal model of fatal monocytotropic ehrlichiosis. J Immunol. 2004;172:1786–1800. doi: 10.4049/jimmunol.172.3.1786. [DOI] [PubMed] [Google Scholar]
- 104.Ismail N, Stevenson HL, Walker DH. Role of tumor necrosis factor alpha (TNF-alpha) and interleukin-10 in the pathogenesis of severe murine monocytotropic ehrlichiosis: increased resistance of TNF receptor p55- and p75-deficient mice to fatal ehrlichial infection. Infect Immun. 2006;74:1846–1856. doi: 10.1128/IAI.74.3.1846-1856.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ismail N, Crossley EC, Stevenson HL, et al. The Relative Importance of T Cell Subsets in Monocytotropic Ehrlichiosis: A Novel Effector Mechanism Involved in Ehrlichia-induced Immunopathology in Murine Ehrlichiosis. Infect Immun. 2007;75(9):4608–20. doi: 10.1128/IAI.00198-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stevenson HL, Jordan JM, Peerwani Z, et al. An intradermal environment promotes a protective type-1 response against lethal systemic monocytotropic ehrlichial infection. Infect Immun. 2006;74:4856–4864. doi: 10.1128/IAI.00246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dierberg KL, Dumler JS. Lymph node hemophagocytosis in rickettsial diseases: a pathogenetic role for CD8 T lymphocytes in human monocytic ehrlichiosis (HME)? BMC Infect Dis. 2006;6:121. doi: 10.1186/1471-2334-6-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Klein MB, Miller JS, Nelson CM, et al. Primary bone marrow progenitors of both granulocytic and monocytic lineages are susceptible to infection with the agent of human granulocytic ehrlichiosis. J Infect Dis. 1997;176:1405–1409. doi: 10.1086/517332. [DOI] [PubMed] [Google Scholar]
- 109.Yoshiie K, Kim HY, Mott J, Rikihisa Y. Intracellular infection by the human granulocytic ehrlichiosis agent inhibits human neutrophil apoptosis. Infect Immun. 2000;68:1125–1133. doi: 10.1128/iai.68.3.1125-1133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Herron MJ, Nelson CM, Larson J, et al. Intracellular parasitism by the human granulocytic ehrlichiosis bacterium through the P-selectin ligand, PSGL-1. Science. 2000;288:1653–1656. doi: 10.1126/science.288.5471.1653. [DOI] [PubMed] [Google Scholar]
- 111.Carlyon JA, Chan WT, Galan J, et al. Repression of rac2 mRNA expression by Anaplasma phagocytophila is essential to the inhibition of superoxide production and bacterial proliferation. J Immunol. 2002;169:7009–7018. doi: 10.4049/jimmunol.169.12.7009. [DOI] [PubMed] [Google Scholar]
- 112.Webster P, Ijdo JW, Chicoine LM, et al. The agent of human granulocytic ehrlichiosis resides in an endosomal compartment. J Clin Invest. 1998;101:1932–1941. doi: 10.1172/JCI1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Banerjee R, Anguita J, Roos D, et al. Cutting edge: Infection by the agent of human granulocytic ehrlichiosis prevents the respiratory burst by down-regulating gp91phox. J Immunol. 2000;164:3946–3949. doi: 10.4049/jimmunol.164.8.3946. [DOI] [PubMed] [Google Scholar]
- 114.Choi K-S, Garyu J, Park J, et al. Diminished adhesion of Anaplasma phagocytophilum-infected neutrophils to endothelial cells is associated with reduced expression of leukocyte surface selectin. Infect Immun. 2003;71:4586–4594. doi: 10.1128/IAI.71.8.4586-4594.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Choi KS, Webb T, Oelke M, et al. Differential innate immune cell activation and proinflammatory response in Anaplasma phagocytophilum infection. Infect Immun. 2007;75(6):3124–30. doi: 10.1128/IAI.00098-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Klein MB, Hu S, Chao CC, et al. The agent of human granulocytic ehrlichiosis induces the production of myelosuppressing chemokines without induction of proinflammatory cytokines. J Infect Dis. 2000;182:200–205. doi: 10.1086/315641. [DOI] [PubMed] [Google Scholar]
- 117.Paddock CD, Childs JE. Ehrlichia chaffeensis: a Prototypical Emerging Pathogen. Clin Microbiol Rev. 2003;16:37–64. doi: 10.1128/CMR.16.1.37-64.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Caldwell CW, Everett ED, McDonald G, et al. Lymphocytosis of gamma/delta T cells in human ehrlichiosis. Am J Clin Pathol. 1995;103:761–766. doi: 10.1093/ajcp/103.6.761. [DOI] [PubMed] [Google Scholar]
- 119.Bitsaktsis C, Huntington J, Winslow G. Production of IFN-gamma by CD4 T cells is essential for resolving Ehrlichia infection. J Immunol. 2004;172:6894–6901. doi: 10.4049/jimmunol.172.11.6894. [DOI] [PubMed] [Google Scholar]
- 120.Bitsaktsis C, Nandi B, Racine R, et al. T-Cell-Independent Humoral Immunity Is Sufficient for Protection against Fatal Intracellular Ehrlichia Infection. Infect Immun. 2007;75:4933–4941. doi: 10.1128/IAI.00705-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bitsaktsis C, Winslow G. Fatal recall responses mediated by CD8 T cells during intracellular bacterial challenge infection. J Immunol. 2006;177:4644–4651. doi: 10.4049/jimmunol.177.7.4644. [DOI] [PubMed] [Google Scholar]
- 122.Winslow GM, Yager E, Shilo K, et al. Antibody-mediated elimination of the obligate intracellular bacterial pathogen Ehrlichia chaffeensis during active infection. Infection and Immunity. 2000;68:2187–2195. doi: 10.1128/iai.68.4.2187-2195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yager E, Bitsaktsis C, Nandi B, et al. Essential role for humoral immunity during Ehrlichia infection in immunocompetent mice. Infect Immun. 2005;73:8009–8016. doi: 10.1128/IAI.73.12.8009-8016.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liddell AM, Sumner JW, Paddock CD, et al. Reinfection with Ehrlichia chaffeensis in a liver transplant recipient. Clin Infect Dis. 2002;34:1644–1647. doi: 10.1086/340523. [DOI] [PubMed] [Google Scholar]
- 125.Thirumalapura NR, Stevenson HL, Walker DH, et al. Protective heterologous immunity against fatal ehrlichiosis and lack of protection following homologous challenge. Infect Immun. 2008;76:1920–1930. doi: 10.1128/IAI.01293-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Paddock CD, Suchard DP, Grumbach KL, et al. Brief report: fatal seronegative ehrlichiosis in a patient with HIV infection. N Engl J Med. 1993;329:1164–1167. doi: 10.1056/NEJM199310143291605. [DOI] [PubMed] [Google Scholar]
- 127.Walker DH. Diagnosing human ehrlichioses: current status and recommendations. ASM News. 2000;66:287–291. [Google Scholar]
- 128.Childs JE, Sumner JW, Nicholson WL, et al. Outcome of diagnostic tests using samples from patients with culture-proven human monocytic ehrlichiosis: implications for surveillance. J Clin Microbiol. 1999;37:2997–3000. doi: 10.1128/jcm.37.9.2997-3000.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Childs JE, McQuiston JH, Sumner JW, et al. Human monocytic ehrlichiosis due to Ehrlichia chaffeensis: how do we count the cases? In: Raoult D, Brouqui P, editors. Rickettsiae and rickettsial diseases at the turn of the third millenium. Elsevier; Paris, France: 1999. pp. 287–293. [Google Scholar]
- 130.Standaert SM, Yu T, Scott MA, et al. Primary isolation of Ehrlichia chaffeensis from patients with febrile illnesses: clinical and molecular characteristics. J Infect Dis. 2000;181:1082–1088. doi: 10.1086/315346. [DOI] [PubMed] [Google Scholar]
- 131.Tan HP, Dumler JS, Maley WR, et al. Human monocytic ehrlichiosis: an emerging pathogen in transplantation. Transplantation. 2001;71:1678–1680. doi: 10.1097/00007890-200106150-00030. [DOI] [PubMed] [Google Scholar]
- 132.Anderson BE, Sumner JW, Dawson JE, et al. Detection of the etiologic agent of human ehrlichiosis by polymerase chain reaction. J Clin Microbiol. 1992;30:775–780. doi: 10.1128/jcm.30.4.775-780.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sumner JW, Nicholson WL, Massung RF. PCR amplification and comparison of nucleotide sequences from the groESL heat shock operon of Ehrlichia species. J Clin Microbiol. 1997;35:2087–2092. doi: 10.1128/jcm.35.8.2087-2092.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sumner JW, Childs JE, Paddock CD. Molecular cloning and characterization of the Ehrlichia chaffeensis variable-length PCR target: an antigen-expressing gene that exhibits interstrain variation. J Clin Microbiol. 1999;37:1447–1453. doi: 10.1128/jcm.37.5.1447-1453.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Doyle CK, Labruna MB, Breitschwerdt EB, et al. Detection of medically important Ehrlichia by quantitative multicolor TaqMan real-time polymerase chain reaction of the dsb gene. J Mol Diagn. 2005;7(4):504–10. doi: 10.1016/S1525-1578(10)60581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bakken JS, Dumler JS. Ehrlichia species. In: Merigan VLYuTC, Jr, Barriere SL., editors. Antimicrobial therapy and vaccines. The Williams & Wilkins Co; Baltimore, Md: 1999. pp. 546–554. [Google Scholar]
- 137.American Academy of Pediatrics. Ehrlichia infections (human ehrlichioses) In: Pickering LK, Baker CJ, Overturf GD, Prober CG, editors. 2003 Red Book: report of the Committee on Infectious Diseases. 26. Elk Grove Village, Illinois: American Academy of Pediatrics, Committee on Infectious Diseases; 2003. pp. 266–9. [Google Scholar]
- 138.Walker DH, Sexton DJ. Rickettsia rickettsii. In: Yu VL, Merigan TC Jr, Barriere SL, editors. Antimicrobial therapy and vaccines. Baltimore: Williams & Wilkins; 1999. pp. 562–8. [Google Scholar]
- 139.Smith Sendev AE, Sehdev PS, Jacobs R, et al. Human monocytic ehrlichiosis presenting as acute appendicitis during pregnancy. Clin Infect Dis. 2002;35:e99–e102. doi: 10.1086/342887. [DOI] [PubMed] [Google Scholar]
- 140.Buitrago MI, IJdo JW, Rinaudo P, et al. Human granulocytic ehrlichiosis during pregnancy treated successfully with rifampin. Clin Infect Dis. 1998;27:213–216. doi: 10.1086/517678. [DOI] [PubMed] [Google Scholar]
- 141.Hongo I, Bloch KC. Ehrlichia infection of the central nervous system. Curr Treat Options Neurol. 2006;8(3):179–84. doi: 10.1007/s11940-006-0008-8. [DOI] [PubMed] [Google Scholar]
- 142.Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43(9):1089–134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 143.Katavolos P, Armstrong PM, Dawson JE, Telford SR., III Duration of tick attachment required for transmission of granulocytic ehrlichiosis. J Infect Dis. 1998;177:1422–5. doi: 10.1086/517829. [DOI] [PubMed] [Google Scholar]
- 144.des Vignes F, Piesman J, Heffernan R, et al. Effect of tick removal on transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis nymphs. J Infect Dis. 2001;183:773–8. doi: 10.1086/318818. [DOI] [PubMed] [Google Scholar]
- 145.Needham GR. Evaluation of five popular methods of tick removal. Pediatrics. 1985;75:997–1002. [PubMed] [Google Scholar]


