Abstract
Schizophrenia postmortem brain is characterized by GABAergic downregulation and by decreased dendritic spine density in frontal cortex. Protracted L-methionine treatment exacerbates schizophrenia symptoms, and our previous work (Tremolizzo et al. and Dong et al.) has shown that L-methionine decreases reelin and GAD67 transcription in mice which is prevented by co-administration of valproate. In this study, we observed a decrease in spine density following L-methionine treatment, which was prevented by co-administration of valproate. Together with our previous findings conducted under the same experimental conditions, we suggest that downregulation of spine density in L-methionine -treated mice may be due to decreased expression of reelin and that valproate may prevent spine downregulation by inhibiting the methylation induced decrease in reelin.
Keywords: reelin, GAD67, dendritic spines, frontal cortex, schizophrenia, valproate, L-methionine, methylation
Introduction
Schizophrenia postmortem brain is characterized by a decrease in the density of spines on dendrites of layer III pyramidal neurons in the prefrontal cortex [1], an area of the brain in which reelin and glutamic acid decarboxylase (GAD67) levels are decreased in distinct populations ofγ aminobutyric acid {GABAergic) neurons [2, 3]. Significantly, spine density is similarly decreased in the cortex and hippocampus of heterozygous reeler mice [4]. In these mice, reelin deficiency is associated with GABAergic downregulation (4), glutamatergic receptor dysfunction [5 ], and behavioral deficits [6].
The observation that schizophrenia symptoms unexpectedly worsened with L-methionine treatment remained unexplained for decades [7]. Based on our studies in mice, we have proposed that L-methionine increases brain levels of S-adenosyl methionine, thereby facilitating methylation of reelin (RELN) and other GABAergic gene promoters, such as GAD67 (GAD1) [7]. The consequence of methylation is a decrease in reelin and GAD67 levels (8,9).
Both L-methionine -treated mice [8] and heterozygous reeler mouse [6] have behavioral abnormalities reminiscent of the negative and cognitive symptoms of schizophrenia which supports this mouse model. Further validation of the L-methionine -treated mouse model for GABAergic downregulation in schizophrenia is provided by our finding that the effects of L-methionine on reelin and GAD67 levels and on behavior in mice are prevented by concurrent administration of the histone deacetylase (HDAC) inhibitors valproate and MS275 [8,9]. The ability of HDAC inhibitors to prevent methylation-induced decreases in GABAergic function may be consistent with the synergistic interactions sometimes observed when valprote is added to an antipsychotic [10].
In the present study, we sought to determine whether L-methionine -treated mice exhibit a decrease in the dendritic spine density of layer III pyramidal neurons in the frontal cortex that is similar to the decrease in spine density reported in schizophrenia postmortem brain and in heterozygous reeler mice. In addition, we investigated whether dendritic spine downregulation by L-methionine could be prevented by co-administration of valproate, an HDAC inhibitor we have previously shown to prevent the effects of L-methionine on the transcription of reelin [8,9].
Materials and methods
Animals
Adult male Swiss Webster albino mice 60-80 days old and weighing ~ 25-30 g were housed five animals to a cage in a temperature and humidity controlled animal laboratory facility on a 12-12 light/dark cycle with food and water ad libitum. Procedures were performed in accordance with the University of Illinois at Chicago Animal Care and Use Committee and federal regulations and policies.
Drugs
L-methionine (Sigma) and valproic acid sodium salt (Sigma) were dissolved in 0.9% saline and injected subcutaneously twice daily (early morning and late afternoon) at 0.1 ml/10 g. The vehicle was 0.9% saline. The dose of L-methionine was 5.2 mmol/kg s.c. twice daily for 7 or 14 days and the dose of valproate was 2 mmol/kg s.c. twice daily for seven days. The dose and duration of L-methionine treatment was selected to induce maximum downregulation of reelin mRNA and protein based on our previous studies in mice [8,9]. The dose of valproate was selected to produce a maximal increase (4-fold) of acetylated histone 3 (H3) two hours after injection based on these prior studies [9]. This dose of valproate administered to mice appears disproportionately larger than the doses of valproate clinically administered. However, pharmacokinetic differences between mice and humans suggest that the dose selected for mice is within the therapeutic range as previously discussed in more detail [8]. For co-administration of L-methionine and valproate, an injection of L-methionine was immediately followed by an injection of valproate, and control mice received the same number of injections.
Golgi-impregnation
Two hours after the last injection, the brain was extracted and stored in 4% paraformaldehyde at 4° C. Tissue blocks were cut from the right frontal cortex and cryoprotected in 30% sucrose in PBS for three days. The Golgi staining technique was previously described by us [4].
Spine measurement
Microscopic work was conducted completely blind to condition because of the inherent random sampling and subjectivity involved in identifying and counting spines. Three-to-five pyramidal neurons from layer III were selected per mouse using a Zeiss Axioskop equipped with a 100x objective and an optovor lens connected to a live image monitor (Samba system) (total magnification = 3,000). The criteria used in selecting neurons were: (i) complete impregnation relatively unobscured by other neurons or artifacts, (ii) clear image, and (iii) an apical and at least three basilar dendrites. For each neuron, the apical and three basilar dendrites (and their branches) were traced from the center of the cell body to their natural or artificial ends. We used the Sholl ring method [11] to measure spine density within each 20 μm ring starting from the center of the pyramidal neuron (density = number of spines counted/length of dendrite). Spine density data for the three basilar dendrites per mouse were averaged.
The method used to count spines does not allow for adequate measurement in the z-plane. However, the sample of actually counted spines to the total population of spines that could be theoretically counted would be expected to be a constant across experimental conditions since measurements were conducted blind to condition. To assess the reliability of measurement, counts were repeated in five vehicle-treated mice by an independent investigator. The agreement between counts was high (r = 0.95 or better).
Statistical Analysis
Each experiment was conducted with an n of four-to-five mice/group, and experiments were replicated two or three times. Two-way ANOVA with distance along the dendrite as the repeated measure, t-tests, or one-way ANOVA with post-hoc comparisons were conducted on raw scores for each experiment separately for the apical and basilar dendrites. Since the results across experiments were consistent, data were combined and a statistical analysis was conducted on z-scores using SAS 9.1. A significance criterion of at least p < 0.05, 2-tailed, was chosen in advance with Bonferroni corrections where appropriate.
Results
Spine density following protracted L-methionine administration
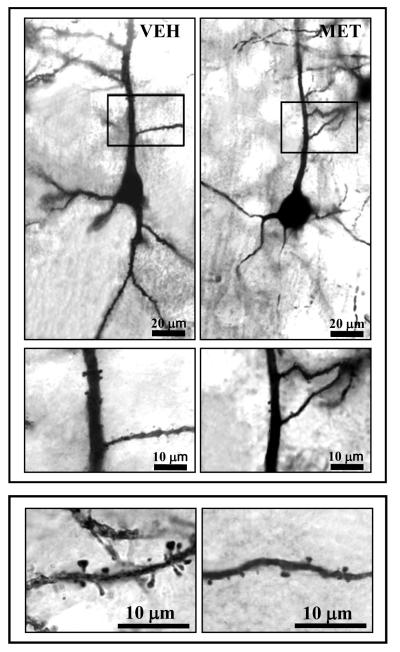
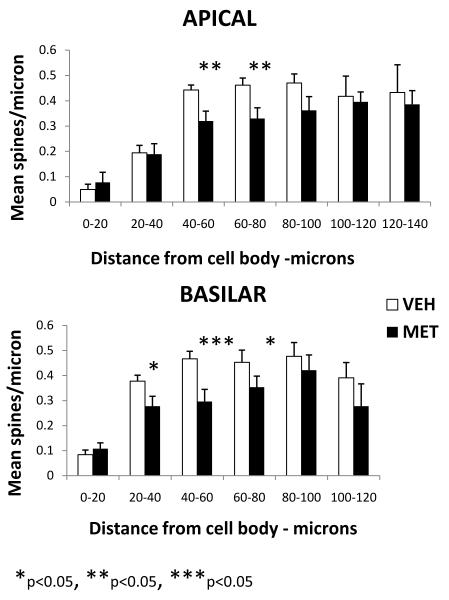
L-methionine administration for seven days decreased dendritic spine density. Figure 1 shows representative Golgi stained pyramidal neurons in layer III of frontal cortex at different magnifications. The distribution of spine density along the apical and basilar dendrites based on quantitative spine counts is shown in Figure 2. In vehicle-treated mice, apical spine density increased from an average density of 0.04 spines/μm in the first 20 μm segment to an average density of 0.47 spines/μm in the 80-100 μm segment. Basilar spine density showed a similar pattern with the increase shifted closer to the cell body.
Figure 1.
Microscope digital photographs of Golgi stained layer III pyramidal neurons in mouse frontal cortex. Top panel: 10x objective; Middle panel: 20x Bottom panel: 100x. Vehicle (VEH); L-methionine (MET)
Figure 2.
Mean spine density per micron as a function of distance from the pyramidal cell body shown separately for the apical and basilar dendrites. (Vehicle (VEH) n=13; L-methionine (MET) n=12). The dose of L-methioinine was 5.2. mmol/kg s.c. twice daily for seven days.
L-methionine -treated animals expressed lower levels of spine density at intermediate distances from the cell body in a region of the dendrite showing high spine density in vehicle-treated mice; the maximum depression of spine density was located in a region 40-80 μm from the cell body for the apical dendrite and 20-80 μm from the cell body for basilar dendrites. A 2-factor ANOVA with distance as a repeated measure effect revealed that the effect of condition (L-methionine vs. vehicle) was significantly different for both the apical dendrite (F 1,152 = 7.31, p = 0.007) and the basilar dendrite (F 1,124 = 24.03, p < 0.0001). The difference in mean spine density between L-methionine and vehicle for each Sholl ring was assessed and differences were significant for the apical dendrite in the 40-60 μm ring (p=0.0043) and the 60-80 μm ring ( p=0.0075) with a trend for the 80-100 μm ring (p=0.0923). For the basilar dendrite, a difference was found for the 20-40 μm ring (p=0.0175), the 40-60 μm ring (p=0.0005), and the 60-80 μm ring (p=0.0474).
Spine density was also decreased in these same regions of the dendrite in mice treated with L-methionine for 14 days. No difference was observed in mean spine density between mice treated with L-methionine for 7 versus 14 days (APICAL at 60-80 μm: L-methionine 7 days =0.28±0.04; L-methionine 14 days = 0.30±0.03; BASILAR at 40-60 μm: L-methionine 7 days = 0.25±0.05; L-methionine 14 days = 0.26±0.05, n=5/group)
Recovery of spine density after withdrawal from L-methionine
After L-methionine treatment for seven days (5.2. mmol/kg s.c. twice daily), L-methionine was withdrawn and mice were injected with vehicle for an additional 12 days to assess recovery from L-methionine -induced spine density downregulation. Spine density recovered to the level of vehicle -treated mice within 12 days of withdrawal (Table 1).
Table 1.
Spine density (M ± SE) for dendritic region where experimental differences were largest
| Apical (40-80 μm) |
Basilar (20 – 80 μm) |
|
|---|---|---|
| Vehicle (n = 13) | 0.45 ± 0.02* | 0.43 ± 0.03* |
| L-methionine (n = 12) | 0.32 ± 0.04 | 0.27 ± 0.04 |
| L-methionine + valproate (n = 4) | 0.58 ± 0.09* | 0.54 ± 0.08* |
| Valproate (n = 9) | 0.61 ± 0.06* | 0.52 ± 0.04* |
| L-methionine withdrawal (n = 5) | 0.51 ± 0.04* | 0.44 ± 0.07* |
Significantly different from L-methionine (Bonferroni multiple comparisons (p<0.01). One-way ANOVA: Apical F = 9.68, p<0.0001 Basilar F = 12.41, P<0.0001.
Co-administration of L-methionine and valproate
As shown in Table 1 for dendritic regions associated with maximal differences, spine density in mice treated with a combination of L-methionine (5.2 mmol/mg s.c. twice daily) and valproate (2 mmol/kg s.c. twice daily) for seven days was similar to spine density in mice treated with vehicle or valproate alone and did not show the decrease in spine density induced by L-methionine alone. The distribution of spine density along the apical and basilar dendrites associated with co-administration of L-methionine and valproate was similar to the distribution of spine density associated with administration of vehicle (Figure 2) or administration of valproate alone.
Discussion
In this study, protracted administration of L-methionine decreased dendritic spine density of layer III pyramidal neurons in frontal cortex of Swiss albino mice. Under the same laboratory conditions, L-methionine was shown in our previous studies of B6C3Fc and albino Swiss mice to upregulate the methyl donor S-adenosyl methionine, hypermethylate GABAergic gene promoters, and decrease the expression of reelin [8: Fig 4 and 9: Fig 1].
The decrease in spine density was maximal at a distance of 40 to 80 μm from the cell body for the apical dendrite and 20-to-80 μm for the basilar dendrite. The effect of L-methionine was not evident closer to the cell body or at more distal regions of the dendrite. Importantly, the decrease in spine density associated with protracted L-methionine treatment is in the same region of the dendrite where a decrease in spine density was detected in heterozygous reeler mouse [4] and in schizophrenia postmortem brain [12].
Dendritic compartmentalization of L-methionine’s effect is consistent with reports that inputs to the layer III pyramidal cell dendritic tree are diverse and specific to dendritic subregions [13,14]. In the distal regions, upper layer GABAergic interneurons (horizontal and bitufted cells) synthesize and secrete reelin into the extracellular matrix by a constitutive process in large amounts [15]. Diffuse extracellular matrix reelin immunostaining is visible with a gradient that declines with each deeper cortical layer [2,3]. In addition to the reelin extracellular matrix gradient, there are other factors that could explain the dendritic compartmentalization of the effect of L-methionine on spine density, e.g., the gamma wave microcircuitry in layer III which involves GABAergic basket cells, large inputs to layer III from the mediodorsal region of the thalamus, and axonal feedback from other layers in the cortical column (14).
Role of reelin and GABAergic tone in the L-methionine-induced downregulation of dendritic spine density
Reelin is decreased by approximately 50% in psychotic postmortem brain and in heterozygous reeler mouse, and is also significantly decreased in mice treated with L-methionine [8,9]. In the cortex and hippocampus, reelin is released constitutively into the extracellular matrix by GABA interneurons where it adheres to spines and shafts of glutamatergic pyramidal neurons [15, 16]. There are several reports indicating that adequate reelin levels are necessary for spine formation and normal glutamatergic function essential for normal cognition and synaptic plasticity [5,15,16,17,18]. These observations provide evidence that reelin and its receptors may act as trophic modulators of spine formation in cortical pyramidal neurons.
Although reelin may play a pivotal role in spine formation, a more direct trophic role for GABA at the dendritic spine level cannot be excluded. Downregulation by L-methionine of GAD67, a major enzyme for GABA synthesis, and a widespread decrease in inhibitory tone in the neural circuitry underlying schizophrenia, together with the reelin deficit, could be responsible for the L-methionine-induced decrease in spine density found in this study. However, we have preliminary data indicating that neither reelin nor spine density are decreased in the frontal cortex of GAD67 heterozygous mice [4]. Nevertheless, the role of GAD67 and a generalized decrease in GABAergic tone in the dendritic spine downregulation induced by L-methionine requires further study.
In addition to reelin and GAD67, promoter regions of other genes involved in synaptic function and spine dynamics may be susceptible to promoter methylation by L-methionine. For example, a role for brain-derived neurotrophic factor (BDNF) downregulation in L-methionine -treated mice cannot be excluded, but cortical dendritic spine density is not decreased in BDNF knockout mice [19]. Additionally, our unpublished data and the data of others [20] indicate that BDNF expression is not reduced in heterozygous reeler mice, but these reelin deficient mice exhibit decreased spine density.
L-methionine downregulation of spine density is prevented by co-administration of valproate
Valproate when administered in combination with L-methionine for seven days prevented the downregulation of spine density induced by L-methionine. These data correspond to our previous finding that co-administration of valproate and L-methionine is associated with promoter demethylation and prevents the downregulation of reelin and GAD67 expression and the behavioral deficits observed in MET-treated mice [8: Table 2] and with our finding that valproate accelerates reelin and GAD 67 promoter demethylation after L-methionine withdrawal [9: Fig 4].
These results suggest that an interplay exists between L-methionine and valproate with respect to influences on spine density. We and others have reported that valproate, by inhibiting histone deacetylases and increasing acetylated histone 3 and lysine 9,14 brain levels [8], remodels chromatin, and thereby 1) induces promoter demethylation [9], 2) facilitates GABAergic gene transcription [9, 21], and 3) as found in this study, prevents spine downregulation in L-methionine -treated mice. Consistent with our hypothesis that valproate prevents the downregulation of spine density by L-methionine by inhibiting histone deacetylases (HDACs) [22], is a recent report that overexpression of HDAC2 decreases spine density in mouse hippocampal pyramidal neurons [23]. However, valproate, in addition to its established HDAC inhibitory activity, is also known to have other actions. These actions include inhibition of the activity of GSK3β, a negative regulator of the Wnt/β catenin signaling pathway [24], which is known to be involved in dendritic spine morphogenesis and synaptic plasticity [25]. Hence, before definitive conclusions on the role of HDACs and chromatin remodeling in the mechanism of action of valproate on spine formation can be made, further studies with other HDAC inhibitors such as MS-275 should be conducted, preferably with a direct comparison of the proximal and distal portions of the apical dendrite.
Further study using the L-methionine mouse model might be aimed at determining whether specific antipsychotics enhance spine density and whether spine density can be further upregulated by valproate co-administration, as we would predict from our finding that clozapine and sulpiride but not haloperidol or olanzapine accelerate demethylation of reelin and GAD67 promoters by valproate in mice [10]. It would also be important to establish whether changes in spine density induced by L-methionine are present in brain areas other than frontal cortex.
Conclusion
Mice treated for seven days with L-methionine are characterized by decreased dendritic spine density of layer III pyramidal neurons in frontal cortex that is similar to the dendritic spine downregulation found in schizophrenia postmortem brain and in heterozygous reeler mice. This spine density downregulation induced by L-methionine was prevented by co-administration of valproate. These results are consistent with reelin’s reported influence on spine function and morphology and with our previously published data on the effect of L-methionine and valproate on reelin transcription in mice.
Acknowledgments
We thank Wen Sheng Liu for assistance with the Golgi staining and microscopic procedures and Qiaoyan Hu for statistical assistance.
Support: NIH grant MH071667 to E. Costa and MH070855 to A. Guidotti
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature References
- 1.Glanz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 2.Guidotti A, Auta J, Davis JM, DiGiorgi-Cerenini V, Dwivedi J, Grayson DR, et al. Decreased reelin and glutamic acid decarboxylase 67 (GAD67) expression in schizophrenia and bipolar disorders a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 3.Ruzicka WB, Zhubi A, Veldic M, Grayson DR, Costa E, Guidotti A. Selective epigenetic alteration of layer 1 GABAergic neurons isolated from prefrontal cortex of schizophrenia patients using laser-assisted microdissection. Mol Psychiatry. 2007;12:385–397. doi: 10.1038/sj.mp.4001954. [DOI] [PubMed] [Google Scholar]
- 4.Liu WS, Pesold C, Rodriguez MA, Carboni G, Auta J, Lacor P, et al. Down-regulation of dendrite spine and glutamic acid decarboxylase 67 expressions in the reelin haploinsufficient heterozygous reeler mouse. Proc Nat Acad Sci USA. 2001;98:3477–3482. doi: 10.1073/pnas.051614698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levenson JM, Qiu S, Weeber EJ. The role of reelin in adult synaptic function and the genetic and epigenetic regulation of the reelin gene. Biochem Biophys Acta. 2008;1779:422–431. doi: 10.1016/j.bbagrm.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Tueting P, Doueiri M-S, Guidotti A, Davis JM, Costa E. Reelin down-regulation in mice and psychosis endophenotypes. Neurosci Biobehav Rev. 2006;30:1065–1077. doi: 10.1016/j.neubiorev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Grayson DR, Chen Y, Dong E, Kundakovic M, Guidotti A. From trans-methylation to cyotsine methylation evolution of the methylation hypothesis of schizophrenia. Epigenetics. 2009;4:144–149. doi: 10.4161/epi.4.3.8534. [DOI] [PubMed] [Google Scholar]
- 8.Tremolizzo L, Doueiri M-S, Dong E, Grayson DR, Davis JM, Pinna G, et al. Valproate corrects the schizophrenia-like epigenetic behavioral modifications induced by methionine in mice. Biol Psychiatry. 2005;57:500–509. doi: 10.1016/j.biopsych.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 9.Dong E, Guidotti A, Grayson DR, Costa E. Histone hyperacetylation induces demethylation of reelin and 67-kDa glutamic acid decarboxylase promoters. Proc Nat Acad Sci USA. 2007;104(11):4676–4681. 13b. doi: 10.1073/pnas.0700529104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong E, Nelson M, Grayson DR, Costa E, Guidotti A. Clozapine and sulpiride but not haloperidol or olanzapine activate brain DNA demethylation. Proc Nat Acad Sci USA. 2008;105:13614–13619. doi: 10.1073/pnas.0805493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- 12.Rosoklija G, Toomayan G, Ellis SP, Keilp J, Mann J, Latov N, et al. Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders. Arch Gen Psychiatry. 2000;57:349–356. doi: 10.1001/archpsyc.57.4.349. [DOI] [PubMed] [Google Scholar]
- 13.Benes FM. Searching for unique endophenotypes for schizophrenia and bipolar disorder within neural circuits and their molecular regulatory mechanism. Schizophrenia Bull. 2007;33:932–939. doi: 10.1093/schbul/sbm064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis DA, Gonzalez-Burgos GG. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez MA, Pesold C, Liu WS, Kriho V, Guidotti A. Colocalization of integrin receptors and reelin in dendritic spine postsynaptic densities of adult nonhuman primate cortex. Proc Nat Acad Sci USA. 2000;97:3550–3555. doi: 10.1073/pnas.050589797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa E, Davis JM, Grayson DR, Guidotti A, Pappas GD, Pesold C. Dendritic spine hypoplasticity and downregulation of reelin and GABAergic tone in schizophrenia vulnerability. Neurobiol Dis. 2001;8:723–742. doi: 10.1006/nbdi.2001.0436. [DOI] [PubMed] [Google Scholar]
- 17.Chameau P, Inta D, Vitalis T, Monyer H, Wadman WJ, vanHooft JA. The N-terminal region of reelin regulates postnatal dendritic maturation of cortical pyramidal neurons. Proc Nat Acad Sci USA. 2009;106:7227–7232. doi: 10.1073/pnas.0810764106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong E, Caruncho H, Liu WS, Smalheiser NR, Grayson DR, Costa E, et al. A reelin-integrin receptor interaction regulates Arc mRNA translation in synaptoneurosomes. Proc Nat Acad Sci USA. 2003;100:5479–5484. doi: 10.1073/pnas.1031602100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill JJ, Kolluri N, Hashimoto T, Wu Q, Sampson AR, Monteggia LM, et al. Analysis of pyramidal neuron morphology in an inducible knockout of brain-derived neurotrophic factor. Biol Psychiatry. 2005;57:932–934. doi: 10.1016/j.biopsych.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Pillai A, Mahadik SP. Increased truncated TrkB receptor expression and decreased BDNF/TrkB signaling in the frontal cortex of reeler mouse model of schizophrenia. Schizophr Res. 2008;100:325–333. doi: 10.1016/j.schres.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 21.Milutinovic S, D’Alessio AC, Detich N, Szyf S. Valproate induces widespread epigenetic reprogramming which involves demethylation of specific genes. Carcinogenesis. 2007;28:560–571. doi: 10.1093/carcin/bgl167. [DOI] [PubMed] [Google Scholar]
- 22.Guidotti A, Dong E, Kundakovic M, Satta R, Grayson DR, Costa E. Characterization of the action of antipsychotic subtypes on valproate-induced chromatin remodeling. Trends Pharm Sci. 2009;30:55–60. doi: 10.1016/j.tips.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Guan J-S, Haggarty SJ, Giacometti E, Dannenberg J-H, Joseph N, Gao J. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fatemi SH, Reutiman TJ, Folsom TD. Chronic psychotropic drug treatment causes differential expression of reelin signaling system in freontal cortex of rats. Schizophr Res. 2009;111:38–52. doi: 10.1016/j.schres.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Okuda T, Yu LMY, Cingolani LA, Kemler R, Goda Y. β-catenin regulates excitatory postsynaptic strength at hippocampal synapses. Proc Nat Acad Sci USA. 2007;104:13479–13484. doi: 10.1073/pnas.0702334104. [DOI] [PMC free article] [PubMed] [Google Scholar]