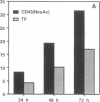
Abstract
A human hematopoietic disorder designated as Tn syndrome or permanent mixed-field polyagglutinability has been ascribed to a stem cell mutation leading to a specific deficiency of UDP-Gal:GalNAc alpha 1-O-Ser/Thr beta 1-3 galactosyltransferase (beta 3 Gal-T) activity in affected cells. To test for the possibility that an allele of the beta 3Gal-T gene might be repressed instead of mutated, we have investigated whether 5-azacytidine or sodium n-butyrate, both inducers of gene expression, would reactivate expression of beta 3Gal-T in cloned enzyme-deficient T cells derived from a patient affected by the Tn syndrome. Flow cytometry revealed that a single treatment induced de novo expression of the Thomsen-Friedenreich antigen (Gal beta 1-3GalNAc-R), the product of beta 3Gal-T activity. In addition, a sialylated epitope on CD43 (leukosialin), which is present on normal but not on beta 3Gal-T-deficient T cells, was also reexpressed. Although no beta 3Gal-T activity was detectable in untreated Tn syndrome T cells, after exposure to 5-azaC,beta 3Gal-T activity reached nearly normal values. Both agents failed to reactivate beta 3Gal-T in Jurkat T leukemic cells, which also lack beta 3Gal-T activity. These data demonstrate that Tn syndrome T cells contain an intact beta 3Gal-T gene copy and that the enzyme deficiency in this patient is due to a persistent and complete but reversible repression of a functional allele. In contrast, the cause of beta 3Gal-T deficiency appears to be different in Jurkat T cells.
Full text
PDF

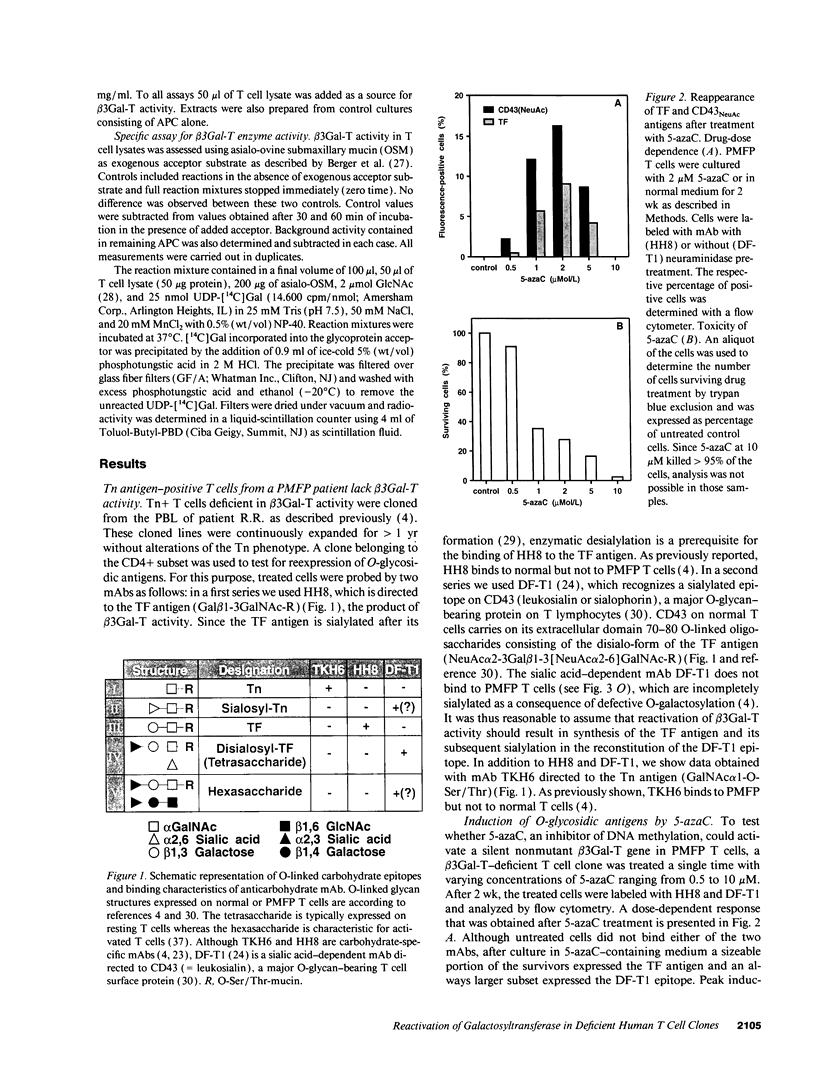
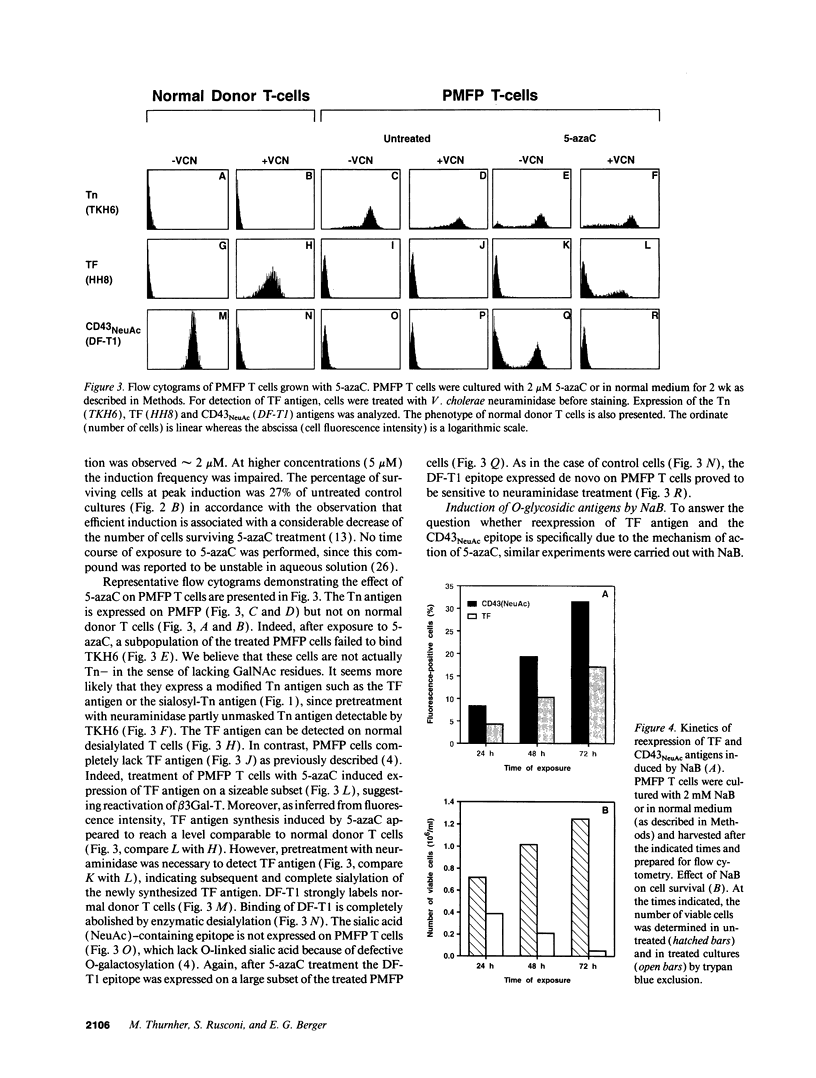
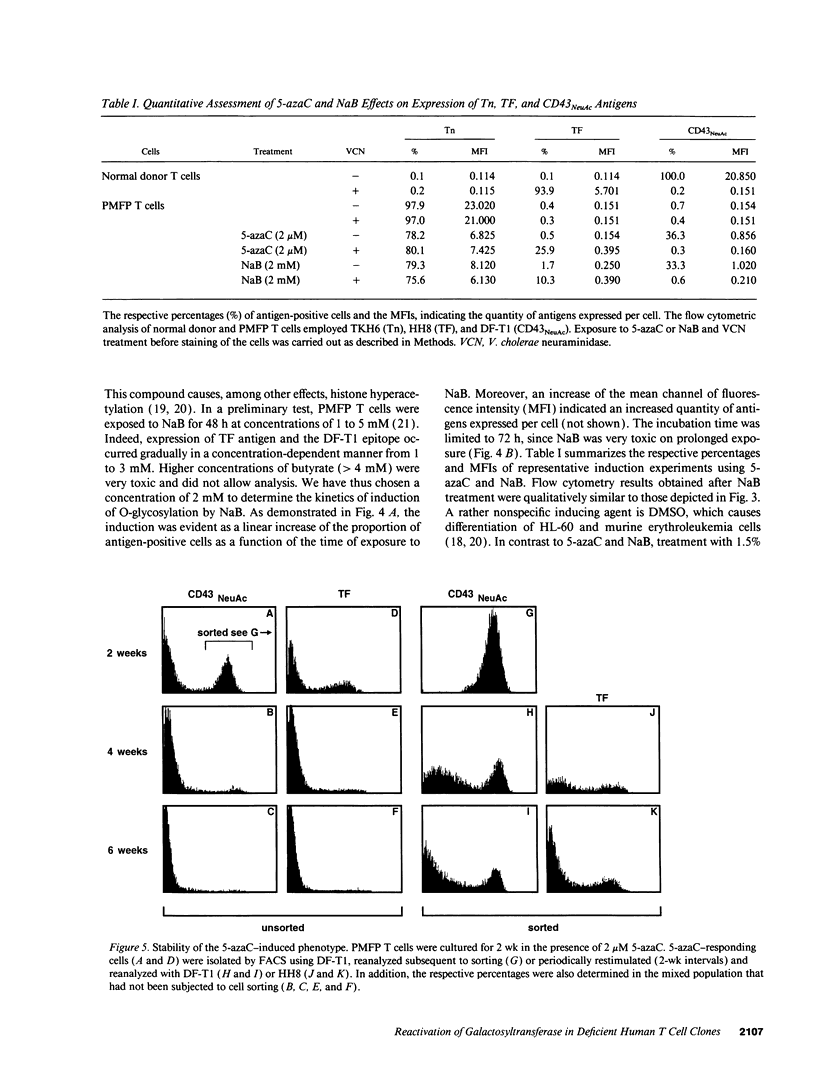


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballas Z. K. The use of 5-azacytidine to establish constitutive interleukin 2-producing clones of the EL4 thymoma. J Immunol. 1984 Jul;133(1):7–9. [PubMed] [Google Scholar]
- Ben-Sasson S. A., Klein G. Activation of the Epstein-Barr virus genome by 5-aza-cytidine in latently infected human lymphoid lines. Int J Cancer. 1981 Aug 15;28(2):131–135. doi: 10.1002/ijc.2910280204. [DOI] [PubMed] [Google Scholar]
- Berger E. G., Kozdrowski I. Permanent mixed-field polyagglutinable erythrocytes lack galactosyltransferase activity. FEBS Lett. 1978 Sep 1;93(1):105–108. doi: 10.1016/0014-5793(78)80815-1. [DOI] [PubMed] [Google Scholar]
- Berger E. G., Kozdrowski I., Weiser M. M., van den Eijnden D. H., Schiphorst W. E. Human serum galactosyltransferase: distinction, separation and product identification of two galactosyltransferase activities. Eur J Biochem. 1978 Oct;90(2):213–222. doi: 10.1111/j.1432-1033.1978.tb12593.x. [DOI] [PubMed] [Google Scholar]
- Bird A. The essentials of DNA methylation. Cell. 1992 Jul 10;70(1):5–8. doi: 10.1016/0092-8674(92)90526-i. [DOI] [PubMed] [Google Scholar]
- Bird G. W., Shinton N. K., Wingham J. Persistent mixed-field polyagglutination. Br J Haematol. 1971 Oct;21(4):443–453. doi: 10.1111/j.1365-2141.1971.tb02705.x. [DOI] [PubMed] [Google Scholar]
- Borche L., Lozano F., Vilella R., Vives J. CD43 monoclonal antibodies recognize the large sialoglycoprotein of human leukocytes. Eur J Immunol. 1987 Oct;17(10):1523–1526. doi: 10.1002/eji.1830171023. [DOI] [PubMed] [Google Scholar]
- Boyd A. W., Schrader J. W. Derivation of macrophage-like lines from the pre-B lymphoma ABLS 8.1 using 5-azacytidine. Nature. 1982 Jun 24;297(5868):691–693. doi: 10.1038/297691a0. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cartron J. P., Nurden A. T. Galactosyltransferase and membrane glycoprotein abnormality in human platelets from Tn-syndrome donors. Nature. 1979 Dec 6;282(5739):621–623. doi: 10.1038/282621a0. [DOI] [PubMed] [Google Scholar]
- Clausen H., Stroud M., Parker J., Springer G., Hakomori S. Monoclonal antibodies directed to the blood group A associated structure, galactosyl-A: specificity and relation to the Thomsen-Friedenreich antigen. Mol Immunol. 1988 Feb;25(2):199–204. doi: 10.1016/0161-5890(88)90068-5. [DOI] [PubMed] [Google Scholar]
- Dahr W., Uhlenbruck G., Gunson H. H., Van Der Hart M. Molecular basis of Tn-polyagglutinability. Vox Sang. 1975;29(1):36–50. doi: 10.1111/j.1423-0410.1975.tb00475.x. [DOI] [PubMed] [Google Scholar]
- Fischkoff S. A., Rossi R. M. Lineage directed HL-60 cell sublines as a model system for the study of early events in lineage determination of myeloid cells. Leuk Res. 1990;14(11-12):979–988. doi: 10.1016/0145-2126(90)90111-l. [DOI] [PubMed] [Google Scholar]
- Fukuda M. Leukosialin, a major O-glycan-containing sialoglycoprotein defining leukocyte differentiation and malignancy. Glycobiology. 1991 Sep;1(4):347–356. doi: 10.1093/glycob/1.4.347. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Peltokorpi L., Andersson L. C. B lymphoblastoid cell lines with normal and defective O-glycosylation established from an individual with blood group Tn. Blood. 1986 Apr;67(4):973–979. [PubMed] [Google Scholar]
- Gasson J. C., Ryden T., Bourgeois S. Role of de novo DNA methylation in the glucocorticoid resistance of a T-lymphoid cell line. Nature. 1983 Apr 14;302(5909):621–623. doi: 10.1038/302621a0. [DOI] [PubMed] [Google Scholar]
- Glauber J. G., Wandersee N. J., Little J. A., Ginder G. D. 5'-flanking sequences mediate butyrate stimulation of embryonic globin gene expression in adult erythroid cells. Mol Cell Biol. 1991 Sep;11(9):4690–4697. doi: 10.1128/mcb.11.9.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub E. I., Li G. R., Volsky D. J. Induction of dormant HIV-1 by sodium butyrate: involvement of the TATA box in the activation of the HIV-1 promoter. AIDS. 1991 Jun;5(6):663–668. [PubMed] [Google Scholar]
- Harris M. Induction of thymidine kinase in enzyme-deficient Chinese hamster cells. Cell. 1982 Jun;29(2):483–492. doi: 10.1016/0092-8674(82)90165-9. [DOI] [PubMed] [Google Scholar]
- Holliday R. The inheritance of epigenetic defects. Science. 1987 Oct 9;238(4824):163–170. doi: 10.1126/science.3310230. [DOI] [PubMed] [Google Scholar]
- Jähner D., Jaenisch R. Retrovirus-induced de novo methylation of flanking host sequences correlates with gene inactivity. Nature. 1985 Jun 13;315(6020):594–597. doi: 10.1038/315594a0. [DOI] [PubMed] [Google Scholar]
- Kruh J. Effects of sodium butyrate, a new pharmacological agent, on cells in culture. Mol Cell Biochem. 1982 Feb 5;42(2):65–82. doi: 10.1007/BF00222695. [DOI] [PubMed] [Google Scholar]
- Lee K. H., Kinashi T., Tohyama K., Tashiro K., Funato N., Hama K., Honjo T. Different stromal cell lines support lineage-selective differentiation of the multipotential bone marrow stem cell clone LyD9. J Exp Med. 1991 May 1;173(5):1257–1266. doi: 10.1084/jem.173.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notari R. E., DeYoung J. L. Kinetics and mechanisms of degradation of the antileukemic agent 5-azacytidine in aqueous solutions. J Pharm Sci. 1975 Jul;64(7):1148–1157. doi: 10.1002/jps.2600640704. [DOI] [PubMed] [Google Scholar]
- Piller F., Piller V., Fox R. I., Fukuda M. Human T-lymphocyte activation is associated with changes in O-glycan biosynthesis. J Biol Chem. 1988 Oct 15;263(29):15146–15150. [PubMed] [Google Scholar]
- Piller V., Piller F., Fukuda M. Biosynthesis of truncated O-glycans in the T cell line Jurkat. Localization of O-glycan initiation. J Biol Chem. 1990 Jun 5;265(16):9264–9271. [PubMed] [Google Scholar]
- Richardson B., Kahn L., Lovett E. J., Hudson J. Effect of an inhibitor of DNA methylation on T cells. I. 5-Azacytidine induces T4 expression on T8+ T cells. J Immunol. 1986 Jul 1;137(1):35–39. [PubMed] [Google Scholar]
- Sawai M., Takase K., Teraoka H., Tsukada K. Reversible G1 arrest in the cell cycle of human lymphoid cell lines by dimethyl sulfoxide. Exp Cell Res. 1990 Mar;187(1):4–10. doi: 10.1016/0014-4827(90)90108-m. [DOI] [PubMed] [Google Scholar]
- Schachter H., Brockhausen I. The biosynthesis of branched O-glycans. Symp Soc Exp Biol. 1989;43:1–26. [PubMed] [Google Scholar]
- Siminovitch L. On the nature of hereditable variation in cultured somatic cells. Cell. 1976 Jan;7(1):1–11. doi: 10.1016/0092-8674(76)90249-x. [DOI] [PubMed] [Google Scholar]
- Springer G. F. T and Tn, general carcinoma autoantigens. Science. 1984 Jun 15;224(4654):1198–1206. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]
- Taylor S. M., Jones P. A. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 1979 Aug;17(4):771–779. doi: 10.1016/0092-8674(79)90317-9. [DOI] [PubMed] [Google Scholar]
- Thurnher M., Clausen H., Fierz W., Lanzavecchia A., Berger E. G. T cell clones with normal or defective O-galactosylation from a patient with permanent mixed-field polyagglutinability. Eur J Immunol. 1992 Jul;22(7):1835–1842. doi: 10.1002/eji.1830220724. [DOI] [PubMed] [Google Scholar]
- Vainchenker W., Vinci G., Testa U., Henri A., Tabilio A., Fache M. P., Rochant H., Cartron J. P. Presence of the Tn antigen on hematopoietic progenitors from patients with the Tn syndrome. J Clin Invest. 1985 Feb;75(2):541–546. doi: 10.1172/JCI111730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Eijnden D. H., Schiphorst W. E., Berger E. G. Specific detection of N-acetylglucosamine-containing oligosaccharide chains on ovine submaxillary asialomucin. Biochim Biophys Acta. 1983 Jan 4;755(1):32–39. doi: 10.1016/0304-4165(83)90269-6. [DOI] [PubMed] [Google Scholar]
- Visvader J., Begley C. G., Adams J. M. Differential expression of the LYL, SCL and E2A helix-loop-helix genes within the hemopoietic system. Oncogene. 1991 Feb;6(2):187–194. [PubMed] [Google Scholar]
- Wallner M., Waldner R. Tn polyagglutinability occurring in a patient with B cell lymphoma. Blut. 1985 Nov;51(5):355–360. doi: 10.1007/BF00320046. [DOI] [PubMed] [Google Scholar]
- Yamamoto F., Clausen H., White T., Marken J., Hakomori S. Molecular genetic basis of the histo-blood group ABO system. Nature. 1990 May 17;345(6272):229–233. doi: 10.1038/345229a0. [DOI] [PubMed] [Google Scholar]