Abstract
The unique granular proteins of eosinophils may have a pathogenetic role in asthma and in the defense against parasitic infestations. However, the mechanisms regulating eosinophil degranulation are largely unknown. We examined the hypothesis that release of these proteins is regulated by endogenous activation of phospholipase A2. Human eosinophils (HE) were isolated from the peripheral blood of 42 subjects either by Percoll density separation or by negative-selection immunomagnetic fractionation. Eosinophil activation was initiated in vitro with 10(-6) M FMLP and 5 micrograms/ml cytochalasin B and was assessed by measurement of eosinophil peroxidase (EPO), leukotriene C4 (LTC4) and superoxide radical (.O2-) secretion. Treatment of HE with 100 microM mepacrine before activation blocked EPO release (2.0 +/- 0.2 vs 10.2 +/- 2.1% cell content for activated HE, P < 0.004, n = 9), .O2- generation (2.6 +/- 0.9 vs 44.2 +/- 10.8 nmol/ml per 10(6) HE, P < 0.002, n = 5), and LTC4 secretion (68.2 +/- 32.2 vs 1,125.2 +/- 526.8 pg/ml per 10(6) HE, P < 0.04, n = 8). Pretreatment of HE with 100 microM 4-bromophenacyl bromide before activation similarly blocked EPO release, .O2- generation and LTC4 secretion. Addition of AA to HE after treatment with 100 microM mepacrine and before subsequent activation reversed the inhibition of both EPO (10.4 +/- 2.2% with 1 microM AA vs 2.0 +/- 0.2% for mepacrine, n = 5, P < 0.02) and LTC4 secretion (695.1 +/- 412.9 with 10 microM AA vs 68.2 +/- 32.2 pg/ml per 10(6) HE for mepacrine, n = 8, P < 0.04), but did not reverse inhibition of .O2- generation by mepacrine. We demonstrate that secretion of preformed cytotoxic proteins and .O2- by eosinophils is regulated endogenously by phospholipase A2.
Full text
PDF

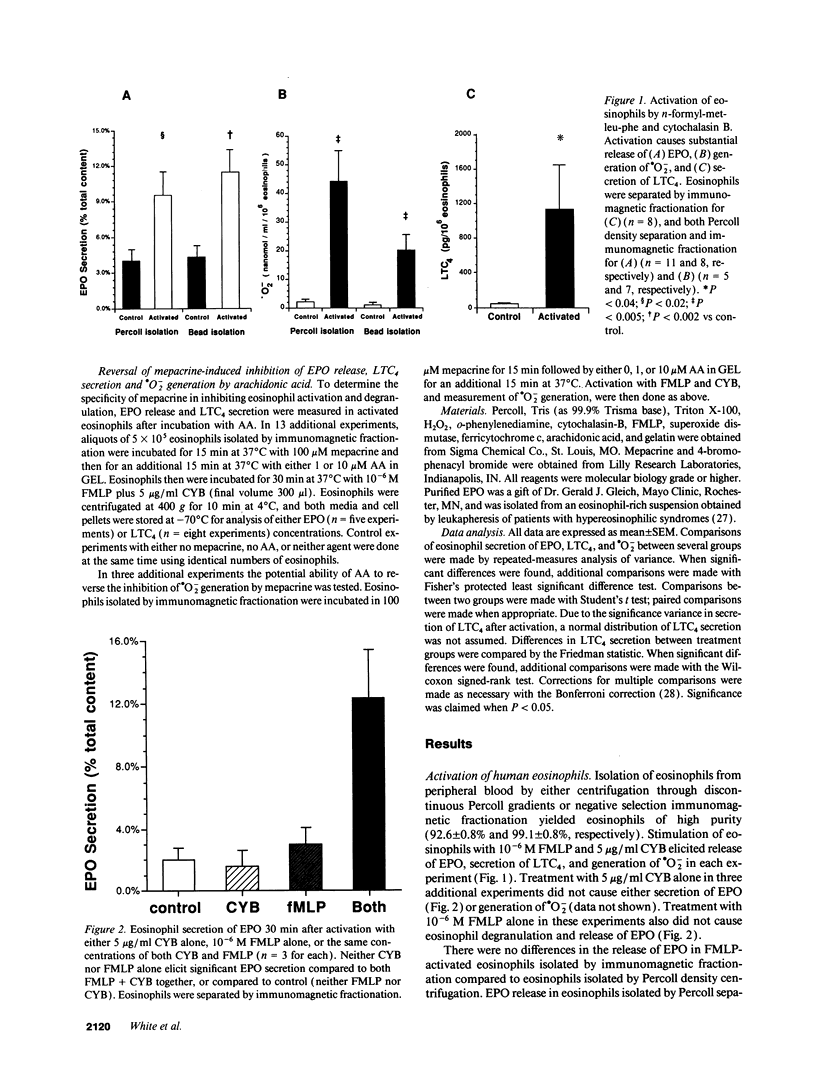
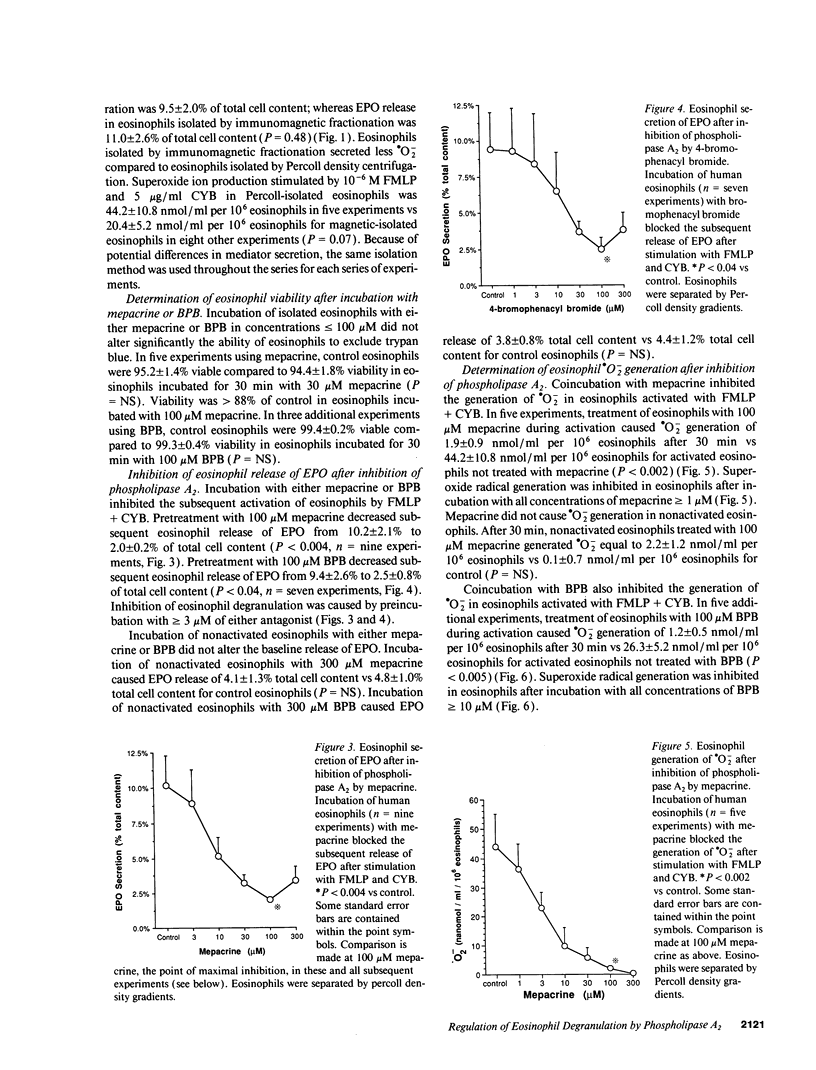
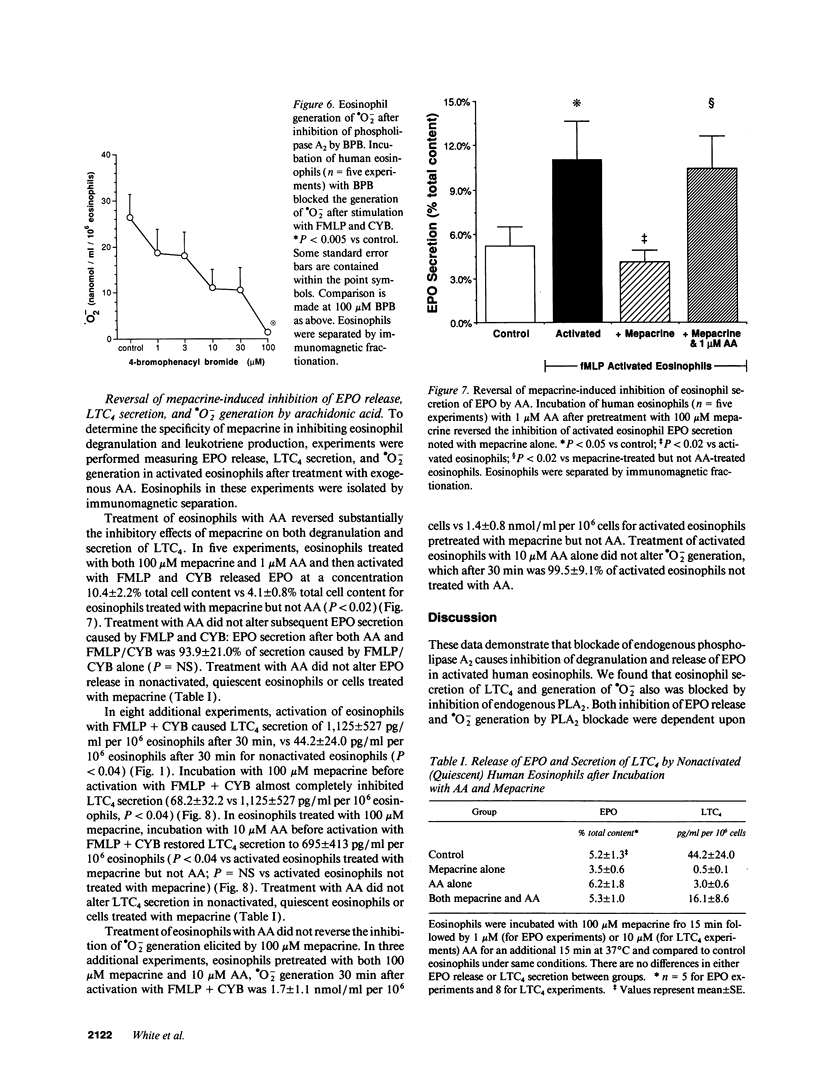
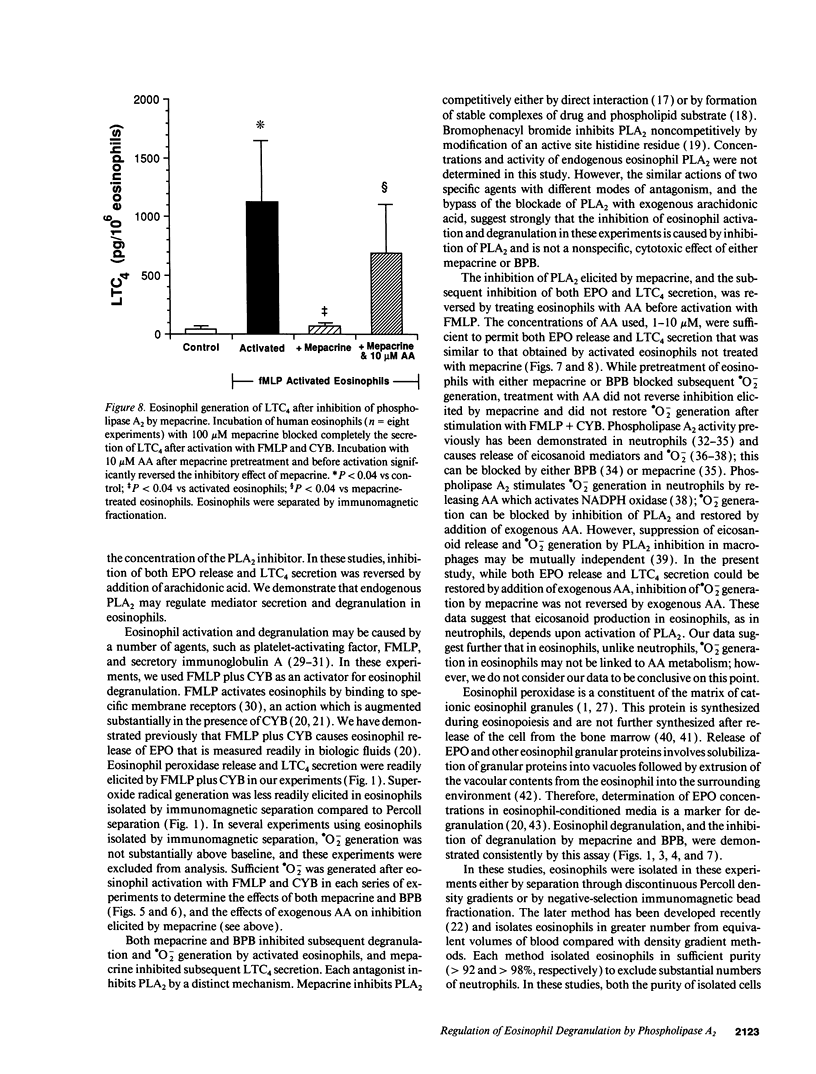


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu-Ghazaleh R. I., Fujisawa T., Mestecky J., Kyle R. A., Gleich G. J. IgA-induced eosinophil degranulation. J Immunol. 1989 Apr 1;142(7):2393–2400. [PubMed] [Google Scholar]
- Adelroth E., Rosenhall L., Johansson S. A., Linden M., Venge P. Inflammatory cells and eosinophilic activity in asthmatics investigated by bronchoalveolar lavage. The effects of antiasthmatic treatment with budesonide or terbutaline. Am Rev Respir Dis. 1990 Jul;142(1):91–99. doi: 10.1164/ajrccm/142.1.91. [DOI] [PubMed] [Google Scholar]
- Agosti J. M., Altman L. C., Ayars G. H., Loegering D. A., Gleich G. J., Klebanoff S. J. The injurious effect of eosinophil peroxidase, hydrogen peroxide, and halides on pneumocytes in vitro. J Allergy Clin Immunol. 1987 Mar;79(3):496–504. doi: 10.1016/0091-6749(87)90368-x. [DOI] [PubMed] [Google Scholar]
- Ayars G. H., Altman L. C., McManus M. M., Agosti J. M., Baker C., Luchtel D. L., Loegering D. A., Gleich G. J. Injurious effect of the eosinophil peroxide-hydrogen peroxide-halide system and major basic protein on human nasal epithelium in vitro. Am Rev Respir Dis. 1989 Jul;140(1):125–131. doi: 10.1164/ajrccm/140.1.125. [DOI] [PubMed] [Google Scholar]
- Balsinde J., Diez E., Schüller A., Mollinedo F. Phospholipase A2 activity in resting and activated human neutrophils. Substrate specificity, pH dependence, and subcellular localization. J Biol Chem. 1988 Feb 5;263(4):1929–1936. [PubMed] [Google Scholar]
- Bauldry S. A., Wykle R. L., Bass D. A. Phospholipase A2 activation in human neutrophils. Differential actions of diacylglycerols and alkylacylglycerols in priming cells for stimulation by N-formyl-Met-Leu-Phe. J Biol Chem. 1988 Nov 15;263(32):16787–16795. [PubMed] [Google Scholar]
- Bomalaski J. S., Baker D. G., Brophy L., Resurreccion N. V., Spilberg I., Muniain M., Clark M. A. A phospholipase A2-activating protein (PLAP) stimulates human neutrophil aggregation and release of lysosomal enzymes, superoxide, and eicosanoids. J Immunol. 1989 Jun 1;142(11):3957–3962. [PubMed] [Google Scholar]
- Chang J., Musser J. H., McGregor H. Phospholipase A2: function and pharmacological regulation. Biochem Pharmacol. 1987 Aug 1;36(15):2429–2436. doi: 10.1016/0006-2952(87)90512-0. [DOI] [PubMed] [Google Scholar]
- Daniels R. H., Finnen M. J., Hill M. E., Lackie J. M. Recombinant human monocyte IL-8 primes NADPH-oxidase and phospholipase A2 activation in human neutrophils. Immunology. 1992 Jan;75(1):157–163. [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M. Enzymes metabolizing phospholipids: from infancy to middle age. Adv Exp Med Biol. 1978;101:1–13. doi: 10.1007/978-1-4615-9071-2_1. [DOI] [PubMed] [Google Scholar]
- Dennis E. A., Rhee S. G., Billah M. M., Hannun Y. A. Role of phospholipase in generating lipid second messengers in signal transduction. FASEB J. 1991 Apr;5(7):2068–2077. doi: 10.1096/fasebj.5.7.1901288. [DOI] [PubMed] [Google Scholar]
- Djukanović R., Wilson J. W., Britten K. M., Wilson S. J., Walls A. F., Roche W. R., Howarth P. H., Holgate S. T. Quantitation of mast cells and eosinophils in the bronchial mucosa of symptomatic atopic asthmatics and healthy control subjects using immunohistochemistry. Am Rev Respir Dis. 1990 Oct;142(4):863–871. doi: 10.1164/ajrccm/142.4.863. [DOI] [PubMed] [Google Scholar]
- Duque R. E., Fantone J. C., Kramer C., Marasco W. A., Phan S. H. Inhibition of neutrophil activation by p-bromophenacyl bromide and its effects on phospholipase A2. Br J Pharmacol. 1986 Jun;88(2):463–472. doi: 10.1111/j.1476-5381.1986.tb10225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franson R., Beckerdite S., Wang P., Waite M., Elsbach P. Some properties of phospholipases of alveolar macrophages. Biochim Biophys Acta. 1973 Feb 14;296(2):365–373. doi: 10.1016/0005-2760(73)90094-5. [DOI] [PubMed] [Google Scholar]
- Frigas E., Loegering D. A., Gleich G. J. Cytotoxic effects of the guinea pig eosinophil major basic protein on tracheal epithelium. Lab Invest. 1980 Jan;42(1):35–43. [PubMed] [Google Scholar]
- Fukuda T., Ackerman S. J., Reed C. E., Peters M. S., Dunnette S. L., Gleich G. J. Calcium ionophore A23187 calcium-dependent cytolytic degranulation in human eosinophils. J Immunol. 1985 Aug;135(2):1349–1356. [PubMed] [Google Scholar]
- Griffin E., Håkansson L., Formgren H., Jörgensen K., Peterson C., Venge P. Blood eosinophil number and activity in relation to lung function in patients with asthma and with eosinophilia. J Allergy Clin Immunol. 1991 Feb;87(2):548–557. doi: 10.1016/0091-6749(91)90014-f. [DOI] [PubMed] [Google Scholar]
- Hamann K. J., Barker R. L., Ten R. M., Gleich G. J. The molecular biology of eosinophil granule proteins. Int Arch Allergy Appl Immunol. 1991;94(1-4):202–209. doi: 10.1159/000235362. [DOI] [PubMed] [Google Scholar]
- Hansel T. T., De Vries I. J., Iff T., Rihs S., Wandzilak M., Betz S., Blaser K., Walker C. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J Immunol Methods. 1991 Dec 15;145(1-2):105–110. doi: 10.1016/0022-1759(91)90315-7. [DOI] [PubMed] [Google Scholar]
- Henderson L. M., Chappell J. B., Jones O. T. Superoxide generation is inhibited by phospholipase A2 inhibitors. Role for phospholipase A2 in the activation of the NADPH oxidase. Biochem J. 1989 Nov 15;264(1):249–255. doi: 10.1042/bj2640249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges M. K., Weller P. F., Gerard N. P., Ackerman S. J., Drazen J. M. Heterogeneity of leukotriene C4 production by eosinophils from asthmatic and from normal subjects. Am Rev Respir Dis. 1988 Oct;138(4):799–804. doi: 10.1164/ajrccm/138.4.799. [DOI] [PubMed] [Google Scholar]
- Horn B. R., Robin E. D., Theodore J., Van Kessel A. Total eosinophil counts in the management of bronchial asthma. N Engl J Med. 1975 May 29;292(22):1152–1155. doi: 10.1056/NEJM197505292922204. [DOI] [PubMed] [Google Scholar]
- Jain M. K., Streb M., Rogers J., DeHaas G. H. Action of phospholipase A2 on bilayers containing lysophosphatidylcholine analogs and the effect of inhibitors. Biochem Pharmacol. 1984 Aug 15;33(16):2541–2551. doi: 10.1016/0006-2952(84)90622-1. [DOI] [PubMed] [Google Scholar]
- Kita H., Abu-Ghazaleh R. I., Gleich G. J., Abraham R. T. Role of pertussis toxin-sensitive G proteins in stimulus-dependent human eosinophil degranulation. J Immunol. 1991 Nov 15;147(10):3466–3473. [PubMed] [Google Scholar]
- Kroegel C., Giembycz M. A., Barnes P. J. Characterization of eosinophil cell activation by peptides. Differential effects of substance P, melittin, and FMET-Leu-Phe. J Immunol. 1990 Oct 15;145(8):2581–2587. [PubMed] [Google Scholar]
- Kroegel C., Yukawa T., Dent G., Chanez P., Chung K. F., Barnes P. J. Platelet-activating factor induces eosinophil peroxidase release from purified human eosinophils. Immunology. 1988 Jul;64(3):559–561. [PMC free article] [PubMed] [Google Scholar]
- Lewis D. M., Lewis J. C., Loegering D. A., Gleich G. J. Localization of the guinea pig eosinophil major basic protein to the core of the granule. J Cell Biol. 1978 Jun;77(3):702–713. doi: 10.1083/jcb.77.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motojima S., Frigas E., Loegering D. A., Gleich G. J. Toxicity of eosinophil cationic proteins for guinea pig tracheal epithelium in vitro. Am Rev Respir Dis. 1989 Mar;139(3):801–805. doi: 10.1164/ajrccm/139.3.801. [DOI] [PubMed] [Google Scholar]
- Owen W. F., Jr, Soberman R. J., Yoshimoto T., Sheffer A. L., Lewis R. A., Austen K. F. Synthesis and release of leukotriene C4 by human eosinophils. J Immunol. 1987 Jan 15;138(2):532–538. [PubMed] [Google Scholar]
- Pick E., Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46(2):211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- Roberts M. F., Deems R. A., Mincey T. C., Dennis E. A. Chemical modification of the histidine residue in phospholipase A2 (Naja naja naja). A case of half-site reactivity. J Biol Chem. 1977 Apr 10;252(7):2405–2411. [PubMed] [Google Scholar]
- Sedgwick J. B., Vrtis R. F., Gourley M. F., Busse W. W. Stimulus-dependent differences in superoxide anion generation by normal human eosinophils and neutrophils. J Allergy Clin Immunol. 1988 May;81(5 Pt 1):876–883. doi: 10.1016/0091-6749(88)90945-1. [DOI] [PubMed] [Google Scholar]
- Shaw R. J., Cromwell O., Kay A. B. Preferential generation of leukotriene C4 by human eosinophils. Clin Exp Immunol. 1984 Jun;56(3):716–722. [PMC free article] [PubMed] [Google Scholar]
- Tai P. C., Spry C. J. The mechanisms which produce vacuolated and degranulated eosinophils. Br J Haematol. 1981 Oct;49(2):219–226. doi: 10.1111/j.1365-2141.1981.tb07218.x. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Urakami M., Takanaka K. Effects of various drugs on superoxide generation, arachidonic acid release and phospholipase A2 in polymorphonuclear leukocytes. Jpn J Pharmacol. 1988 Mar;46(3):275–284. doi: 10.1254/jjp.46.275. [DOI] [PubMed] [Google Scholar]
- Tsunawaki S., Nathan C. F. Release of arachidonate and reduction of oxygen. Independent metabolic bursts of the mouse peritoneal macrophage. J Biol Chem. 1986 Sep 5;261(25):11563–11570. [PubMed] [Google Scholar]
- Vadas P., Pruzanski W. Role of secretory phospholipases A2 in the pathobiology of disease. Lab Invest. 1986 Oct;55(4):391–404. [PubMed] [Google Scholar]
- Verhagen J., Bruynzeel P. L., Koedam J. A., Wassink G. A., de Boer M., Terpstra G. K., Kreukniet J., Veldink G. A., Vliegenthart J. F. Specific leukotriene formation by purified human eosinophils and neutrophils. FEBS Lett. 1984 Mar 12;168(1):23–28. doi: 10.1016/0014-5793(84)80199-4. [DOI] [PubMed] [Google Scholar]
- Vigo C., Lewis G. P., Piper P. J. Mechanisms of inhibition of phospholipase A2. Biochem Pharmacol. 1980 Feb 15;29(4):623–627. doi: 10.1016/0006-2952(80)90386-x. [DOI] [PubMed] [Google Scholar]
- White S. R., Kulp G. V., Spaethe S. M., Van Alstyne E., Leff A. R. A kinetic assay for eosinophil peroxidase activity in eosinophils and eosinophil conditioned media. J Immunol Methods. 1991 Nov 22;144(2):257–263. doi: 10.1016/0022-1759(91)90094-v. [DOI] [PubMed] [Google Scholar]
- White S. R., Ohno S., Munoz N. M., Gleich G. J., Abrahams C., Solway J., Leff A. R. Epithelium-dependent contraction of airway smooth muscle caused by eosinophil MBP. Am J Physiol. 1990 Oct;259(4 Pt 1):L294–L303. doi: 10.1152/ajplung.1990.259.4.L294. [DOI] [PubMed] [Google Scholar]
- van GELDER B., SLATER E. C. The extinction coefficient of cytochrome c. Biochim Biophys Acta. 1962 Apr 23;58:593–595. doi: 10.1016/0006-3002(62)90073-2. [DOI] [PubMed] [Google Scholar]


