Abstract
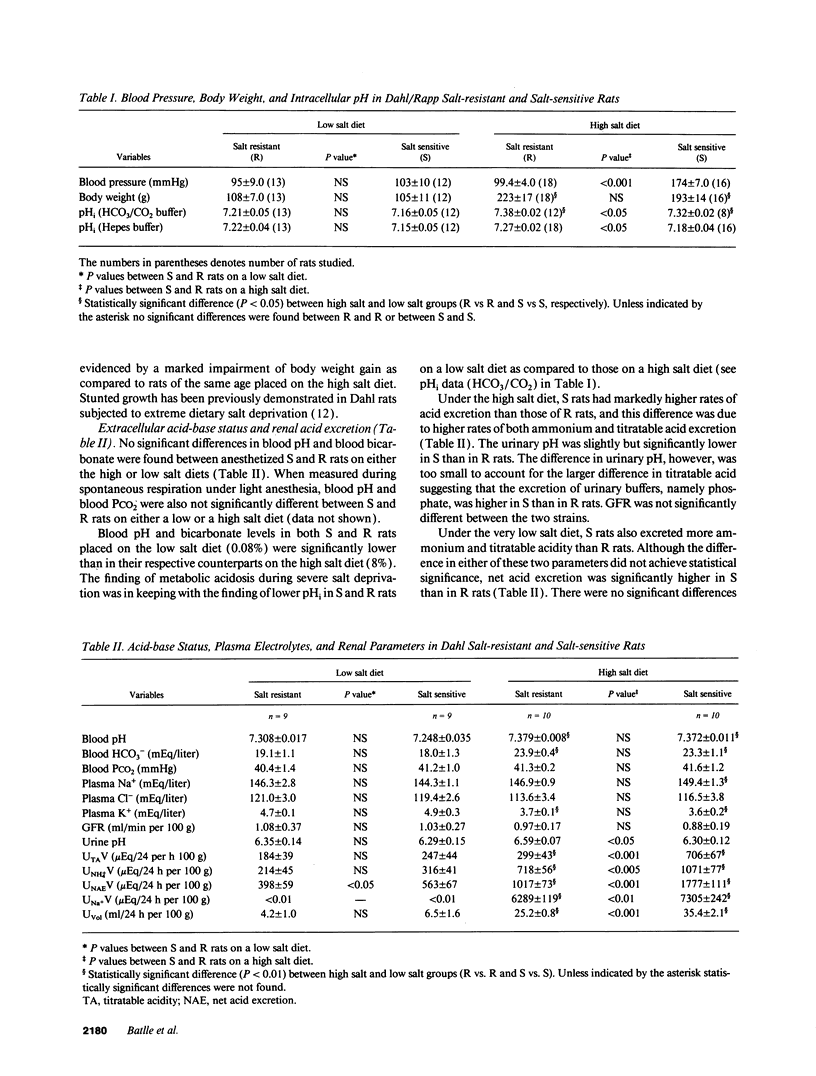
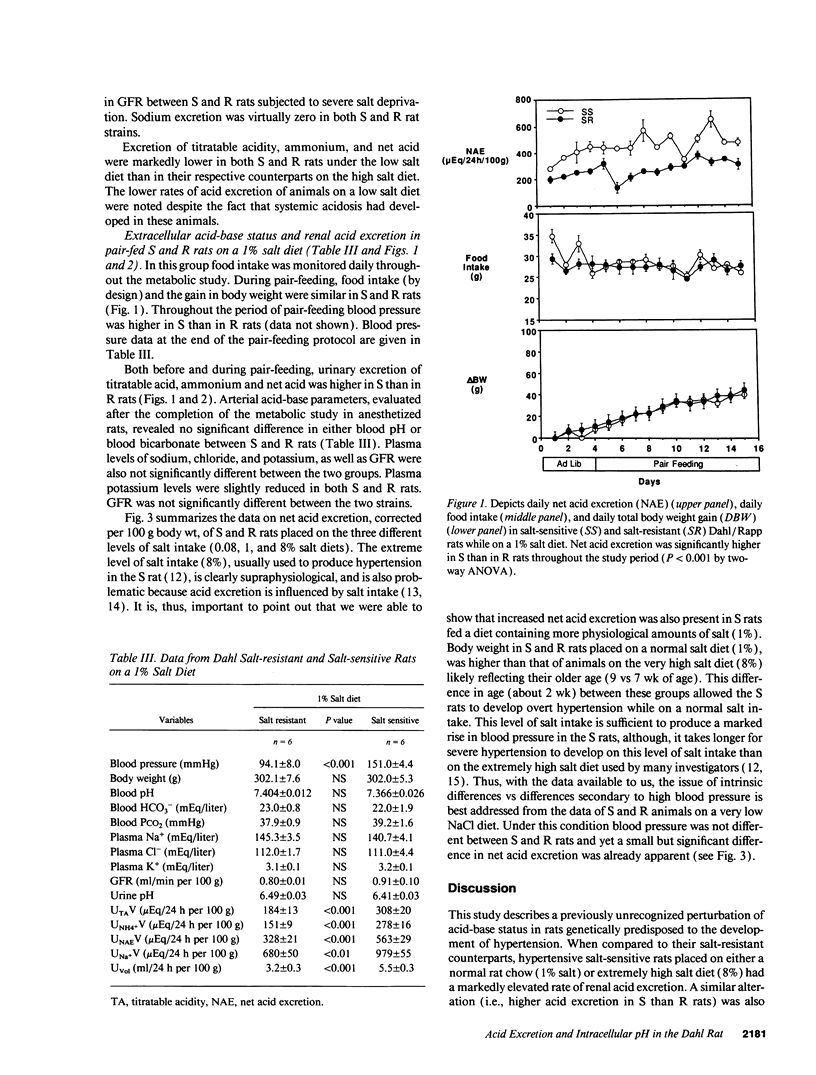
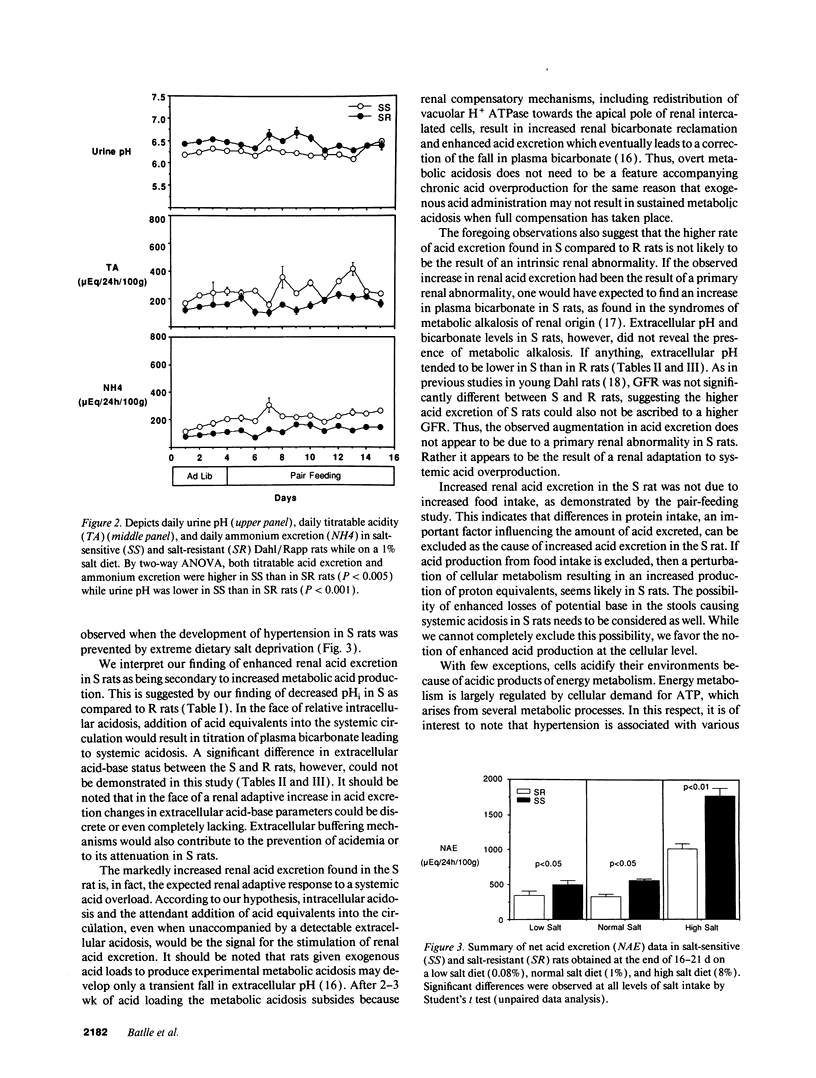
Acid-base status and renal acid excretion were studied in the Dahl/Rapp salt-sensitive (S) rat and its genetically salt-resistant counterpart (R). S rats developed hypertension while on a very high salt diet (8%) and while on a more physiological salt diet (1%) and remained normotensive while on a very low salt diet (0.08%). Under the high salt diet, intracellular pH measured in freshly isolated thymic lymphocytes using 2',7'-bis (carboxyethyl)-5 (6)-carboxyfluorescein acetomethyl ester, a pH-sensitive dye, was lower in S than in R rats both when measured in the presence of HCO3/CO2 (7.32 +/- 0.02 vs. 7.38 +/- 0.02, respectively, P < 0.05) and in its absence (7.18 +/- 0.04 vs. 7.27 +/- 0.02, respectively, P < 0.05). Under the high salt diet, net acid excretion was higher in S than R rats (1,777 +/- 111 vs. 1,017 +/- 73 muEq/24 h per 100 g body wt, respectively, P < 0.001), and this difference was due to higher rates of both titratable acid and ammonium excretion. Directionally similar differences in intracellular pH and net acid excretion between S and R rats were also observed in salt-restricted animals. In S and R rats placed on a normal salt intake (1%) and strictly pair-fed to control food intake as a determinant of dietary acid, net acid excretion was also higher in S than in R rats (562 +/- 27 vs. 329 +/- 21 muEq/24 h per 100 g, respectively, P < 0.01). No significant difference in either blood pH or bicarbonate levels were found between S and R rats on either the 0.08%, 1%, or 8% salt diets. We conclude that renal acid excretion is augmented in the salt-sensitive Dahl/Rapp rat. Enhanced renal acid excretion may be a marker of increased acid production by cells from subjects with salt-sensitive hypertension.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiba T., Rocco V. K., Warnock D. G. Parallel adaptation of the rabbit renal cortical sodium/proton antiporter and sodium/bicarbonate cotransporter in metabolic acidosis and alkalosis. J Clin Invest. 1987 Aug;80(2):308–315. doi: 10.1172/JCI113074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastani B., Purcell H., Hemken P., Trigg D., Gluck S. Expression and distribution of renal vacuolar proton-translocating adenosine triphosphatase in response to chronic acid and alkali loads in the rat. J Clin Invest. 1991 Jul;88(1):126–136. doi: 10.1172/JCI115268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle D. C., Downer M., Gutterman C., Kurtzman N. A. Relationship of urinary and blood carbon dioxide tension during hypercapnia in the rat. Its significance in the evaluation of collecting duct hydrogen ion secretion. J Clin Invest. 1985 May;75(5):1517–1530. doi: 10.1172/JCI111856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle D. C., Janss G., LaPointe M., Llibre J., Saleh A. Cytosolic calcium in T lymphocytes from the spontaneously hypertensive rat. Am J Hypertens. 1990 May;3(5 Pt 1):343–348. doi: 10.1093/ajh/3.5.343. [DOI] [PubMed] [Google Scholar]
- Batlle D. C., Saleh A., Rombola G. Reduced intracellular pH in lymphocytes from the spontaneously hypertensive rat. Hypertension. 1990 Jan;15(1):97–103. doi: 10.1161/01.hyp.15.1.97. [DOI] [PubMed] [Google Scholar]
- Berk B. C., Vallega G., Muslin A. J., Gordon H. M., Canessa M., Alexander R. W. Spontaneously hypertensive rat vascular smooth muscle cells in culture exhibit increased growth and Na+/H+ exchange. J Clin Invest. 1989 Mar;83(3):822–829. doi: 10.1172/JCI113964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. Y., Sanders P. W. L-arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J Clin Invest. 1991 Nov;88(5):1559–1567. doi: 10.1172/JCI115467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig P. U., D'Occhio M. A., Boylan J. W. Lymphocyte membrane sodium-proton exchange in spontaneously hypertensive rats. Hypertension. 1987 Mar;9(3):282–288. doi: 10.1161/01.hyp.9.3.282. [DOI] [PubMed] [Google Scholar]
- Gesek F. A., Schoolwerth A. C. Hormone responses of proximal Na(+)-H+ exchanger in spontaneously hypertensive rats. Am J Physiol. 1991 Sep;261(3 Pt 2):F526–F536. doi: 10.1152/ajprenal.1991.261.3.F526. [DOI] [PubMed] [Google Scholar]
- Graber M., Barry C., Dipaola J., Hasagawa A. Intracellular pH in OK cells. II. Effects of temperature on cell pH. Am J Physiol. 1992 May;262(5 Pt 2):F723–F730. doi: 10.1152/ajprenal.1992.262.5.F723. [DOI] [PubMed] [Google Scholar]
- Greene A. S., Yu Z. Y., Roman R. J., Cowley A. W., Jr Role of blood volume expansion in Dahl rat model of hypertension. Am J Physiol. 1990 Feb;258(2 Pt 2):H508–H514. doi: 10.1152/ajpheart.1990.258.2.H508. [DOI] [PubMed] [Google Scholar]
- Kurtz T. W., Al-Bander H. A., Morris R. C., Jr "Salt-sensitive" essential hypertension in men. Is the sodium ion alone important? N Engl J Med. 1987 Oct 22;317(17):1043–1048. doi: 10.1056/NEJM198710223171702. [DOI] [PubMed] [Google Scholar]
- Landsberg L. Diet, obesity and hypertension: an hypothesis involving insulin, the sympathetic nervous system, and adaptive thermogenesis. Q J Med. 1986 Dec;61(236):1081–1090. [PubMed] [Google Scholar]
- Lucas P. A., Lacour B., McCarron D. A., Drüeke T. Disturbance of acid-base balance in the young spontaneously hypertensive rat. Clin Sci (Lond) 1987 Aug;73(2):211–215. doi: 10.1042/cs0730211. [DOI] [PubMed] [Google Scholar]
- Luft F. C., Steinberg H., Ganten U., Meyer D., Gless K. H., Lang R. E., Fineberg N. S., Rascher W., Unger T., Ganten D. Effect of sodium chloride and sodium bicarbonate on blood pressure in stroke-prone spontaneously hypertensive rats. Clin Sci (Lond) 1988 Jun;74(6):577–585. doi: 10.1042/cs0740577. [DOI] [PubMed] [Google Scholar]
- Moe O. W., Miller R. T., Horie S., Cano A., Preisig P. A., Alpern R. J. Differential regulation of Na/H antiporter by acid in renal epithelial cells and fibroblasts. J Clin Invest. 1991 Nov;88(5):1703–1708. doi: 10.1172/JCI115487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morduchowicz G. A., Sheikh-Hamad D., Jo O. D., Nord E. P., Lee D. B., Yanagawa N. Increased Na+/H+ antiport activity in the renal brush border membrane of SHR. Kidney Int. 1989 Oct;36(4):576–581. doi: 10.1038/ki.1989.233. [DOI] [PubMed] [Google Scholar]
- Nara Y., Kihara M., Nabika T., Mano M., Horie R., Yamori Y. Dietary effect on platelet aggregation in men with and without a family history of essential hypertension. Hypertension. 1984 May-Jun;6(3):339–343. doi: 10.1161/01.hyp.6.3.339. [DOI] [PubMed] [Google Scholar]
- Parenti P., Hanozet G. M., Bianchi G. Sodium and glucose transport across renal brush border membranes of Milan hypertensive rats. Hypertension. 1986 Oct;8(10):932–939. doi: 10.1161/01.hyp.8.10.932. [DOI] [PubMed] [Google Scholar]
- Preisig P. A., Alpern R. J. Chronic metabolic acidosis causes an adaptation in the apical membrane Na/H antiporter and basolateral membrane Na(HCO3)3 symporter in the rat proximal convoluted tubule. J Clin Invest. 1988 Oct;82(4):1445–1453. doi: 10.1172/JCI113750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J. M., Wilmoth F. R. Elevated body temperature and increased blood vessel sensitivity in spontaneously hypertensive rats. Am J Physiol. 1990 Apr;258(4 Pt 2):H946–H953. doi: 10.1152/ajpheart.1990.258.4.H946. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Kerbey A. L., Espinal J. Mechanisms decreasing glucose oxidation in diabetes and starvation: role of lipid fuels and hormones. Diabetes Metab Rev. 1988 Nov;4(7):623–638. doi: 10.1002/dmr.5610040702. [DOI] [PubMed] [Google Scholar]
- Rapp J. P. Dahl salt-susceptible and salt-resistant rats. A review. Hypertension. 1982 Nov-Dec;4(6):753–763. doi: 10.1161/01.hyp.4.6.753. [DOI] [PubMed] [Google Scholar]
- Reaven G. M., Twersky J., Chang H. Abnormalities of carbohydrate and lipid metabolism in Dahl rats. Hypertension. 1991 Nov;18(5):630–635. doi: 10.1161/01.hyp.18.5.630. [DOI] [PubMed] [Google Scholar]
- Saleh A. M., Batlle D. C. Kinetic properties of the Na+/H+ antiporter of lymphocytes from the spontaneously hypertensive rat: role of intracellular pH. J Clin Invest. 1990 Jun;85(6):1734–1739. doi: 10.1172/JCI114629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmouder R. L., Weder A. B. Platelet sodium-proton exchange is increased in essential hypertension. J Hypertens. 1989 Apr;7(4):325–330. [PubMed] [Google Scholar]
- Sharma A. M., Kribben A., Schattenfroh S., Cetto C., Distler A. Salt sensitivity in humans is associated with abnormal acid-base regulation. Hypertension. 1990 Oct;16(4):407–413. doi: 10.1161/01.hyp.16.4.407. [DOI] [PubMed] [Google Scholar]
- St Lezin E., Simonet L., Pravenec M., Kurtz T. W. Hypertensive strains and normotensive 'control' strains. How closely are they related? Hypertension. 1992 May;19(5):419–424. doi: 10.1161/01.hyp.19.5.419. [DOI] [PubMed] [Google Scholar]
- Tokudome G., Tomonari H., Gardner J. P., Aladjem M., Fine B. P., Lasker N., Gutkin M., Byrd L. H., Aviv A. Variations in the apparent pH set point for activation of platelet Na-H antiport. Hypertension. 1990 Aug;16(2):180–189. doi: 10.1161/01.hyp.16.2.180. [DOI] [PubMed] [Google Scholar]
- Tsai C. J., Ives H. E., Alpern R. J., Yee V. J., Warnock D. G., Rector F. C., Jr Increased Vmax for Na+/H+ antiporter activity in proximal tubule brush border vesicles from rabbits with metabolic acidosis. Am J Physiol. 1984 Aug;247(2 Pt 2):F339–F343. doi: 10.1152/ajprenal.1984.247.2.F339. [DOI] [PubMed] [Google Scholar]
- Wright G. L., Knecht E., Badger D., Samueloff S., Toraason M., Dukes-Dobos F. Oxygen consumption in the spontaneously hypertensive rat. Proc Soc Exp Biol Med. 1978 Dec;159(3):449–452. doi: 10.3181/00379727-159-40368. [DOI] [PubMed] [Google Scholar]


