Abstract
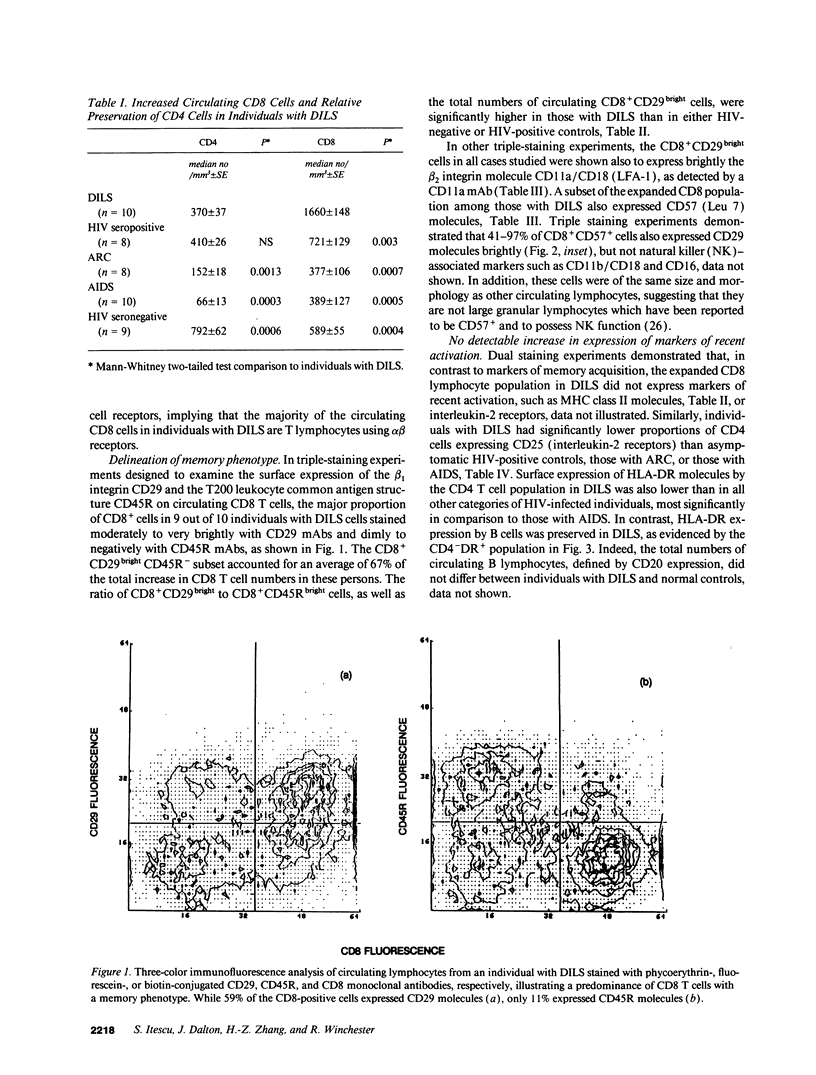
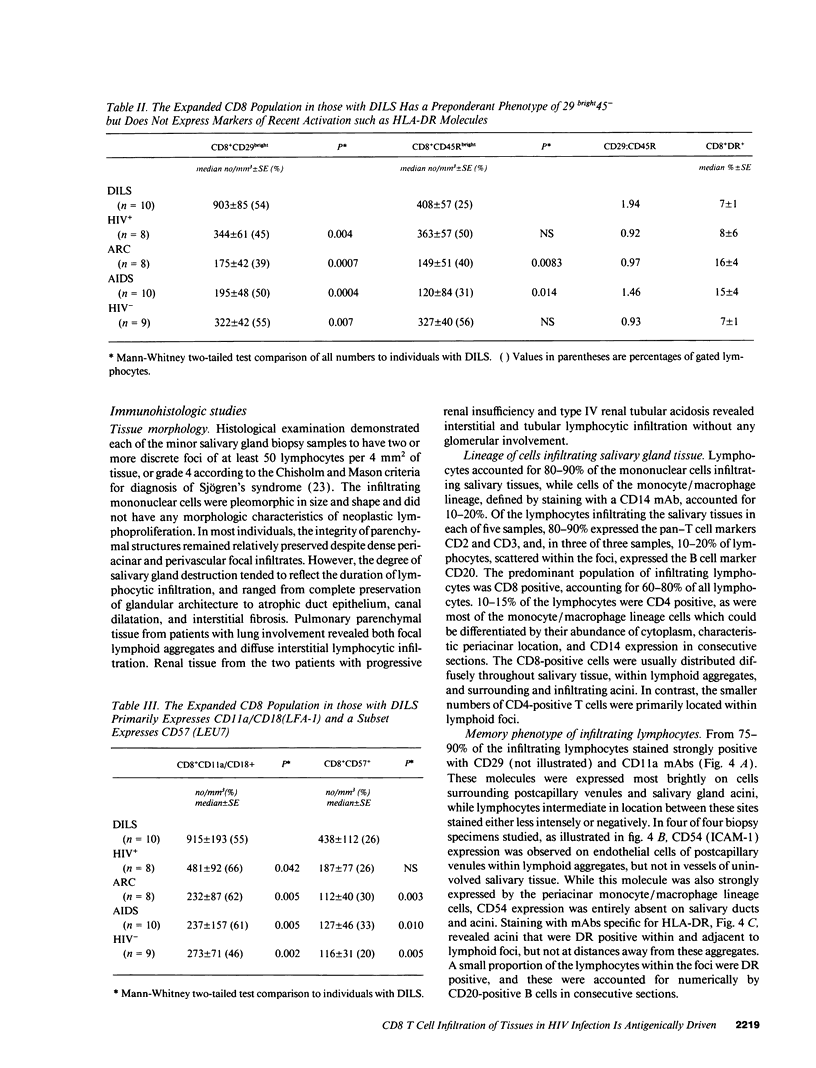
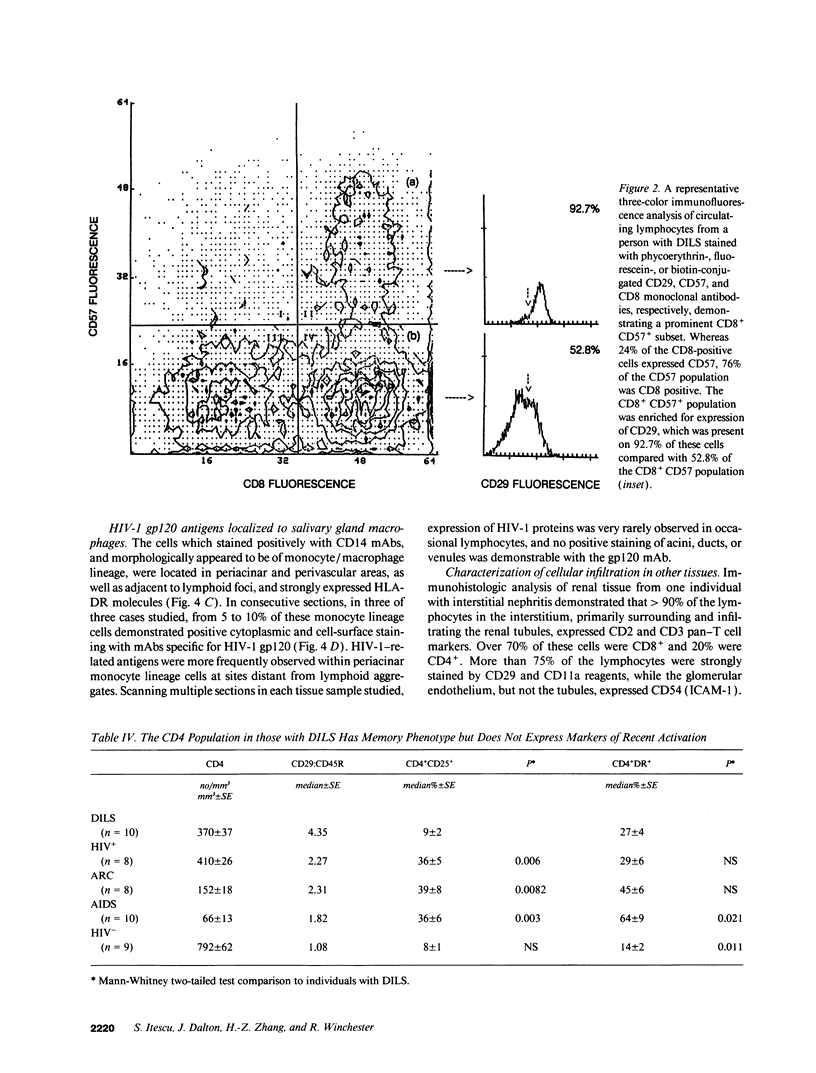
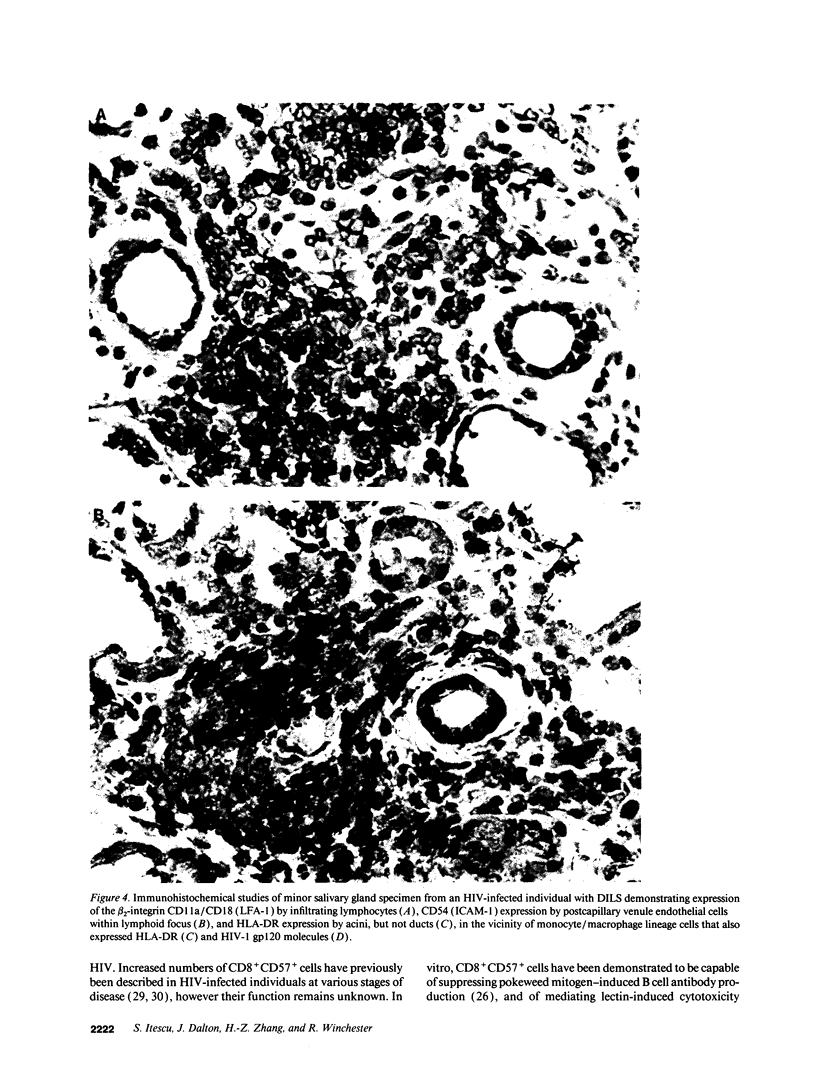
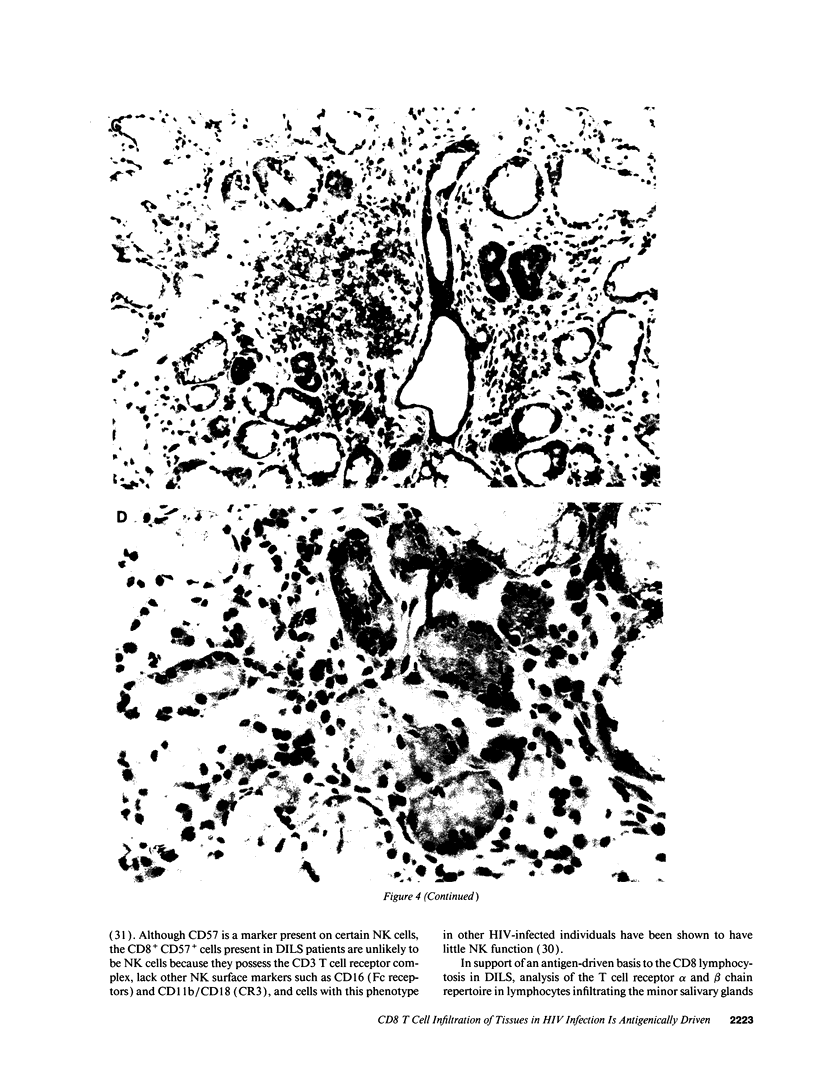
HIV-1 infection may initiate to an HLA-associated response designated diffuse infiltrative lymphocytosis syndrome, characterized by increased numbers of circulating CD8 T cells that infiltrate salivary glands, lungs, gastrointestinal tract, and kidneys. Since this response could either be an antigenically driven process induced by HIV-1 or a lymphoproliferation of cells with neoplastic or unusual features, we sought to define the phenotype of the cellular populations, the nature of tissue derangement, and the tissue localization of virus in diffuse infiltrative lymphocytosis syndrome. Circulating CD8 T cells were greatly increased while CD4 T cell numbers remained in the range found in asymptomatic seropositive persons. The majority of CD8 and CD4 T cells in both blood and tissues had the memory phenotype of CD29+ (beta 1 integrin) and CD11a+/CD18 (beta 2 integrin) expression, but lacked markers of recent activation. A proportion of the circulating CD8 T cells also expressed CD57 (Leu 7) but not other markers of natural killer cells. HIV-encoded proteins were identified in tissue macrophages located in periacinar areas of the salivary glands. CD54 (intercellular adhesion molecule-1), a ligand for the CD11a integrin, was strongly expressed on postcapillary venule endothelium within lymphoid foci, and HLA-DR molecules were found on limited regions of ductular epithelium adjacent to lymphoid aggregates. These findings suggest that (a) the visceral lymphocytic infiltration in diffuse infiltrative lymphocytosis syndrome is an antigen-driven, and MHC-determined, host immune response to an element associated with HIV-1 infection, and (b) that the specific adhesive molecule interactions mediating the cellular influx, as well as the subsequent tissue damage, reflect altered patterns of gene expression in tissues undergoing an immune response.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson T. C., 3rd, Fox R. I., Frisman D. M., Howell F. V. Immunohistologic analysis of lymphoid infiltrates in primary Sjogren's syndrome using monoclonal antibodies. J Immunol. 1983 Jan;130(1):203–208. [PubMed] [Google Scholar]
- Berkower I., Smith G. E., Giri C., Murphy D. Human immunodeficiency virus 1. Predominance of a group-specific neutralizing epitope that persists despite genetic variation. J Exp Med. 1989 Nov 1;170(5):1681–1695. doi: 10.1084/jem.170.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd R. C., Cerottini J. C., MacDonald H. R. Phenotypic identification of memory cytolytic T lymphocytes in a subset of Lyt-2+ cells. J Immunol. 1987 Feb 15;138(4):1009–1013. [PubMed] [Google Scholar]
- Celada F., Cambiaggi C., Maccari J., Burastero S., Gregory T., Patzer E., Porter J., McDanal C., Matthews T. Antibody raised against soluble CD4-rgp120 complex recognizes the CD4 moiety and blocks membrane fusion without inhibiting CD4-gp120 binding. J Exp Med. 1990 Oct 1;172(4):1143–1150. doi: 10.1084/jem.172.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm D. M., Mason D. K. Labial salivary gland biopsy in Sjögren's disease. J Clin Pathol. 1968 Sep;21(5):656–660. doi: 10.1136/jcp.21.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement L. T., Grossi C. E., Gartland G. L. Morphologic and phenotypic features of the subpopulation of Leu-2+ cells that suppresses B cell differentiation. J Immunol. 1984 Nov;133(5):2461–2468. [PubMed] [Google Scholar]
- Ensoli B., Lusso P., Schachter F., Josephs S. F., Rappaport J., Negro F., Gallo R. C., Wong-Staal F. Human herpes virus-6 increases HIV-1 expression in co-infected T cells via nuclear factors binding to the HIV-1 enhancer. EMBO J. 1989 Oct;8(10):3019–3027. doi: 10.1002/j.1460-2075.1989.tb08452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. D., Briggs M., Ward P. A., Tedder R. S. Human herpesvirus 6 in salivary glands. Lancet. 1990 Sep 8;336(8715):590–593. doi: 10.1016/0140-6736(90)93392-3. [DOI] [PubMed] [Google Scholar]
- Haverkos H. W., Gotlieb M. S., Killen J. Y., Edelman R. Classification of HTLV-III/LAV-related diseases. J Infect Dis. 1985 Nov;152(5):1095–1095. doi: 10.1093/infdis/152.5.1095. [DOI] [PubMed] [Google Scholar]
- Itescu S., Brancato L. J., Buxbaum J., Gregersen P. K., Rizk C. C., Croxson T. S., Solomon G. E., Winchester R. A diffuse infiltrative CD8 lymphocytosis syndrome in human immunodeficiency virus (HIV) infection: a host immune response associated with HLA-DR5. Ann Intern Med. 1990 Jan 1;112(1):3–10. doi: 10.7326/0003-4819-112-1-3. [DOI] [PubMed] [Google Scholar]
- Itescu S., Brancato L. J., Winchester R. A sicca syndrome in HIV infection: association with HLA-DR5 and CD8 lymphocytosis. Lancet. 1989 Aug 26;2(8661):466–468. doi: 10.1016/s0140-6736(89)92085-0. [DOI] [PubMed] [Google Scholar]
- Itescu S., Mathur-Wagh U., Skovron M. L., Brancato L. J., Marmor M., Zeleniuch-Jacquotte A., Winchester R. HLA-B35 is associated with accelerated progression to AIDS. J Acquir Immune Defic Syndr. 1992;5(1):37–45. [PubMed] [Google Scholar]
- Jalkanen S., Reichert R. A., Gallatin W. M., Bargatze R. F., Weissman I. L., Butcher E. C. Homing receptors and the control of lymphocyte migration. Immunol Rev. 1986 Jun;91:39–60. doi: 10.1111/j.1600-065x.1986.tb01483.x. [DOI] [PubMed] [Google Scholar]
- Lane H. C., Fauci A. S. Immunologic abnormalities in the acquired immunodeficiency syndrome. Annu Rev Immunol. 1985;3:477–500. doi: 10.1146/annurev.iy.03.040185.002401. [DOI] [PubMed] [Google Scholar]
- Lewis D. E., Puck J. M., Babcock G. F., Rich R. R. Disproportionate expansion of a minor T cell subset in patients with lymphadenopathy syndrome and acquired immunodeficiency syndrome. J Infect Dis. 1985 Mar;151(3):555–559. doi: 10.1093/infdis/151.3.555. [DOI] [PubMed] [Google Scholar]
- Martz E. LFA-1 and other accessory molecules functioning in adhesions of T and B lymphocytes. Hum Immunol. 1987 Jan;18(1):3–37. doi: 10.1016/0198-8859(87)90110-8. [DOI] [PubMed] [Google Scholar]
- Meltzer M. S., Skillman D. R., Gomatos P. J., Kalter D. C., Gendelman H. E. Role of mononuclear phagocytes in the pathogenesis of human immunodeficiency virus infection. Annu Rev Immunol. 1990;8:169–194. doi: 10.1146/annurev.iy.08.040190.001125. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Schlossman S. F., Reinherz E. L. Clonal analysis of human cytotoxic T lymphocytes: T4+ and T8+ effector T cells recognize products of different major histocompatibility complex regions. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4395–4399. doi: 10.1073/pnas.79.14.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Boyd A. W., Hagan M., Brown H. M., Kornacki M. M., Schlossman S. F. The isolation and characterization of the human helper inducer T cell subset. J Immunol. 1985 Jun;134(6):3762–3769. [PubMed] [Google Scholar]
- Phillips J. H., Lanier L. L. Lectin-dependent and anti-CD3 induced cytotoxicity are preferentially mediated by peripheral blood cytotoxic T lymphocytes expressing Leu-7 antigen. J Immunol. 1986 Mar 1;136(5):1579–1585. [PubMed] [Google Scholar]
- Plaeger-Marshall S., Spina C. A., Giorgi J. V., Mitsuyasu R., Wolfe P., Gottlieb M., Beall G. Alterations in cytotoxic and phenotypic subsets of natural killer cells in acquired immune deficiency syndrome (AIDS). J Clin Immunol. 1987 Jan;7(1):16–23. doi: 10.1007/BF00915420. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Sharrow S. O., Stephany D., Springer T. A., Young H. A., Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988 Mar 1;140(5):1401–1407. [PubMed] [Google Scholar]
- Siekevitz M., Josephs S. F., Dukovich M., Peffer N., Wong-Staal F., Greene W. C. Activation of the HIV-1 LTR by T cell mitogens and the trans-activator protein of HTLV-I. Science. 1987 Dec 11;238(4833):1575–1578. doi: 10.1126/science.2825351. [DOI] [PubMed] [Google Scholar]
- Siliciano R. F., Lawton T., Knall C., Karr R. W., Berman P., Gregory T., Reinherz E. L. Analysis of host-virus interactions in AIDS with anti-gp120 T cell clones: effect of HIV sequence variation and a mechanism for CD4+ cell depletion. Cell. 1988 Aug 12;54(4):561–575. doi: 10.1016/0092-8674(88)90078-5. [DOI] [PubMed] [Google Scholar]
- Smith S. H., Brown M. H., Rowe D., Callard R. E., Beverley P. C. Functional subsets of human helper-inducer cells defined by a new monoclonal antibody, UCHL1. Immunology. 1986 May;58(1):63–70. [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Dustin M. L., Kishimoto T. K., Marlin S. D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- Thesleff I., Viinikka L., Saxén L., Lehtonen E., Perheentupa J. The parotid gland is the main source of human salivary epidermal growth factor. Life Sci. 1988;43(1):13–18. doi: 10.1016/0024-3205(88)90231-7. [DOI] [PubMed] [Google Scholar]
- Tsubota H., Lord C. I., Watkins D. I., Morimoto C., Letvin N. L. A cytotoxic T lymphocyte inhibits acquired immunodeficiency syndrome virus replication in peripheral blood lymphocytes. J Exp Med. 1989 Apr 1;169(4):1421–1434. doi: 10.1084/jem.169.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollger L. W., Tuck D. T., Springer T. A., Haynes B. F., Singer K. H. Thymocyte binding to human thymic epithelial cells is inhibited by monoclonal antibodies to CD-2 and LFA-3 antigens. J Immunol. 1987 Jan 15;138(2):358–363. [PubMed] [Google Scholar]
- Walker B. D., Chakrabarti S., Moss B., Paradis T. J., Flynn T., Durno A. G., Blumberg R. S., Kaplan J. C., Hirsch M. S., Schooley R. T. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987 Jul 23;328(6128):345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- Walker C. M., Moody D. J., Stites D. P., Levy J. A. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986 Dec 19;234(4783):1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- Zolla-Pazner S., Des Jarlais D. C., Friedman S. R., Spira T. J., Marmor M., Holzman R., Mildvan D., Yancovitz S., Mathur-Wagh U., Garber J. Nonrandom development of immunologic abnormalities after infection with human immunodeficiency virus: implications for immunologic classification of the disease. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5404–5408. doi: 10.1073/pnas.84.15.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]






