Abstract
BACKGROUND
Previous studies relying on clinical care data have suggested that atrial fibrillation is less common in African Americans than Caucasians, but the mechanism remains unknown. Clinical care may itself vary by race, potentially affecting the accuracy of atrial fibrillation ascertainment in studies relying on clinical data. We sought to examine racial differences in atrial fibrillation prevalence determined by protocol-driven electrocardiograms (ECGs) obtained in prospective cohort studies and to study racial differences in echocardiographic characteristics.
METHODS
We pooled primary data from 3 cohort studies with atrial fibrillation adjudicated from study protocol ECGs and documentation of potentially important confounders: the Heart and Soul Study (n = 1014), the Heart and Estrogen-Progestin Replacement Study (n = 2673), and The Osteoporotic Fractures in Men Sleep Study (n = 2911). Left atrial anatomic dimensions were compared among races from sinus rhythm echocardiograms in the Heart and Soul Study.
RESULTS
Of the 6611 participants, 268 (4%) had atrial fibrillation: Caucasians had the highest prevalence (5%), and African Americans had the lowest (1%; P <.001 for each compared with all other races). After adjustment for potential confounders, Caucasians had a 3.8-fold greater odds of having atrial fibrillation than African Americans (95% confidence interval, 1.6–8.8, P = .002). Although ventricular and atrial volumes and function were similar in Caucasians and African Americans, Caucasians had a 2 mm larger anterior-posterior left atrial diameter after adjusting for potential confounders (95% confidence interval, 1–3 mm, P <.001).
CONCLUSION
ECG confirmed atrial fibrillation is more common in Caucasians than in African Americans, which might be related to the larger left atrial diameter observed in Caucasians.
Keywords: African American, Atrial fibrillation, Black, Caucasian, Left atrium, Race, White
Although atrial fibrillation is the most common arrhythmia encountered in clinical practice and is responsible for significant morbidity and mortality, the cause of the disease remains largely unknown.1 Cross-sectional studies using potentially haphazard outcome data derived from clinical practice (using chart review and International Classification of Diseases, Ninth Revision, codes) have shown that African American patients might have less atrial fibrillation than Caucasians,2,3 suggesting that genetic differences across races may influence the propensity to atrial fibrillation. However, no studies using electrocardiographically confirmed atrial fibrillation derived from data collected in a uniform fashion—such that all races would be evaluated in the same manner—have focused specifically on the question of race and atrial fibrillation, and the mechanism by which differences across races may occur has not been examined.
Race might influence the way that clinical care is delivered, 4–7 potentially biasing results of studies that rely on clinical records (eg, International Classification of Diseases, Ninth Revision, codes). Therefore, we sought to determine the prevalence of atrial fibrillation in cohort studies wherein data were collected on the basis of predetermined study protocols and the arrhythmia was confirmed by electrocardiogram (ECG). As a second phase of the study, differences in left atrial characteristics among races were derived from echocardiograms in subjects enrolled in one of these cohorts.
MATERIALS AND METHODS
Subjects
Three cohorts were included in this analysis, representing different populations (as below) with documentation of potentially important confounders and ECG-confirmed atrial fibrillation. Data from these 3 cohorts were combined to obtain summary estimates both before and after adjustment for potential confounders.
The Heart and Soul Study
The Heart and Soul Study is a prospective cohort study of health outcomes in patients with coronary artery disease.8 Between September 2000 and December 2002, 1024 outpatients with stable coronary artery disease were recruited using administrative databases from 2 Department of Veterans Affairs Medical Centers (San Francisco, Calif, and Palo Alto, Calif), 1 university medical center (University of California, San Francisco), and 9 public health clinics in the Community Health Network of San Francisco. Patients were eligible if they had a history of myocardial infarction, angiographic evidence of ≥ 50% stenosis in 1 or more coronary vessels, evidence of exercise-induced ischemia by treadmill or nuclear testing, or prior coronary revascularization. Patients were not eligible if they had an acute coronary syndrome within the past 6 months, could not walk 1 block, or were planning to move out of the local area within 3 years.
The Heart and Estrogen-Progestin Replacement Study
The Heart and Estrogen-Progestin Replacement (HERS) study design has been described previously. 9 Briefly, 2763 postmenopausal women less than 80 years of age with known coronary heart disease were randomized to receive either 0.625 mg conjugated equine estrogens plus 2.5 mg medroxyprogesterone acetate in 1 daily pill (n = 1380) or identical placebo (n = 1383). Coronary heart disease was documented in the form of myocardial infarction in 39% of study participants, ≥; 50% narrowing of ≥ 1 coronary artery in 82%, catheter-based coronary revascularization in 39%, and surgical coronary revascularization in 37%. Participants were seen annually for an average of 4.1 years.
The Osteoporotic Fractures in Men Sleep Study
The Osteoporotic Fractures in Men (MrOS) study is a prospective study of community dwelling men age 65 years or older at the baseline examination in 2000–2002.10 The MrOS Sleep Study, an ancillary study of the parent MrOS cohort, was conducted between December 2003 and March 2005 and recruited 3135 of these participants (exceeding the goal of 3000 participants) for a comprehensive sleep assessment. Of these, 2911 had usable polysomnography data from which an atrial fibrillation or atrial flutter diagnosis could be made (polysomnography was not performed in 179, polysomnography failed in 38 because of inadequate signals, and 7 had no clinical data available).
Atrial Fibrillation Diagnosis
For the Heart and Soul Study, the cardiac rhythm was determined from a baseline 12-lead ECG at standard amplitudes and 25 mm/s paper speed obtained at the time of enrollment. The diagnosis of atrial fibrillation was adjudicated by 2 physicians, and discrepancies were resolved by a third physician reviewer. Only patients whose rhythm was atrial fibrillation, sinus rhythm, or an atrial paced rhythm were included in the analysis. Patients with atrial flutter (n = 3), a multifocal atrial rhythm (n = 1), ventricular arrhythmia (n = 0), or a regular supraventricular tachycardia (n = 1) on their baseline ECG were excluded.
For the HERS trial, presence of atrial fibrillation was assessed by standard 12-lead ECG obtained at study enrollment and subsequently at yearly follow-up visits (with any diagnosis of atrial fibrillation counted toward prevalent atrial fibrillation during the period of study). Subjects with atrial flutter (n = 11), wandering atrial pacemaker, supraventricular tachycardia, a paced rhythm, uncertain rhythm classification, or an uncertain detection of P waves (n = 79 for all these other arrhythmias) were excluded. ECG interpretation was performed using NOVACODE software, which produces the classic Minnesota Code Classification.11
For the MrOS Sleep Study, subjects underwent single-lead ECG monitoring using lead II of a standard 12 lead ECG during a home sleep study (range 4–8 hours in duration). A certified ECG technician reviewed all of the strips and designated whether any atrial fibrillation or atrial flutter was present, but did not distinguish between the 2. All atrial arrhythmias and any questionable events were reviewed by a board-certified Critical Care physician to confirm accuracy of the diagnosis. These 2 reviewers have been shown to have excellent interobserver reliability.12
Race and Other Covariates
Race, age, and medical history were determined in each cohort by questionnaire or patient interview. Race was divided into self-identified white, black, Asian, Latino, or other. Body mass index (BMI) (kilograms/meters squared) was calculated by height and weight measured by study personnel. A history of heart failure was recorded, but there were insufficient data from all cohorts to distinguish systolic from diastolic heart failure. All participants were instructed to bring their medication bottles to the study appointment, and medications were recorded. The institutional review board at each of the sites approved this protocol. All participants provided written informed consent.
Echocardiographic Measurements
All participants in the Heart and Soul study underwent a complete resting echocardiogram using a commercially available ultrasound system with harmonic imaging (Acuson Sequoia; Siemens Corp, Mountain View, Calif). Patients with atrial fibrillation were excluded from this analysis. By protocol, left atrial size was maximized in both 2- and 4-chamber views. A single cardiologist (N.B.S.) blinded to clinical and laboratory information evaluated each echocardiogram at rest. This reader’s reproducibility was previously established.13 Left atrial volume was measured from standard apical 2- and 4-chamber views at end systole. Left atrial borders were traced using planimetry. The biplane method of disks was used to calculate left atrial volume.14 Left atrial ejection fraction was calculated as (left atrial systolic volume-left atrial diastolic volume)/left atrial systolic volume. Left ventricular ejection fraction was calculated using the biplane method of disks from apical 4- and 2-chamber views. Diastolic dysfunction was categorized according to the classification by Khouri et al.15
Statistical Analysis
Normally distributed continuous variables are displayed as means ± standard deviation. Not normally distributed continuous variables are displayed as medians and interquartile ranges. Univariate analyses between continuous variables were performed using t tests and Wilcoxon rank sum tests as appropriate. Categoric variables were compared using the chi-square test. Selection of covariates for inclusion in multivariate models was based on either established or conventionally considered confounders (eg, sex) or covariates that were associated with both the predictor and outcome of interest in univariate analysis with a P value ≤.1. Multivariate analysis of dichotomous outcomes (eg, atrial fibrillation) was performed using logistic regression, and multivariate analysis of continuous outcomes (eg, left atrial diameter) was performed using linear regression. Two-tailed P values <.05 were considered statistically significant. Statistical analyses were performed using Stata version 9.2 (College Station, Tex).
RESULTS
Pooled Analysis
A total of 6611 participants were included, 268 of whom had ECG-proved atrial fibrillation: 42 of 1014 (4%) from the Heart and Soul Study, 88 of 2673 (3%) from HERS, and 138 of 2911 (5%) from MrOS. The baseline characteristics of those with and without atrial fibrillation are shown in Table 1, demonstrating that prevalence of atrial fibrillation differed significantly by race, age, sex, presence of heart failure, treatment with angiotensin-converting enzyme inhibitor or angiotensin receptor blockers, and use of statins. The atrial fibrillation prevalence by race for each study is shown in Table 2. Within HERS, the proportions of blacks and whites with follow-up ECGs were nearly identical at each follow-up visit (P = .91 for heterogeneity). Baseline atrial fibrillation risk was similar across the 3 studies (P = 0.25).
Table 1.
Clinical Characteristics of Patients with and without Atrial Fibrillation, All 3 Studies Combined
| Atrial Fibrillation (n = 268) |
No Atrial Fibrillation (n = 6343) |
P Value | |
|---|---|---|---|
| Race | |||
| Caucasian | 251 (94%) | 5367 (85%) | |
| African American | 6 (2%) | 470 (7%) | |
| Asian | 6 (3%) | 215 (3%) | |
| Latino | 4 (2%) | 194 (3%) | |
| Other | 1 (0.4%) | 83 (1%) | .002 |
| Age | 74 ± 8 | 71 ± 9 | <.001 |
| Male | 180 (67%) | 3745 (59%) | .009 |
| BMI (kg/m2) | 29 ± 6 | 28 ± 5 | .08 |
| Hypertension | 156 (59%) | 3583 (57%) | .50 |
| Diabetes | 55 (21%) | 1204 (19%) | .51 |
| Previous myocardial infarction |
88 (33%) | 2322 (37%) | .23 |
| Heart failure | 71 (27%) | 665 (11%) | <.001 |
| Statin drug use | 95 (36%) | 2753 (44%) | .009 |
| ACE inhibitor or ARB usea |
117 (45%) | 1876 (30%) | <.001 |
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; BMI = body mass index.
Only data on ACE inhibitors (and not ARBs) were available in the Heart and Estrogen-Progestin Replacement Study.
Table 2.
Proportion with Atrial Fibrillation by Race Displayed as Percent (N with Atrial Fibrillation/Total N in Race Category) for Each Study
| Caucasians | African Americans |
Other Races | P Value | |
|---|---|---|---|---|
| Heart and | 6% (36/610) | 0% (0/165) | 3% (6/238) | .001 |
| Soul | ||||
| HERS | 4% (82/2285) | 2% (4/212) | 2% (2/94) | .38 |
| MrOS Sleep | 5% (133/2641) | 2% (2/99) | 2% (3/171) | .064 |
| Total | 5% (251/5618) | 1% (6/476) | 2% (11/503) | <.001 |
HERS = Heart and Estrogen-Progestin Replacement; MrOS = Osteoporotic Fractures in Men.
Caucasians had significantly more atrial fibrillation when compared with all the other races combined (P <.001), and African Americans had significantly less atrial fibrillation when compared with all other races combined (P = .001). However, Asians did not exhibit a significantly different prevalence of atrial fibrillation (3% of Asians vs 4% of all other groups combined, P = .30), nor did Latinos (2% vs 3% of all other groups combined, P = .14). The largest difference was seen in comparing Caucasians with African Americans: 251 (5%) of all Caucasians had atrial fibrillation compared with 6 (1%) of all African Americans, P = .001.
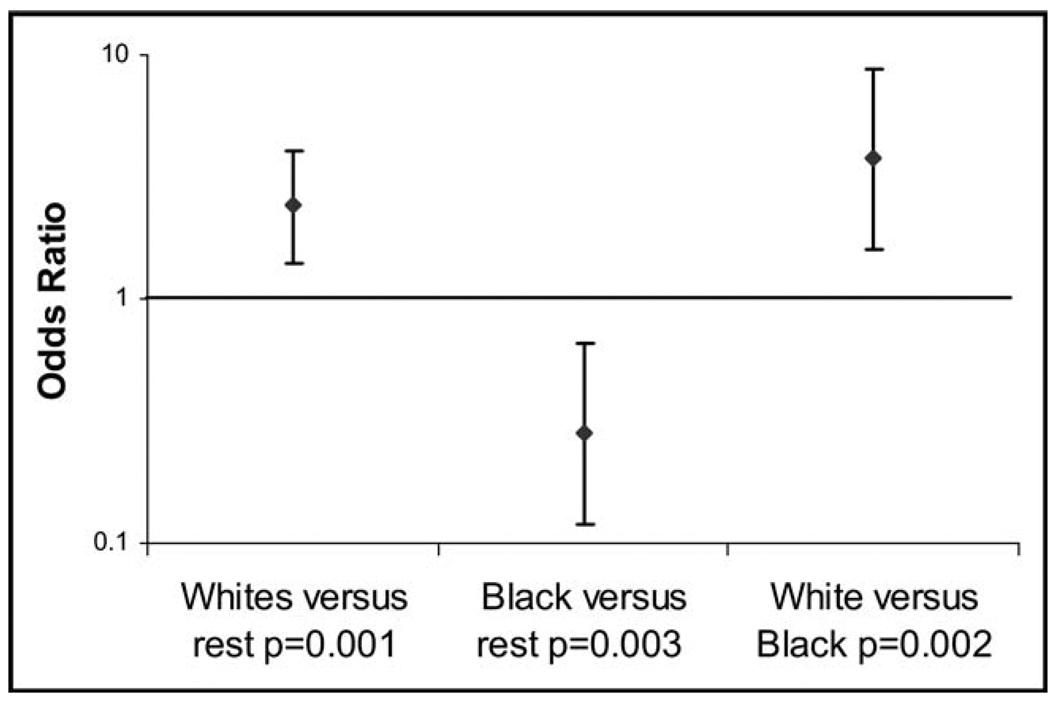
In logistic regression analysis adjusting for potential confounders, including age, sex, BMI, heart failure, statin use, and angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use, Caucasians had a statistically significant 240% greater odds of having atrial fibrillation compared with all other races combined, and African Americans had a statistically significant 72% lesser odds of having atrial fibrillation compared with all other races combined (Figure 1). Caucasians had a statistically significant 3.8-fold greater odds of having atrial fibrillation than African Americans. Finally, there were no meaningful changes in the summary estimates after adjusting for the cohort study in which each individual was enrolled.
Figure 1.
Log odds of atrial fibrillation by race after adjusting for age, gender, BMI, heart failure, statin use, and angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use. Y error bars denote 95% confidence intervals.
Echocardiographic Study
Among patients in sinus rhythm in the Heart and Soul study, although Caucasians had a larger left atrial systolic volume (mean 63 ± 21 mL) compared with all other races combined (mean 60 ± 20 mL, P = .0048), African Americans did as well (65 ± 22 mL in African Americans vs 61 ± 21 mL in all other races, P = .045). Differences in clinical characteristics are shown in Table 3. When comparing Caucasians with African Americans, the only difference in atrial size or function was that Caucasians had a 7 mm larger average left atrial diameter (Table 4). The only difference in ventricular characteristics between Caucasians and African Americans (including measures of diastolic dysfunction) was that African Americans had greater ventricular thickness (Table 4).
Table 3.
Clinical Characteristics of Caucasians Compared with African Americans in the Heart and Soul Study
| Caucasian (n = 550) |
African American (n = 161) |
P Value | |
|---|---|---|---|
| Age | 67 ± 11 | 63 ± 10 | <.001 |
| BMI (kg/m2) | 28 ± 5 | 29 ± 6 | .46 |
| Hypertension | 362 (66%) | 128 (81%) | <.001 |
| Diabetes | 125 (20%) | 53 (32%) | .002 |
| Previous myocardial infarction |
310 (57%) | 76 (48%) | .07 |
| NYHA class III or IV | 107 (20%) | 52 (32%) | .001 |
BMI = body mass index; NYHA = New York Heart Association.
Table 4.
Echocardiographic Characteristics of Caucasians and African Americans in the Heart and Soul Study
| Caucasian (n = 550) |
African American (n = 161) |
P Value | |
|---|---|---|---|
| Left atrial | |||
| Systolic volume (mL) | 64 ± 21 | 65 ± 22 | .47 |
| Diastolic volume (mL) |
29 ± 14 | 30 ± 16 | .28 |
| Diameter (cm) | 4.33 ± 0.6 | 4.26 ± 0.6 | .0026 |
| Ejection fraction (%) | 55 ± 11 | 55 ± 12 | .48 |
| Mitral valve | |||
| Stenosis | 1 (0.2%) | 0 | .59 |
| Prolapse | 5 (1%) | 2 (1%) | .70 |
| Moderate to severe regurgitation |
104 (18%) | 31 (19%) | .92 |
| A-wave velocity (m/s) | 0.78 ± 0.25 | 0.78 ± 0.28 | .87 |
| Right atrial | |||
| Systolic volume (mL) | 45 ± 18 | 45 ± 19 | .86 |
| Diastolic volume (mL) |
23 ± 14 | 24 ± 12 | .42 |
| Left ventricle: | |||
| Systolic volume (mL) | 36 (27–48)a | 33 (25–47)a | .24 |
| Diastolic volume (mL) |
100 (81–121)a | 92 (76–150)a | .20 |
| Ejection fraction (%) | 61 ± 10 | 61 ± 10 | .96 |
| Mass index g/m2 | 99 ± 38 | 104 ± 30 | .10 |
| Septal thickness (cm) |
1.17 ± 0.22 | 1.30 ± 0.31 | <.001 |
| Diastolic function | |||
| Normal | 290 (60%) | 99 (67%) | |
| Impaired relaxation | 124 (26%) | 34 (23%) | |
| Pseudonormal | 37 (8%) | 10 (7%) | |
| Restrictive | 29 (6%) | 4 (3%) | .30 |
The only continuous variables not normally distributed are displayed as medians (interquartile ranges); all other continuous variables are expressed as means ± standard deviation.
After adjustment for potential confounders, including age, hypertension, BMI, and ventricular septal thickness, Caucasians on average had a 2 mm larger left atrial diameter (95% confidence interval, 0.9–3.0 mm, P < .001) than African Americans. Adding left atrial systolic volume to the left atrial diameter multivariate model did not meaningfully change the results (ie, left atrial diameter was larger in Caucasians independently of an effect of left atrial systolic volume).
DISCUSSION
By combining data from 3 prospective cohort studies, we found that Caucasian race was associated with greater atrial fibrillation prevalence and that African American race was associated with substantially lower atrial fibrillation prevalence. These differences were not materially changed after adjustment for potential confounders. To our knowledge, this is the first study to use data derived from standardized protocols and electrocardiographically proven atrial fibrillation to focus specifically on the question of race and atrial fibrillation. By comparing echocardiographic characteristics in the Heart and Soul subgroup without atrial arrhythmias, we found that left atrial diameter was significantly larger in Caucasians than in African Americans.
The initial suggestion that atrial fibrillation might be less common in African Americans arose from studies of patients with stroke.16,17 Go et al2 then demonstrated through an analysis of medical records and International Classification of Diseases, Ninth Revision, codes that African Americans enrolled in the Kaiser Permanente system were less likely to have atrial fibrillation. Other studies have been consistent with this,18,19 but no previous study has data derived from predefined and uniform study protocols to confirm these findings. Given that race may influence the way an individual undergoing clinical care is treated,4–7 the data collected in a clinical setting may bias findings connecting diagnoses and race. Within each of the studies included in our analysis, all participants had the same evaluation regardless of race, with data collected in a uniform fashion according to predetermined protocols.
To investigate possible mechanisms by which African Americans might be protected from atrial fibrillation, we performed an analysis of echocardiograms in subjects enrolled in the Heart and Soul Study. Because atrial arrhythmias can cause atrial remodeling, we excluded participants with known atrial fibrillation from this analysis. Of note, no African Americans in the Heart and Soul Study had atrial fibrillation. The ventricular septum was thicker in African Americans, a finding that, if anything, would be expected to be associated with a higher risk of atrial fibrillation: A thicker ventricular septum can represent cardiac remodeling due to hypertension (and therefore might represent occult or more severe forms of hypertension) or a higher left ventricular end-diastolic pressure, both factors that would generally increase one’s predisposition to atrial fibrillation. Despite no differences in atrial volumes or function, Caucasians had a larger left atrial diameter, even after adjusting for potential confounders. Left atrial diameter is a well-established risk factor for atrial fibrillation,20–23 but the larger diameter in Caucasians compared with African Americans is surprising given that atrial volumes, also known to predict atrial fibrillation, 24 were not significantly different. Of note, previous studies have shown that left atrial diameter predicts atrial fibrillation independent of volume,24 and a recent study in Japanese subjects suggests that left atrial diameter might be more important than left atrial volume in predicting recurrence of paroxysmal atrial fibrillation.25 The association between Caucasians and larger left atrial diameter also was maintained after adjusting for left atrial volume. The only previous study that compared echocardiographic atrial characteristics between Caucasians and African Americans used M-mode only,26 but also found a 1.9 mm greater left atrial diameter among Caucasians after adjusting for confounders. Their statistically significant finding was similar to our finding of a 2-mm difference. It is therefore possible that African Americans have less atrial fibrillation because of a smaller left atrial diameter, potentially due to genes that govern left atrial shape or the shape of anterior or posterior structures (eg, the aorta, chest wall, or spine) that may restrict anterior–posterior atrial expansion.
It also is possible that our finding related to left atrial volume reflects a type I error (a false-positive association). In fact, whereas the difference in atrial fibrillation prevalence is substantial (Caucasians having an ~4-fold greater odds of atrial fibrillation after multivariable adjustment), the difference in left atrial diameter was small. However, in the Framingham study, the difference in mean left atrial diameter between those with and without incident atrial fibrillation was 2.6 mm, suggesting that small changes in left atrial diameter may be clinically relevant.22
The remarkable difference in atrial fibrillation prevalence is especially interesting given that African Americans had more hypertension, a greater septal thickness, and no other differences in ventricular or atrial measurements. The largely negative echocardiographic findings might point to differences in tissue composition rather than chamber size or shape as an explanation for racial differences in atrial fibrillation.
LIMITATIONS
This study has several limitations. First, race was self-reported. Although this method is frequently used,2,3 it is well known that the racial make-up of any given individual can be complex.27,28 However, these limitations would make it more difficult to find a difference between self-identified races and should not result in false-positive associations. Diagnosis of the primary outcome, atrial fibrillation, was specific but not sensitive: ECG evidence of atrial fibrillation was required, but there may have been undetected paroxysmal atrial fibrillation—once again, this would only decrease our sensitivity to identify an association between race and atrial fibrillation and would not result in any false-positive associations. However, although it would seem unlikely, we cannot exclude the possibility that African Americans have the same frequency or potentially even more paroxysmal atrial fibrillation than Caucasians. The sensitivity of atrial fibrillation detection likely differed by cohort given the heterogeneous methods of atrial fibrillation detection: a single baseline ECG in Heart and Soul (least sensitive), continuous monitoring over several hours in MrOS, and repeated annual ECGs in HERS (likely the most sensitive). Because all of the women included came from the HERS trial, the sensitivity of atrial fibrillation detection was likely greater for women than men. However, the proportions of each race with atrial fibrillation were not meaningfully different across cohorts, and, most important, the method of atrial fibrillation detection did not significantly differ by race within each cohort—therefore, these differences in sensitivity should not have affected our findings regarding differences in atrial fibrillation prevalence across races.
CONCLUSIONS
Caucasians have a substantially greater risk of developing atrial fibrillation than African Americans. After adjusting for potential confounders, atrial volumes and function are similar in the 2 races, but atrial diameter is significantly larger in Caucasians. The difference is small, but there is evidence in other studies that differences of this magnitude can be a cause of atrial fibrillation.
CLINICAL SIGNIFICANCE.
Despite exhibiting common risk factors for atrial fibrillation, African Americans have substantially less atrial fibrillation than other races.
Caucasian race is independently associated with atrial fibrillation.
The mechanism by which African Americans are protected against atrial fibrillation remains unknown, but it may be related to a smaller left atrial diameter.
Acknowledgments
Funding: This work was made possible by grant number KL2 RR024130 (G.M.M.) from the National Center for Research Resources, a component of the National Institutes of Health. The Heart and Soul Study was funded by grants from the department of Veterans Affairs (Epidemiology Merit Review Program), Washington, DC; the National Heart Lung and Blood Institute grant R01 HL079235, the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholar Program), Princeton, NJ; the American Federation for Aging Research (Paul Beeson Faculty Scholars in Aging Research Program), New York, NY; and the Ischemia Research and Education Foundation, San Bruno, CA. The Osteoporotic Fractures in Men (MrOS) study is supported by National Institutes of Health funding from the following institutes: the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institute on Aging, the National Center for Research Resources, and National Institutes of Health Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140. The National Heart, Lung, and Blood Institute provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839.
Footnotes
Conflict of Interest: None of the authors have any conflicts of interest associated with the work presented in this manuscript. None of the authors have any conflicts of interest to disclose.
Authorship: All authors had access to the data and played a role in writing this manuscript.
References
- 1.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) J Am Coll Cardiol. 2006;48:854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.Ruo B, Capra AM, Jensvold NG, Go AS. Racial variation in the prevalence of atrial fibrillation among patients with heart failure: the Epidemiology, Practice, Outcomes, and Costs of Heart Failure (EPOCH) study. J Am Coll Cardiol. 2004;43:429–435. doi: 10.1016/j.jacc.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 4.Bach PB, Pham HH, Schrag D, et al. Primary care physicians who treat blacks and whites. N Engl J Med. 2004;351:575–584. doi: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
- 5.Bradley EH, Herrin J, Wang Y, et al. Racial and ethnic differences in time to acute reperfusion therapy for patients hospitalized with myocardial infarction. JAMA. 2004;292:1563–1572. doi: 10.1001/jama.292.13.1563. [DOI] [PubMed] [Google Scholar]
- 6.Gwyn K, Bondy ML, Cohen DS, et al. Racial differences in diagnosis, treatment, and clinical delays in a population-based study of patients with newly diagnosed breast carcinoma. Cancer. 2004;100:1595–1604. doi: 10.1002/cncr.20169. [DOI] [PubMed] [Google Scholar]
- 7.Lillie-Blanton M, Maddox TM, Rushing O, Mensah GA. Disparities in cardiac care: rising to the challenge of Healthy People 2010. J Am Coll Cardiol. 2004;44:503–508. doi: 10.1016/j.jacc.2004.04.043. [DOI] [PubMed] [Google Scholar]
- 8.Ruo B, Rumsfeld JS, Hlatky MA, et al. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290:215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grady D, Applegate W, Bush T, et al. Heart and Estrogen/progestin Replacement Study (HERS): design, methods, and baseline characteristics. Control Clin Trials. 1998;19:314–335. doi: 10.1016/s0197-2456(98)00010-5. [DOI] [PubMed] [Google Scholar]
- 10.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the Osteoporotic Fractures in Men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Rautaharju PM, Calhoun HP, Chaitman BR. NOVACODE serial ECG classification system for clinical trials and epidemiologic studies. J Electrocardiol. 1992;24 Suppl:179–187. doi: 10.1016/s0022-0736(10)80041-x. [DOI] [PubMed] [Google Scholar]
- 12.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Resp Crit Care Med. 2006;173:910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kircher B, Abbott JA, Pau S, et al. Left atrial volume determination by biplane two-dimensional echocardiography: validation by cine computed tomography. Am Heart J. 1991;121(3 Pt 1):864–871. doi: 10.1016/0002-8703(91)90200-2. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Khouri SJ, Maly GT, Suh DD, Walsh TE. A practical approach to the echocardiographic evaluation of diastolic function. J Am Soc Echocardiogr. 2004;17:290–297. doi: 10.1016/j.echo.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Hajat C, Dundas R, Stewart JA, et al. Cerebrovascular risk factors and stroke subtypes: differences between ethnic groups. Stroke. 2001;32:37–42. doi: 10.1161/01.str.32.1.37. [DOI] [PubMed] [Google Scholar]
- 17.Sacco RL, Kargman DE, Zamanillo MC. Race-ethnic differences in stroke risk factors among hospitalized patients with cerebral infarction: the Northern Manhattan Stroke Study. Neurology. 1995;45:659–663. doi: 10.1212/wnl.45.4.659. [DOI] [PubMed] [Google Scholar]
- 18.Borzecki AM, Bridgers DK, Liebschutz JM, et al. Racial differences in the prevalence of atrial fibrillation among males. J Natl Med Assoc. 2008;100:237–245. doi: 10.1016/s0027-9684(15)31212-8. [DOI] [PubMed] [Google Scholar]
- 19.Kelley GP, Stellingworth MA, Broyles S, Glancy DL. Electrocardiographic findings in 888 patients > or = 90 years of age. Am J Cardiol. 2006;98:1512–1514. doi: 10.1016/j.amjcard.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 20.Nakai T, Lee RJ, Schiller NB, et al. The relative importance of left atrial function versus dimension in predicting atrial fibrillation after coronary artery bypass graft surgery. Am Heart J. 2002;143:181–186. doi: 10.1067/mhj.2002.120294. [DOI] [PubMed] [Google Scholar]
- 21.Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 22.Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89:724–730. doi: 10.1161/01.cir.89.2.724. [DOI] [PubMed] [Google Scholar]
- 23.Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 24.Tsang TS, Barnes ME, Bailey KR, et al. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc. 2001;76:467–475. doi: 10.4065/76.5.467. [DOI] [PubMed] [Google Scholar]
- 25.Cui Q, Zhang W, Wang H, et al. Left and right atrial size and the occurrence predictors in patients with paroxysmal atrial fibrillation. Int J Cardiol. 2008;130:69–71. doi: 10.1016/j.ijcard.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Manolio TA, Gottdiener JS, Tsang TS, Gardin JM. Left atrial dimensions determined by M-mode echocardiography in black and white older (> or = 65 years) adults (The Cardiovascular Health Study) Am J Cardiol. 2002;90:983–987. doi: 10.1016/s0002-9149(02)02665-6. [DOI] [PubMed] [Google Scholar]
- 27.Liu XQ, Paterson AD, John EM, Knight JA. The role of self-defined race/ethnicity in population structure control. Ann Hum Genet. 2006;70(Pt 4):496–505. doi: 10.1111/j.1469-1809.2005.00255.x. [DOI] [PubMed] [Google Scholar]
- 28.Reiner AP, Ziv E, Lind DL, et al. Population structure, admixture, and aging-related phenotypes in African American adults: the Cardiovascular Health Study. Am J Hum Genet. 2005;76:463–477. doi: 10.1086/428654. [DOI] [PMC free article] [PubMed] [Google Scholar]