Abstract
Synthesis of heat shock proteins (HSPs) in response to heat shock (HS) is essential for thermotolerance. The effect of a Ca2+ chelator, EGTA, was investigated before a lethal HS treatment in soybean (Glycine max) seedlings with acquired thermotolerance induced by preheating. Such seedlings became non-thermotolerant with EGTA treatment. The addition of Ca2+, Sr2+ or Ba2+ to the EGTA-treated samples rescued the seedlings from death by preventing the increased cellular leakage of electrolytes, amino acids, and sugars caused by EGTA. It was confirmed that EGTA did not affect HSP accumulation and physiological functions but interfered with the recovery of HS-released Ca2+ concentration which was required for thermotolerance. Pectin methylesterase (PME, EC 3.1.1.11), a cell wall remodelling enzyme, was activated in response to HS, and its elevated activity caused an increased level of demethylesterified pectin which was related to the recovery of the HS-released Ca2+ concentration. Thus, the recovery of HS-released Ca2+ in Ca2+-pectate reconstitution through PME activity is required for cell wall remodelling during HS in soybean which, in turn, retains plasma membrane integrity and co-ordinates with HSPs to confer thermotolerance.
Keywords: Ca2+, heat shock, pectin, pectin methylesterase, thermotolerance
Introduction
Organisms develop a large number of strategies to mitigate the effects of environmental changes. The expression of a specialized set of proteins called heat shock proteins (HSPs) elicits one of the best-characterized responses called the heat shock response (HSR), which plays an important role in acquired thermotolerance (Alexandrov, 1994). Plant HSPs are synthesized in response to a heat shock (HS) and during various developmental processes including pollen maturation, embryogenesis, and seed maturation (zur Nieden et al., 1995; Wehmeyer et al., 1996; Waters and Vierling, 1999).
Plants express an unusually high amount and multiplicity of small HSPs (sHSPs) relative to other organisms. In pea and soybean, the level of sHSPs is up to ∼1% of the total protein (DeRocher et al., 1991; Hsieh et al., 1992). Some sHSPs are selectively localized in cellular organelles during HS and relocalized to the cytoplasm after the recovery from HS in a temperature-dependent manner, which is associated with the acquisition of thermotolerance in soybean (Lin et al., 1984; Chou et al., 1989; Jinn et al., 1997, 2004). Biochemical evidence has shown that HSPs work primarily as molecular chaperones to prevent aggregation and to promote the refolding of denatured proteins caused by HS (Parsell and Lindquist, 1993). Thermal injury has also been suggested to alter membrane fluidity, which results in increased cellular leakage (Levitt, 1980). In addition to their chaperone function, sHSPs in membranes can also regulate membrane fluidity and preserve membrane integrity during thermal stress (Tsvetkova et al., 2002).
A wide variety of stresses stimulate a transient Ca2+ content in cells, which presumably results in acquired tolerance/resistance to environmental stresses (Sanders et al., 1999). Ca2+ acts as an intracellular messenger in coupling a wide range of extracellular signals for specific responses to heat, chilling, salinity, drought, and anaerobic stress (for a review see Trewavas and Malho, 1998). In addition, apoplastic Ca2+ is essential for the control of cell integrity, cell wall cohesion, and plasma membrane permeability; low apoplastic Ca2+ concentration increases plasma membrane permeability (Hirschi, 2004). Gong et al. (1998) showed that pretreatment with 10 mM EGTA under HS reduced cell viability in tobacco (Nicotiana plumbaginifolia), whereas 10 mM Ca2+ enhanced the cell survival rate. Larkindale and Knight (2002) indicated that Ca2+ treatment could improve cell survival in Arabidopsis thaliana after severe HS. Genetic evidence in Arabidopsis supports HS causing a transient increase in the level of cytosolic Ca2+, and a calmodulin, AtCaM3, mediates the Ca2+ signal which is involved in the induction of HSP gene expression (Zhang et al., 2009).
Pectin is a major component of the cell wall and is important for both cellular adhesion and cell wall plasticity. The middle lamella, a pectinaceous interface, which depends on the formation of intermolecular links between pectin molecules, is important for the adhesion of neighbouring cells (for a review see Jarvis et al., 2003). Pectin modification is, in general, catalysed by a large enzyme family of pectin methylesterases (PMEs) residing in the cell wall, which modulates apoplastic Ca2+ content in response to stresses for both the assembly and disassembly of the pectic network (for a review see Micheli, 2001; Pelloux et al., 2007). Homogalacturonan is a major component of pectin and has a conformational flexibility that can be influenced by growth, development, and environmental cues (for a review see Willats et al., 2001). During cell wall formation, homogalacturonan is demethylesterified by the activity of PME, which results in contiguous and random patterns of free carboxylic residues. The contiguous demethylesterified homogalacturonan binds with Ca2+ to promote the formation of the ‘egg-box’ structures and plays a significant role in the structural rigidity of the cell wall. Demethylesterification randomly releases protons, which become a target for pectin-degrading enzymes such as polygalacturonase (PG, EC 3.2.1.15). PG acts co-operatively with PME to disassemble the pectin polymer networks and contributes to cell wall weakening (Micheli, 2001). The degree of methylesterification of pectin affects Ca2+ cross-linking to form a pectate gel involved in growth and defence reactions in numerous plants (Pelloux et al., 2007). The highly complex structures of plant cell walls are critically involved in cell elongation, the drought response, and plant–pathogen interactions. Each process is affected by the chemical composition and physical architecture of the cell wall (Micheli, 2001).
Plant cells can monitor the functional integrity of cell walls, and the maintenance of cell wall integrity is an important process to relieve the stresses (Hamann et al., 2009). The cleavage of Ca2+ bridges between pectic carboxyl groups is considered to play an important role in cell wall remodelling, which is a strategy to avoid tearing the plasma membrane from the cell wall and to retain cell integrity during drought stress (Farrant and Sherwin, 1998). Solecka et al. (2008) showed pectin content associated with temperature-dependent modifications, and the demethylesterification degree is also involved with resistance to freezing and pathogen infection. A vacuum-impregnation experiment with PME plus Ca2+ also showed increased rigidity of the pectin network and the mechanical rigidity of the cell wall structures (Guillemin et al., 2008). Thus, the tight control of the methylesterification status of pectin appears to play a major role in plant growth and acts as a regulator in response to environmental cues. Modification of the biophysical properties of cell walls, such as through PME activity, could be a key component in responding to environmental injuries.
PMEs may have potential in the development of thermotolerance by maintaining apoplastic Ca2+ homeostasis. To date, this has not been investigated. It is shown here that EGTA inhibited the development of thermotolerance in soybean by preventing the recovery of the HS-released Ca2+ concentration. HS-induced Ca2+ mobilization, PME and PG activity, and pectin methylesterification status during the HSR and with EGTA treatment were investigated. It is suggested that the HS-activated PME participates in cell wall remodelling which, in turn, retains plasma membrane integrity and co-ordinates with HSPs to confer thermotolerance in soybean.
Materials and methods
Plant growth and cellular leakage analysis
Soybean (Glycine max cv. Kaohsiung No. 8) seeds were germinated in moist paper towels at 28 °C for 2 d in a dark growth chamber, and etiolated seedlings with the embryonic axis of ∼1.5–3 cm were used (Lin et al., 1984). For ion and EGTA treatment, seedlings were incubated in 25 ml Milli-Q-purified water with a final concentration of 15 mM CaCl2, SrCl2, BaCl2, MgCl2, KCl, or EGTA (adjusted to pH 6.8) in a 250 ml flask and incubated in a shaking water bath under various treatments. Before treatment with a 45 °C HS for 2 h, the incubation medium was drained and replaced with Milli-Q-purified water. Electrolytes were measured by the use of a conductivity meter (Model SC 170; SUNTEX, Taipei, Taiwan) (Lin et al., 1985). Amino acids and total soluble sugars were quantified by the ninhydrin method (Moore and Stein, 1954) and the phenol–sulphuric acid method (Dubois et al., 1956), with leucine and glucose used as standards, respectively.
Post-ribosomal supernatant (PRS) preparation and fractionation
Soybean seedlings without cotyledons were incubated with 5 mM potassium phosphate buffer (pH 6.0) containing 1% (w/v) sucrose, 50 μg ml−1 chloramphenicol, and 20 μCi ml−1 [35S]Met (Amersham Pharmacia Biotech, Piscatway, NJ, USA) at 28 °C for 2 h with gentle shaking. Seedlings were harvested and homogenized with 0.2 M TRIS-HCl buffer (pH 8.8) containing 0.5 M sucrose, 0.1 M KCl, 30 mM MgCl2, 1 mM DTT, and 1 mM PMSF. The PRS was collected and fractionated to 70–100% (w/v) saturation with ammonium sulphate to enrich class-I sHSP levels (Jinn et al., 1989).
Assay for thermal denaturation of soluble proteins
One mg of the 70–100% ammonium sulphate-saturated fraction was added to 1 mg [35S]-labelled PRS (∼1×106 cpm) of non-HS seedlings, and the mixture was heated to 55 °C for 30 min with shaking (Jinn et al., 1989, 1993). The denatured proteins were pelleted at 16 000 g for 15 min, and the radioactivity in the pellet was measured after suspension in Laemmli's sample buffer (Laemmli, 1970).
Quantitation of class-I small heat shock protein (sHSP) levels
The cross-reaction of proteins with class-I sHSP antibodies was visualized by reacting with 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium according to the manufacturer's specifications (Bio-Rad, Hercules, CA, USA) and quantified by scanning the PVDF membrane by use of a densitometer (Model SI; Molecular Dynamics, Sunnyvale, CA, USA) and ImageQuant software (Molecular Dynamics) (Hsieh et al., 1992). Two-day-old seedlings treated at 40 °C for 2 h, as an internal standard, contains 0.98 μg class-I sHSP 100 μg−1 of total proteins is sufficient to confer thermoprotection (Hsieh et al., 1992).
Ion analysis
Soybean seedlings were incubated in 20 ml Milli-Q-purified water in a shaking water bath with various treatments. A Zeeman graphite furnace atomic absorption spectrophotometer (Hitachi Z-2300, Tokyo, Japan) was used to determine the concentration of ions leaked into Milli-Q-purified water.
Pectin methylesterase (PME) activity analysed by acidic continuous native-PAGE
Proteins were extracted from the root elongation zone of soybean seedlings at 4 °C with phosphate citrate buffer (pH 5.0) (a 100 ml mixture containing 24.3 ml of 0.1 M citric acid and 25.7 ml of 0.2 M Na2HPO4) containing 1 M NaCl, and PME activity was analysed by acidic continuous native-PAGE (Ren and Kermode, 2000). After electrophoresis, gel was equilibrated with phosphate citrate buffer (pH 6.3) (a 100 ml mixture containing 12.5 ml of 0.1 M citric acid and 25 ml of 0.2 M Na2HPO4) for 5 min and incubated for 1.5 h at 37 °C in the same buffer, with 0.5% (w/v) esterified pectin (90% purity) (Sigma, St Louis, MO, USA) used as a substrate. After a brief rinse with water, gel was stained with 0.02% (w/v) ruthenium red and destained with water, and only a single activity band was detected. The activity was quantified by scanning the gel with the densitometer and using ImageQuant software as described previously.
Polygalacturonase (PG) activity assay
Protein was extracted from the root elongation zone of soybean seedlings (Pressey, 1986). PG activity was measured spectrophotometrically by the formation of reducing groups from polygalacturonic acid (poly-GalUA) (Sigma). The sample solution in 50 μl was incubated with 180 μl of 0.1% (w/v) poly-GalUA (in 40 mM sodium acetate buffer, pH 4.4) at 35 °C for 10 min. The reaction was stopped by the addition of 1.2 ml of 0.1 M borate buffer (pH 9.0) and 240 μl of 1% 2-cyanoacetamide and was heated at 100 °C for 10 min. The absorbance of the solution was measured spectrophotometrically at 276 nm (Honda et al., 1980). A blank was determined in the same way by replacing the protein extract with sodium acetate buffer solution.
Histochemical analysis of pectin by ruthenium red (RR) staining
Cross-sections of the hypocotyls of soybean seedlings were stained with 0.05% (w/v) RR for 30 min at room temperature, and then destained with water and observed by light microscopy (Jensen, 1962). RR reacts primarily with the carboxyl groups of acidic sugars to stain tissues from pink (less demethylesterified pectin) to red (more demethylesterified pectin) (Iwai et al., 1999). Treatment with 1 N NaOH can remove methyl groups of pectin and lead to free carboxyl groups, which allows the localization of demethylesterified pectin in primary cell walls to be shown. Thus, a comparison of RR staining with and without alkali could reflect the status of demethylesterified pectin (Asahina et al., 2002).
Immunolocalization of Ca2+-demethylated homogalacturonan
The monoclonal antibody 2F4, obtained from PlantProbes (University of Leeds, Leeds, UK), specifically recognizes a Ca2+-induced supramolecular conformation of homogalacturonan, the ‘egg-box’ shaped structures as described by Liners (1992), with slight modification. Cross-sections of hypocotyls of soybean seedlings were treated with 50 mM NaOH (pH 12) for 1 h, incubated with 0.5% (w/v) bovine serum albumin in T/Ca/Na buffer (20 mM TRIS pH 8.2, 0.5 mM CaCl2, and 150 mM NaCl) for 1 h to minimize non-specific binding, and then probed with the 2F4 antibody and T/Ca/Na buffer in a 1:250 dilution at room temperature for 1 h and rinsed thoroughly with T/Ca/Na buffer three times for 10 min each. Sections were incubated with the fluorescein isothiocyanate (FITC)-conjugated anti-mouse antibodies (Sigma) in a 1:200 dilution for 1 h and washed as described above, and then mounted with T/Ca/Na buffer/glycerol (1:1 v/v). Fluorescence signals were observed with a laser scanning confocal microscope (TCS-SP2; Leica Lasertechnik GmbH, Heidelberg, Germany). Sections treated without alkali before immunolocalization displayed a muted fluorescence signal; thus, specimens were incubated with 50 mM NaOH to increase the sensitivity as communicated by Dr Francoise Liners (Unité de Recherche en Biologie Cellulaire Végétale, Facultés Universitaires, Notre-Dame de la Paix, Belgium) (Liners, 1992) and as described previously (Willats et al., 2001; Guillemin et al., 2005).
Statistical analysis
All experiments were independently repeated at least three times, and 25 or 30 2-d-old etiolated soybean seedlings were used for each treatment. Statistical analysis was performed using Student's t test (two-tailed, unpaired).
Results
Effect of EGTA treatment on plant growth and cellular leakage
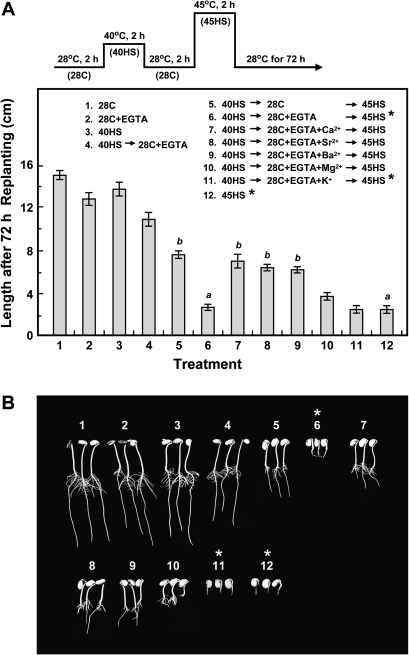
Two-day-old etiolated soybean seedlings which had a preheating treatment at 40 °C for 2 h (40HS), a non-lethal HS, before a lethal treatment of 45 °C for 2 h (45HS) showed thermoprotection (Fig. 1A, compare treatments 5 and 12). Exogenously supplying a Ca2+ chelator, EGTA, during the recovery at 28 °C for 2 h (28C) after 40HS abolished the acquired thermoprotection provided by HSPs in seedlings subjected to 45HS (compare treatments 5 and 6). The addition of Ca2+ or ions of the same periodic group, such as Sr2+ or Ba2+, together with EGTA, significantly rescued the seedlings from death (treatments 7–9); however, Mg2+ was not effective (treatment 10), and K+ (monovalent cation) could not alleviate the inhibitory effect of EGTA (treatment 11). EGTA treatment during 28C was not harmful to seedlings (treatments 2 and 4). However, the addition of Ca2+ after EGTA treatment (i.e. 40HS→28C+EGTA→45HS+Ca2+) protected seedlings against death (data not shown). Figure 1B shows typical seedlings after treatment at 72 h as indicated in Fig. 1A. Seedling length was measured at 24, 48, and 72 h to assess seedling viability after treatment (see Supplementary Table S1 at JXB online).
Fig. 1.
Thermotolerance is abolished by EGTA treatment during the recovery after HS but restored by Ca2+. Two-day-old etiolated soybean seedlings with an embryonic axis of ∼1.5 cm were tested. (A) HS regimen is shown on the top and inside the panel. Treatments 1–5 and 12 were used as references. Thermotolerance was lost with treatment at 40HS→28C+EGTA→45HS (treatment 6) and restored by adding Ca2+, Sr2+ or Ba2+ (treatments 7–9, respectively); Mg2+ was less effective (treatment 10), and K+ was not effective (treatment 11). Seedlings were grown at 28 °C in a dark growth chamber for an additional 72 h after treatment, and the length was measured. Data are means ±SD of three independent replicates, with 30 seedlings for each treatment. a and b represent significantly different values (P <0.05). (B) Typical seedlings were photographed at 72 h after treatment as indicated in (A). An asterisk indicates that the treatment is lethal.
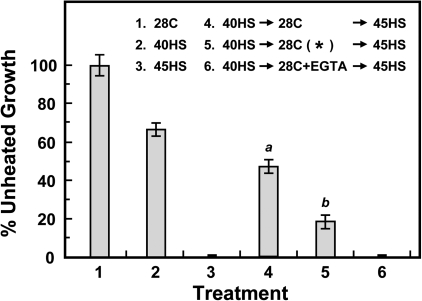
The leakage of electrolytes, amino acids, and sugars into the seedling incubation medium were also measured (Fig. 2). A small amount of leakage occurred after 40HS (treatments 2 and 3), and 40HS pretreatment prevented the considerable amount of leakage that was shown with 45HS (compare treatments 4 and 7). Protection against cellular leakage with 40HS pretreatment was abolished by EGTA (treatment 5), whereas the addition of Ca2+ (treatment 6), Sr2+ or Ba2+ (data not shown) counteracted the EGTA effect. These results suggested that the EGTA-based loss of thermotolerance may be due to the altered permeability of the plasma membrane.
Fig. 2.
EGTA treatment increases cellular leakage of electrolytes, amino acids, and sugars and is counteracted by treatment with Ca2+. Treatments are indicated inside the panel. Data are means ±SD of three independent replicates, with 30 seedlings for each treatment. An asterisk indicates that the treatment is lethal.
Effect of HS-released Ca2+ concentration and its recovery on the development of thermotolerance
It was found that EGTA affected electrolyte leakage in abolishing thermoprotection (Fig. 2), and apoplastic Ca2+ is known to bind phospholipids, stabilize lipid bilayers, and control membrane permeability (Hanson, 1984). In this study, Ca2+ and K+ leakage from seedlings in the incubation medium after HS were measured by atomic absorption spectrophotometry (Fig. 3). In 2-d-old etiolated soybean seedlings, 40HS induced Ca2+ release compared with the control treatment (0.99 versus 0.73 μg Ca2+ seedling−1) (compare treatments 1 and 2). HS triggered the release of Ca2+ at 2.04 μg seedling−1 during the 28C recovery period after the 40HS (treatment 3), and this released Ca2+ was significantly recovered after 45HS in the seedlings with acquired thermotolerance (1.09 μg Ca2+ seedling-1) (treatment 4). However, both K+ release during recovery after 40HS (compare treatments 2 and 3) and K+ recovery after the subsequent 45HS (compare treatments 3 and 4) were not detected. Meanwhile, 40HS pretreatment significantly prevented the release of Ca2+ and K+ at 45HS (compare treatments 4 and 5), which was well associated with lowered conductivity (Fig. 2, compare treatments 4 and 7). Here, the 45HS-lethal treatment caused the release of Ca2+ at 2.57 μg seedling−1 and K+ at 38.76 μg seedling−1 (treatment 5). These results suggested that the recovery of HS-released Ca2+ is essential for the acquisition of thermoprotection to mitigate the lethal HS injury, and EGTA chelates Ca2+ to prevent its recovery to abolish the acquired thermotolerance.
Fig. 3.
Atomic absorption spectrometry of Ca2+ and K+ leakage in response to HS and EGTA treatment. Treatments are indicated inside the panel. Data are means ±SD of 3–5 independent replicates, with 25 seedlings for each treatment. a, b, and c represent significantly different values (P <0.05).
The EGTA incubation medium was replaced with Milli-Q-purified water every 30 min during the 2 h 28 °C recovery period after 40HS (Fig. 4, treatment 5; indicated by an asterisk) for titration of the HS-triggered released and recovered Ca2+ concentration. Acquired thermotolerance, as measured by seedling growth, was significantly lower than that without the replaced medium (compare treatments 4 and 5). These results confirmed that the recovery of HS-released Ca2+ concentration is required for thermotolerance.
Fig. 4.
HS-triggered release of Ca2+ and its recovery is required for the development of thermotolerance. Treatments are indicated inside the panel. The EGTA incubation medium was replaced with Milli-Q-purified water every 30 min during the 2 h 28 °C recovery period after 40HS (treatment 5, indicated by an asterisk). After treatment, seedlings were grown at 28 °C in a dark growth chamber for an additional 48 h, and the length of seedlings was measured. Data are means ±SD of three independent replicates, with 30 seedlings for each treatment. a and b represent significantly different values (P <0.05).
Effect of EGTA on sHSP accumulation and organelle localization in vivo and thermostabilization of soluble proteins in vitro
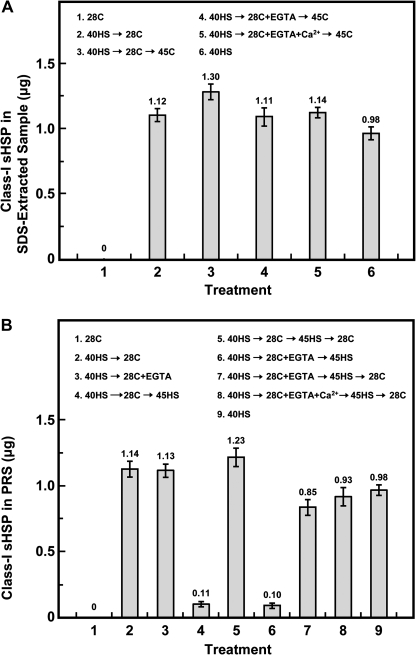
It was analysed whether EGTA affected class-I sHSP accumulation and organelle localization during the HSR (Fig. 5). Soybean seedlings with or without EGTA treatment did not differ in class-I sHSP accumulation to confer thermoprotection (Fig. 5A, treatments 2–5). Meanwhile, 45HS after 40HS→28C ±EGTA decreased the amount of class-I sHSP to ∼10% in the post-ribosomal supernatant (PRS) (Fig. 5B, treatments 4 and 6). The 28C recovery after 45HS caused the organelle-associated class-I sHSP to redistribute into the cytosol (Fig. 5B, treatments 5, 7, and 8), because the accumulation of class-I sHSP in the PRS increased remarkably (>86%).
Fig. 5.
Effect of EGTA on class-I sHSP accumulation, and organelle association and dissociation in response to HS. Treatments are indicated inside the panel. Proteins were extracted by (A) SDS-extraction buffer or (B) were collected from the post-ribosomal supernatant (PRS) after treatment. Extracted proteins were separated by SDS-PAGE, then analysed for class-I sHSP content. 40HS treatment, as an internal standard, contains 0.98 μg class-I sHSP 100 μg-1 of total proteins is sufficient to confer thermoprotection. Data are means ±SD of three independent replicates.
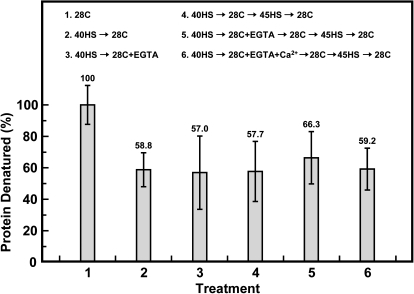
A class-I sHSP-enriched fraction was also prepared from seedlings treated with EGTA and the thermostabilization of soluble proteins by this fraction was tested (Fig. 6). No significant difference was found in the thermostabilization of soluble proteins among these treatments (treatments 2–6). This class-I sHSP-enriched fraction provided ∼40% of the soluble proteins retained after heating. In summary, EGTA did not interfere with class-I sHSP accumulation and physiological functions.
Fig. 6.
Thermostabilization of [35S]-labelled proteins by addition of a class-I sHSP-enriched fraction from different treatments. Treatments are indicated inside the panel. The thermodenatured proteins were quantitated as described in the Materials and methods. The values relative to the 28C control (100%) were presented. Data are means ±SD of three independent replicates.
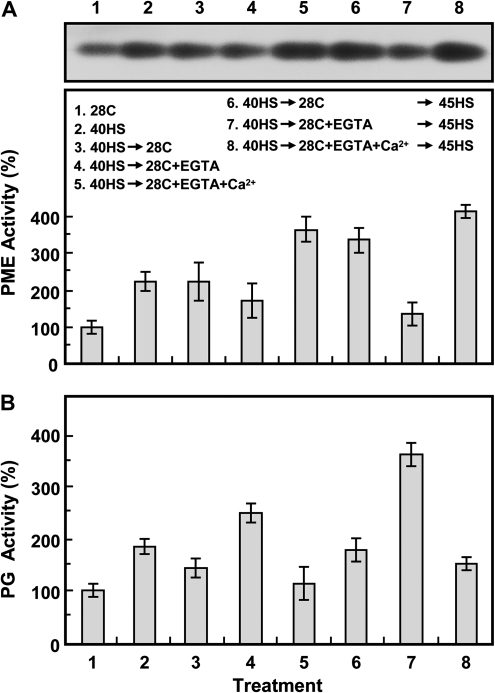
Effect of HS and EGTA treatment on pectin methylesterase (PME) and polygalacturonase (PG) activity
PME and PG activity with HS and EGTA treatment was investigated (Fig. 7). PME activity during the 40HS treatment was higher, by ∼2.2-fold, than that with the 28C control treatment (Fig. 7A, treatments 1–3). The activity of PME was maintained at a steady-state level during recovery after the 40HS (treatment 3). EGTA treatment reduced PME activity, but the addition of Ca2+ with EGTA reinstated the activity in response to HS (treatment 4 compared with treatments 3 and 5; treatment 7 compared with treatments 6 and 8).
Fig. 7.
Pectin methylesterase (PME) and polygalacturonase (PG) activities are affected by HS and EGTA treatment. Treatments are indicated inside the panel. (A) PME activity was analysed by acidic continuous native-PAGE. One typical stained gel is shown (top panel), and the relative activity of PME to 28C control treatment was quantified (bottom panel). (B) Enzymatic analysis of PG was by colorimetric assay. Data are means ±SD of three independent replicates.
However, PG activity was increased with 40HS treatment (∼1.8-fold higher than that with the control treatment) and was slightly reduced during the recovery after 40HS (Fig. 7B, treatments 1–3). EGTA treatment increased the PG activity, but Ca2+ treatment counteracted the EGTA-induced PG activity (treatment 4 compared with treatments 3 and 5; treatment 7 compared with treatments 6 and 8).
Status of demethylesterified pectin in response to HS and EGTA treatment
The status of pectic substances during the HSR and EGTA treatment was analysed histochemically by ruthenium red (RR) staining (Fig. 8). Soybean hypocotyl parenchymal cells under the 28C control conditions showed indistinct RR labelling in the primary cell wall (Fig. 8A). With 40HS, a 2 h recovery period after 40HS, and acquired thermotolerance treatments (Fig. 8B, C, E, respectively), cells showed increased RR labelling as compared with the control treatment (Fig. 8A). EGTA treatment produced no RR staining (Fig. 8D, F), and Ca2+ treatment counteracted the EGTA effect (Fig. 8G). Strong RR staining on the primary cell wall after alkali treatment indicated the abundance of total pectin in the control parenchymal cells (Fig. 8H). EGTA treatment at 28C did not affect RR staining (Fig. 8I). These results showed that the demethylesterified status of pectin is increased, possibly through HS-activated PME activity in response to HS (Fig. 7).
Fig. 8.
Ruthenium red staining characterizes pectic substances in hypocotyl parenchymal cells under HS and EGTA treatment. Treatments were (A) 28C, (B) 40HS, (C) 40HS→28C, (D) 40HS→28C+EGTA, (E) 40HS→28C→45HS, (F) 40HS→28C+EGTA→45HS, and (G) 40HS→28C+EGTA+Ca2+→45HS. (H) 28C control treated with 1 N NaOH before staining. (I) 28C+EGTA treatment. Scale bars, 100 μm.
The ‘egg-box’ model structure, Ca2+-demethylated homogalacturonan, in response to HS and EGTA treatment
Immunolocalization of Ca2+-pectate was performed by probing with the 2F4 monoclonal antibody (Fig. 9) to verify the RR staining results. Hypocotyl parenchymal cells under 28C and 40HS treatment (Fig. 9A, B) showed the typical fluorescence signals of egg-box structures located at tricellular junctions and the corners of intercellular spaces (indicated by arrows). Ca2+ release during 28C recovery and after 40HS slightly affected the egg-box structure (Fig. 9C). The thermoprotected cells showed more reconstructed egg-box structures, with enhanced fluorescence signals (Fig. 9E). EGTA treatment greatly reduced the fluorescence intensity (Fig. 9D, F), but Ca2+ counteracted the EGTA effect and restored the signals (Fig. 9G). EGTA treatment at 28C did not interfere with the fluorescence signals (Fig. 9I). These results agreed with the RR staining results (Fig. 8) and suggested that the recovery of HS-released Ca2+ concentration can be through Ca2+-pectate reconstruction. Also, the formation of egg-box structures may affect the cell wall texture and rigidity for thermotolerance.
Fig. 9.
Immunolocalization of the egg-box structures in hypocotyl parenchymal cells under HS and EGTA treatment. Treatments were (A) 28C, (B) 40HS, (C) 40HS→28C, (D) 40HS→28C+EGTA, (E) 40HS→28C→45HS, (F) 40HS→28C+EGTA→45HS, and (G) 40HS→28C+EGTA+Ca2+→45HS. (H) 28C without prior NaOH treatment, (I) 28C+EGTA, and (J) 28C without the primary antibody were controls. Except for specimens in (H), all specimens were incubated with 50 mM NaOH before immunolocalization. The egg-box structures are indicted by arrows. Scale bars, 50 μm.
Discussion
During the HSR, maintaining apoplastic Ca2+ homeostasis through PME activity is required for cell wall remodelling which, in turn, retains plasma membrane integrity and co-ordinates with HSPs to confer thermotolerance in soybean. Hanson (1984) showed increased leakage of ion and metabolite levels in cells cultured with low Ca2+ solutions, especially in the presence of EGTA. The order of efficiency in displacing Ca2+ from the isolated cell walls was Ca2+, Ba2+, Sr2+ > Mg2+ > K+, Na+ (Young and Kauss, 1983). It was found that EGTA-depleted thermotolerance associated with increased cellular leakage in soybean seedlings, and the thermotolerance was restored by adding divalent cations of Ca2+, Sr2+ or Ba2+ with EGTA during the recovery from HS (Fig. 1; see Supplementary Table S1 at JXB online). Compared with Ca2+, Mg2+ had a relatively weak affinity for the pectin chain and showed lower efficiency in restoring the EGTA effect on thermotolerance (Fig. 1). However, Mg2+ greatly affected root growth but had a lower effect on the inhibition of hypocotyl elongation, which suggests that the EGTA sensitivity of roots to Mg2+ was higher than that of stems. O'Neill and York (2003) indicated that K+ does not lead to the formation of a pectin network. Our results also confirmed that the addition of K+ did not restore the EGTA effect. In addition, the accumulation, localization, and thermostabilization of soluble proteins of class-I sHSP during HSR were unaffected by EGTA (Figs 5, 6). Thus, in addition to HSPs, the homeostasis of apoplastic Ca2+, which is affected by EGTA treatment, also participates in the development of thermotolerance in soybean.
PME is a ubiquitous enzyme functioning in the cell wall and is encoded by 66 genes in the Arabidopsis genome; most genes have tissue- and stress-specific expression patterns. Expression analysis of the predicted PME genes in the Arabidopsis microarray database revealed ∼75% genes with varied expression in response to biotic and abiotic stresses (Pelloux et al., 2007). Among the wealth of PME genes expressed in Arabidopsis pollen, disruption of the VANGUARD1 (VGD1, At2g47040) causes a slight reduction in pollen PME activity and retards pollen tube growth within the style transmitting tract; however, only some pollen-specific PME genes can complement the phenotype of VGD1 (Jiang et al., 2005). In addition, QUARTET1 (QRT1, At5g55590), a PME involved in the degradation of the pollen mother-cell primary wall, promotes wall loosening by making the pectin susceptible to degradation, which is required for pollen separation (Francis et al., 2006). Therefore, the presence of characteristic amino acid sequences and similar expression patterns does not necessarily reflect the functional redundancy within the PME gene family. In our study, a screening of 54 Arabidopsis PME-knockout lines from the Arabidopsis Biological Resource Center (Ohio State University, USA) revealed two PME mutants with lost acquired thermotolerance (H-C Wu, unpublished data). This finding revealed that PME has a novel role in thermotolerance.
Sharma (1986) found that EDTA treatment could enhance the activity of PG but not PME. As well, in tomato (Lycopersicon esculentum), PG can be inactivated under sustained high temperature, whereas PME remains active (Crelier et al., 2001; Fachin et al., 2002). A contrary role for PME and PG activity during the HSR and EGTA treatment was also found (Fig. 7). Phenotype observations also revealed that 2-d-old soybean embryonic roots had a tender-texture phenotype after EGTA treatment, but the effect was ameliorated after the addition of Ca2+ (Fig. 1B, treatments 6 and 7).
In addition, the pectin methylesterification status during the HSR and EGTA treatment was found to be associated with PME activity, as analysed by RR staining and immunolocalization (Figs 8, 9). These observations supported the recovery of HS-released Ca2+ as being required for the formation of a Ca2+ cross-linked pectin network, and EGTA treatment chelates the released Ca2+ to prevent its reconstitution, which revealed a depletion of the ability to acquire thermotolerance in soybean. Thus, HS-activated PME, acting on pectin to remove the methyl groups and restoring the carboxyl groups for Ca2+ recovery, is involved in remodelling the cell wall to retain plasma membrane integrity and to minimize HS injury.
In moss (Physcomitrella patens), the HS-induced entry of extracellular Ca2+ into the cytoplasm mediates the HSR by Ca2+-permeable channels in the plasma membrane (Saidi et al., 2009). In our study, Ca2+ leakage in response to HS could be from both extracellular and intracellular sources (Fig. 3). Compared with Ca2+ and K+ leakage during the recovery period after 40HS (Fig. 3, treatments 2 and 3), Ca2+ was mainly from an apoplastic reservoir, Ca2+-pectate. Thus, the recovery of the HS-triggered released Ca2+ mediates cell wall remodelling and may also increase Ca2+ influx through PME activity to regulate the HSR in soybean.
The HS-increased intracellular Ca2+ level serving as a cellular second messenger through the Ca2+-CaM HS signal transduction pathway for the induction of HSP gene expression has been well described (Gong et al., 1998; Saidi et al., 2009; Zhang et al., 2009). In addition, homeostasis of the apoplastic Ca2+ in the cell wall may have a pronounced effect through pectin remodelling to prevent cellular leakage. The above two mechanisms may interact in the adaptation to thermal stress in soybean.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Table S1. Thermotolerance is lost in the presence of EGTA during recovery from HS and is restored by adding Ca2+, Sr2+ or Ba2+.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Taiwan University (97R0066-39) and partially supported by the National Science Council, Taiwan (94-2311-B-002-014) to T-LJ.
The authors are grateful to Drs Chu-Yung Lin and Bai-Ling Lin (National Taiwan University), Dr Kevin Lease (University of Missouri, Columbia) and Dr Ming-Hwa Benjamin Liang for critical reading and comments; and to Dr Francoise Liners for helpful advice on the immunofluorescence study with 2F4 antibody.
References
- Alexandrov V. Functional aspects of cell response to heat shock. International Review of Cytology. 1994;148:171–227. doi: 10.1016/s0074-7696(08)62408-0. [DOI] [PubMed] [Google Scholar]
- Asahina M, Iwai H, Kikuchi A, Yamaguchi S, Kamiya Y, Kamada H, Satoh S. Gibberellin produced in the cotyledon is required for cell division during tissue reunion in the cortex of cut cucumber and tomato hypocotyls. Plant Physiology. 2002;129:201–210. doi: 10.1104/pp.010886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou M, Chen YM, Lin CY. Thermotolerance of isolated mitochondria associated with heat shock proteins. Plant Physiology. 1989;89:617–621. doi: 10.1104/pp.89.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crelier S, Robert M-C, Claude J, Juillerat M-A. Tomato (Lycopersicon esculentum) pectin methylesterase and polygalacturonase behaviors regarding heat- and pressure-induced inactivation. Journal of Agricultural and Food Chemistry. 2001;49:5566–5575. doi: 10.1021/jf010202u. [DOI] [PubMed] [Google Scholar]
- DeRocher AE, Helm KW, Lauzon LM, Vierling E. Expression of a conserved family of cytoplasmic low molecular weight heat shock proteins during heat stress and recovery. Plant Physiology. 1991;96:1038–1047. doi: 10.1104/pp.96.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for the determination of sugars and related substances. Analytical Chemistry. 1956;28:350–356. [Google Scholar]
- Fachin D, Van Loey A, Nguyen BL, Verlent I, Hendrickx M. Temperature and pressure inactivation of tomato pectinases: a kinetic study. Mededelingen (Rijksuniversiteit te Gent. Fakulteit van de Landbouwkundige en Toegepaste Biologische Wetenschappen) 2002;67:23–26. [PubMed] [Google Scholar]
- Farrant JM, Sherwin HS. Mechanisms of dessication tolerance in seeds and resurrection plants. In: Taylor AG, Huang XL, editors. Progress in seed research. Proceedings of the Second International Conference on Seed Science and Technology. New York: Communication Services of the New York State Agricultural Experiment Station; 1998. pp. 109–120. [Google Scholar]
- Francis KE, Lam SY, Copenhaver GP. Separation of Arabidopsis pollen tetrads is regulated by QUARTET1, a pectin methylesterase gene. Plant Physiology. 2006;142:1004–1013. doi: 10.1104/pp.106.085274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M, van der Luit AH, Knight MR, Trewavas AJ. Heat-shock-induced changes in intracellular Ca2+level in tobacco seedlings in relation to thermotolerance. Plant Physiology. 1998;116:429–437. [Google Scholar]
- Guillemin A, Guillon F, Degraeve P, Rondeau C, Devaux MF, Huber F, Badel E, Saurel R, Lahaye M. Firming of fruit tissues by vacuum-infusion of pectin methylesterase: visualisation of enzyme action. Food Chemistry. 2008;109:368–378. doi: 10.1016/j.foodchem.2007.12.050. [DOI] [PubMed] [Google Scholar]
- Guillemin F, Guillon F, Bonnin E, Devaux MF, Chevalier T, Knox JP, Liners F, Thibault JF. Distribution of pectic epitopes in cell walls of the sugar beet root. Planta. 2005;222:355–371. doi: 10.1007/s00425-005-1535-3. [DOI] [PubMed] [Google Scholar]
- Hamann T, Bennett M, Mansfield J, Somerville C. Identification of cell-wall stress as a hexose-dependent and osmosensitive regulator of plant responses. The Plant Journal. 2009;57:1015–1026. doi: 10.1111/j.1365-313X.2008.03744.x. [DOI] [PubMed] [Google Scholar]
- Hanson JB. The functions of calcium in plant nutrition. In: Tinker PB, Lauchli A, editors. Advances in plant nutrition. Vol. 1. New York: Praeger Publishers; 1984. [Google Scholar]
- Hirschi KD. The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiology. 2004;136:2438–2442. doi: 10.1104/pp.104.046490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S, Matsuda Y, Takahashi M, Kakehi K, Ganno S. Fluorimetric determination of reducing carbohydrates with 2-cyanoacetamide and application to automated-analysis of carbohydrates as borate complexes. Analytical Chemistry. 1980;52:1079–1082. [Google Scholar]
- Hsieh MH, Chen JT, Jinn TL, Chen YM, Lin CY. A class of soybean low molecular weight heat shock proteins: immunological study and quantitation. Plant Physiology. 1992;99:1279–1284. doi: 10.1104/pp.99.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai H, Kikuchi A, Kobayashi T, Kamada H, Satoh S. High levels of non-methylesterified pectins and low levels of peripherally located pectins in loosely attached non-embryogenic callus of carrot. Plant Cell Reports. 1999;18:561–566. [Google Scholar]
- Jarvis MC, Briggs SPH, Knox JP. Intercellular adhesion and cell separation in plants. Plant, Cell and Environment. 2003;26:977–989. [Google Scholar]
- Jensen WA. Pectic substances. In: Freeman WH, editor. Botanical histochemistry: principles and practice. California: San Francisco; 1962. pp. 201–202. [Google Scholar]
- Jiang L, Yang SL, Xie LF, Puah CS, Zhang XQ, Yang WC, Sundaresan V, Ye D. VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. The Plant Cell. 2005;17:584–596. doi: 10.1105/tpc.104.027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinn TL, Chang P, Chen YM, Key JL, Lin CY. Tissue-type-specific heat-shock response and immunolocalization of class I low-molecular-weight heat-shock proteins in soybean. Plant Physiology. 1997;114:429–438. doi: 10.1104/pp.114.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinn TL, Chiu CC, Song WW, Chen YM, Lin CY. Azetidine-induced accumulation of class I small heat shock proteins in the soluble fraction provides thermotolerance in soybean seedlings. Plant and Cell Physiology. 2004;45:1759–1767. doi: 10.1093/pcp/pch193. [DOI] [PubMed] [Google Scholar]
- Jinn TL, Wu SH, Yeh CH, Hsieh MH, Yeh YC, Chen YM, Lin CY. Immunological kinship of class I low molecular weight heat shock proteins and thermostabilization of soluble proteins in vitro among plants. Plant and Cell Physiology. 1993;34:1055–1062. [Google Scholar]
- Jinn TL, Yeh YC, Chen YM, Lin CY. Stabilization of soluble proteins in vitro by heat shock proteins-enriched ammonium sulfate fraction from soybean seedlings. Plant and Cell Physiology. 1989;30:463–469. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larkindale J, Knight MR. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiology. 2002;128:682–695. doi: 10.1104/pp.010320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt J. Responses of plants to environmental stresses: chilling, freezing and high temperatures stresses. In: Kozlowski TT, editor. Physiological ecology: a series of monographs, texts, and treatises. Vol. 1. New York: Academic Press; 1980. pp. 23–64. [Google Scholar]
- Lin CY, Chen YM, Key JL. Solute leakage in soybean seedlings under various heat shock regimes. Plant and Cell Physiology. 1985;26:1493–1498. [Google Scholar]
- Lin CY, Roberts JK, Key JL. Acquisition of thermotolerance in soybean seedlings: synthesis and accumulation of heat shock proteins and their cellular localization. Plant Physiology. 1984;74:152–160. doi: 10.1104/pp.74.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liners F, Thibault J-F, Van Cutsem P. Influence of the degree of polymerization of oligogalacturonates and of esterification pattern of pectin on their recognition by monoclonal antibodies. Plant Physiology. 1992;99:1099–1104. doi: 10.1104/pp.99.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheli F. Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends in Plant Science. 2001;6:414–419. doi: 10.1016/s1360-1385(01)02045-3. [DOI] [PubMed] [Google Scholar]
- Moore S, Stein WH. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. Journal of Biological Chemistry. 1954;211:907–913. [PubMed] [Google Scholar]
- O'Neill MA, York WS. The composition and structure of plant primary cell. In: Jocelyn KCR, editor. The plant cell wall. Vol. 8. Oxford: Blackwell; 2003. pp. 1–54. [Google Scholar]
- Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annual Review of Genetics. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- Pelloux J, Rusterucci C, Mellerowicz EJ. New insights into pectin methylesterase structure and function. Trends in Plant Science. 2007;12:267–277. doi: 10.1016/j.tplants.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Pressey R. Extraction and assay of tomato polygalacturonases. Horticultural Science. 1986;21:490–492. [Google Scholar]
- Ren C, Kermode AR. An increase in pectin methyl esterase activity accompanies dormancy breakage and germination of yellow cedar seeds. Plant Physiology. 2000;124:231–242. doi: 10.1104/pp.124.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidi Y, Finka A, Muriset M, Bromberg Z, Weiss YG, Maathuis FJ, Goloubinoff P. The heat shock response in moss plants is regulated by specific calcium-permeable channels in the plasma membrane. The Plant Cell. 2009;21:2829–2843. doi: 10.1105/tpc.108.065318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D, Brownlee C, Harper JF. Communicating with calcium. The Plant Cell. 1999;11:691–706. doi: 10.1105/tpc.11.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma HSS. Effects of the application of chemical additives to desiccated flax on retting. Biotechnology Letters. 1986;8:219–224. [Google Scholar]
- Solecka D, Zebrowski J, Kacperska A. Are pectins involved in cold acclimation and de-acclimation of winter oil-seed rape plants? Annals of Botany. 2008;101:521–530. doi: 10.1093/aob/mcm329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas AJ, Malho R. Ca2+ signalling in plant cells: the big network! Current Opinion in Plant Biology. 1998;1:428–433. doi: 10.1016/s1369-5266(98)80268-9. [DOI] [PubMed] [Google Scholar]
- Tsvetkova NM, Horváth I, Török Z, et al. Small heat-shock proteins regulate membrane lipid polymorphism. Proceedings of the National Academy of Sciences, USA. 2002;99:13504–13509. doi: 10.1073/pnas.192468399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters ER, Vierling E. The diversification of plant cytosolic small heat shock proteins preceded the divergence of mosses. Molecular Biology and Evolution. 1999;16:127–139. doi: 10.1093/oxfordjournals.molbev.a026033. [DOI] [PubMed] [Google Scholar]
- Wehmeyer N, Hernandez LD, Finkelstein RR, Vierling E. Synthesis of small heat-shock proteins is part of the developmental program of late seed maturation. Plant Physiology. 1996;112:747–757. doi: 10.1104/pp.112.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willats WGT, McCartney L, Mackie W, Knox JP. Pectin: cell biology and prospects for functional analysis. Plant Molecular Biology. 2001;47:9–27. [PubMed] [Google Scholar]
- Young DH, Kauss H. Release of calcium from suspension-cultured Glycine max cells by chitosan, other polycations, and polyamines in relation to effects on membrane permeability. Plant Physiology. 1983;73:698–702. doi: 10.1104/pp.73.3.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhou RG, Gao YJ, Zheng SZ, Xu P, Zhang SQ, Sun DY. Molecular and genetic evidence for the key role of AtCaM3 in heat-shock signal transduction in Arabidopsis. Plant Physiology. 2009;149:1773–1784. doi: 10.1104/pp.108.133744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Nieden U, Neumann D, Bucka A, Nover L. Tissue-specific localization of heat-stress proteins during embryo development. Planta. 1995;196:530–538. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.