Abstract
Objectives
Skeletal muscle mitochondrial dysfunction is associated with aging and diabetes, which decreases respiratory capacity and increases reactive oxygen species. Lipoic acid (LA) possesses antioxidative and antidiabetic properties. Metabolic action of LA ismediated by activation of AMP-activated protein kinase (AMPK), a cellular energy sensor that can regulate PGC-1α, a master regulator of mitochondrial biogenesis. We hypothesized that LA improves energy metabolism and mitochondrial biogenesis by enhancing AMPK-PGC-1α signalling in the skeletal muscle of aged mice.
Methods
C57BL/6 mice (24-month old, male) were supplemented with or without α-LA (0.75% in drinking water) for one month. In addition, metabolic action and cellular signalling of LA were studied in cultured mouse myoblastoma C2C12 cells.
Results
LA supplementation improved body composition, glucose tolerance, and energy expenditure in the aged mice. LA increased skeletal muscle mitochondrial biogenesis with increased phosphorylation of AMPK and mRNA expression of PGC-1α and glucose transporter-4 (GLUT-4). Besides body fat mass, LA decreased lean mass and attenuated phosphorylation of mammalian target of rapamycin (mTOR) signalling in the skeletal muscle. In cultured C2C12 cells, LA increased glucose uptake and palmitate β-oxidation, but decreased protein synthesis, which was associated with increased phosphorylation of AMPK and expression of PGC-1α and GLUT-4, and attenuated phosphorylation of mTOR and p70S6 kinase.
Conclusions
We conclude that LA improves skeletal muscle energy metabolism in the aged mouse possibly through enhancing AMPK-PGC-1α-mediated mitochondrial biogenesis and function. Moreover, LA increases lean mass loss possibly by suppressing protein synthesis in the skeletal muscle by down-regulating the mTOR signalling pathway. Thus, LA may be a promising supplement for treatment of obesity and/or insulin resistance in older patients.
Keywords: Lipoic acid, Aging, Mitochondrial biogenesis, Protein synthesis, Energy metabolism
Introduction
Obesity and related metabolic syndrome continue to be a major public health problem in the developed world. Both obesity and insulin resistance increase with aging, which is associated with reduced mitochondrial mass and function, leading to a defective energy homeostasis 1–3. A substantial decline in mitochondrial oxidative capacity in the skeletal muscle may contribute to the whole body aging process 4. A reduction in respiration rate and mitochondria biogenesis accounts for a defective energy expenditure, which predisposes to obesity, type 2 diabetes, and other metabolic consequences 5. Energy metabolism in the skeletal muscle is finely regulated in healthy subjects; however, such regulation may be impaired in aging and diabetes 6. The mechanisms that regulate body composition and energy homeostasis are not fully understood.
Nutrient supplementation has been applied to slow down the aging process and improve the quality of life. Supplemented lipoic acid (LA)4, an essential cofactor in mitochondrial dehydrogenase complexes, might protect against aging-related mitochondrial dysfunction 7, 8, and increases glucose utilization in type 2 diabetes mellitus in vivo 9, 10. Recently, LA is shown to induce body weight loss by inhibiting hypothalamic AMPK activity, resulting from suppressed food intake and stimulated energy expenditure 11. In addition, LA treatment combined with acetyl-carnitine increases ambulatory activity in aged rats 12, and improves mitochondrial function with attenuated oxidative damage 13. Skeletal muscle is a key tissue and a major contributor to whole-body energy homeostasis in humans 14. However, it is unknown whether LA supplementation increases mitochondrial biogenesis and energy metabolism in skeletal muscle of aged mice.
AMPK is a highly conserved, cellular energy sensor. It appears as an intracellular fuel gauge that is activated by a drop in the ATP/AMP ratio 15. One mechanism by which activated AMPK stimulates glucose uptake, fatty acid oxidation, and mitochondrial biogenesis in skeletal muscle is by increasing GLUT-4 and PGC-1α expression 16–18. Furthermore, metformin treatment in type 2 diabetes mellitus activates AMPK, leading to enhanced glucose disposal in skeletal muscle 19. Interestingly, the increase in AMPK activity results in suppressed skeletal muscle protein synthesis. Lipoic acid has been reported to increase AMPK activity in skeletal muscles in diabetes-prone obese rats and in C2C12 myotubes, which is accompanied by improved glucose metabolism and fatty acid oxidation 20, 21. Previous studies focused on LA-mediated anti-oxidative protective effects in aged mice 22, 23, though its metabolic effects in energy metabolism are also evident in obese and/or diabetic mice 24, 25. Considering LA-mediated activation of AMPK, however, it is not known whether LA supplementation facilitates mitochondrial biogenesis, and/or inhibits protein loss in aged mice. Therefore, we hypothesized that α-LA improves energy metabolism and mitochondrial biogenesis by enhancing AMPK-PGC-1α signalling in the skeletal muscle of aged mice. Our objectives in the present study are to determine whether LA-stimulated energy expenditure and protein loss are mediated by activating the AMPK-PGC-1α signalling pathway in the skeletal muscle of aged mice.
Methods
Experiment procedures
All experiments were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine. The C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) were fed ad libitum (standard rodent diet # 2920, Harlan Teklad) and given free access to water for 24 months. Individual male mice (at the age of 24 months) were provided with water supplemented with 0% (n=10, as control group) or 0.75% α-lipoic acid (Sigma-Aldrich, St. Louis, MO; n=10, as treatment group) for 1 month. Body weight was recorded weekly. During the 3rd week of treatment, food intake of mice was recorded daily for one week. After 1 month the mice were killed under isoflurane anaesthesia, and tissues were taken and frozen immediately in liquid nitrogen.
Body composition
During the 4th week of treatment, the mice in the fed status were anesthetized with isoflurane shortly, and then body composition was assessed in vivo by dual-energy X-ray absorptiometry (DXA; Lunar, Madison, WI). Body fat mass, lean mass and bone density were analyzed using GE Lunar PIXImus software version 1.45 (Lunar, Madison, WI).
Indirect calorimetry
During the 4th week of treatment, the mice were housed in individual chambers at 24 ± 1°C with free access to food and water with/without 0.75% α-LA. Oxygen consumption and CO2 production were measured individually over 48 h using the Columbus Instrument Oxymax System (Columbus Instruments, Columbus, OH). Two time points were sampled per hour. Energy expenditure was calculated using the formula provided by the manufacturer: energy expenditure (kcal) = (3.815 + 1.232VO2/VCO2)·VO2.
Glucose tolerance test
Glucose tolerance test (GTT) was performed during the 4th week of treatment. The mice were fasted for 16 h and then were i.p. injected at 2.0 g D-glucose/kg BW. Blood samples were taken at 0, 30, 60 and 90 min post glucose administration. Blood glucose concentrations were measured by the glucose oxidase method using an Ultrasmart™ Glucose Meter (Life-Scan, Milpitas, Canada). The area under the curve was calculated by an integration method.
RNA extraction and quantitative RT-PCR
Total RNA was isolated from skeletal muscles (gastrocnemius muscles) and C2C12 cells using Trizol reagent (Invitrogen, Carlsbad, CA). RNA concentration was determined by NanoDrop 1000 (NanoDrop products, Wilmington, DE). Reverse transcription was performed with 1 μg of RNA as template using SuperScript-III-First-Strand-Synthesis-SuperMix kit (Invitrogen, Carlsbad, CA). An abundance of mRNA was quantified by real-time qRT-PCR. Primers and probes for PGC-1α were based on GenBank Accession No. NM_008904.1 as follows: Forward, 5′-AGAAGCGGGAGTCTGAAAGG-3′; Reverse, 5′-CAGTTCTGTCCGCGTTGTG-3′; Probe, 5′-FAM-AGAAAGCAATTGAAGAGCGCCGTGTG-TAMRA-3′. Primers for GLUT-4 were based on GenBank Accession No. NM_009204 as follows: Forward, 5′-ATGGCTGTCGCTGGTTTCTC-3′; Reverse, 5′-ACCCATGCCGACAATGAAGT-3′; Probe, Sybergreen (Bio-Rad, Hercules, CA). The housekeeping gene 18S ribosomal RNA was not altered, and thus used as an internal control in the study. Assays were performed in triplicate with an ABI Prism 7900 sequence detector (Applied Biosystems Inc., Foster City, CA). Data were normalized to 18S ribosomal RNA (ΔΔCT analysis).
Mitochondrial biogenesis
Mitochondrial biogenesis was determined by the ratio of mitochondrial DNA (mtDNA) to nuclear DNA (nDNA) contents quantified by real-time qPCR as described 26 assuming that nuclear DNA remains constant. DNA was extracted from gastrocnemius muscles samples using UltraPure™ Phenol:Chloroform:Isoamyl Alcohol (25:24:1)(Invitrogen) followed by ethanol precipitation. For each DNA extract, the nuclear gene for ribosomal protein large p0 and the mitochondrial gene cytochrome c oxidase subunit I (CoxI) were quantified individually by real-time qPCR. The specific primers were used as follows: p0 forward, 5′-GCACTTTCGCTTTCTGGAGGGTGT-3′; p0 reverse, 5′-TGACTTGGTTGCTTTGGCGGGATT-3′; CoxI forward, 5′-TCTACTATTCGGAGCCTGAG-3′; and CoxI reverse, 5′-CTACTGATgCTCCTGCATGG-3′. Sybergreen was used as the probe. Data were normalized to the nuclear gene p0 DNA (ΔΔCT analysis).
Protein extraction and western blotting
Proteins were extracted from mouse gastrocnemius muscles and C2C12 cells. Muscle samples were homogenized on ice in RIPA buffer (50 mM Tris-HCl at pH 7.4, 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 mM sodium orthovanadate, 1mM sodium fluoride) and centrifuged at 10,000 g for 15 min at 4 °C. Protein concentration was determined using BCA protein assay kit (Pierce, Rockford, IL) using BSA as a standard. Samples were boiled at 100 °C for 10 min in 2× sample buffer. Total protein (100 μg per sample) was loaded per lane and electrophoresed in running buffer on a 7.5 ~ 12% Tris-glycine SDS-Polyacrylamide gel. Following the SDS PAGE, proteins were transferred to nitrocellulose membrane. After blocking, the membrane was incubated with primary antibodies. Primary antibodies were purchased from Cell Signalling Technology (Danvers, MA): phosphor-AMPK ( Thr172; 1:1000), phospho-mTOR (Ser2448; 1:750), phospho-p70 S6K1 (Thr389; 1:500), phospho-4E-BP1 (Thr37/46; 1:500), total-AMPK (1:1000), total-mTOR (1:1000), total-p70 S6K1 (1:500), and total-4E-BP1 (1:500). After washing, the membrane was incubated with anti-rabbit IgG horseradish-peroxidase-conjugated secondary antibody (Bio-Rad, 1:3000), and reacted with ECL-Plus chemiluminescent detection HRP reagents (Amersham Biosciences, Piscataway, NJ). Western blot images were scanned and analyzed on a Storm 860 PhosphorImager (GE Healthcare). The densitometry of phosphorylatedprotein was normalized to its total protein.
Cell culture
The mouse myoblastoma C2C12 cells were obtained from the American Type Culture Collection (Manassas, VA). Cells were cultured in six-well plates and maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% heat-inactivated fetal bovine serum (Invitrogen), 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were allowed to differentiate in DMEM with 2% horse serum, 100 U/ml penicillin, and 100 μg/ml streptomycin for 3 days before treatment. Each treatment was repeated at least three times. After treatment as indicated, cells were washed twice with PBS prior to protein extraction with the RIPA buffer, or to RNA extraction using Trizol reagent. The samples were then applied for western blotting or real-time qRT-PCR.
[3H]-phenylalanine incorporation assay
Protein synthesis was determined by [3H]-phenylalanine incorporation assay 27. In brief, C2C12 myotubes were grown in 6-well plates and changed to serum-free DMEM for 1 h. C2C12 cells were then treated with α-LA at 1 mM for 0.5, 2, or 24 h. The stimulated cells were pulsed with 2 μCi/ml [3H]-phenylalanine (Sigma-Aldrich) for additional 1 h prior to harvest. After washed with ice-cold PBS, the cells were scraped in 0.5 ml PBS, lysed and precipited in 0.5 ml 20% trichloroacetic acid for 30 min on ice, and centrifuged at 10,000 g for 15 min at 4 °C. The pellets (precipited proteins) were washed twice with 10% trichloroacetic acid, and solubilised in 1 ml of 0.3 N NaOH for 1 h. An aliquot (400 μl) was taken to determine the incorporated radioactivity by liquid scintillation counter (Beckman LS 3801, Fullerton, CA). Incorporation rate of [3H]-phenylalanine into total protein was expressed as DPM/μg protein mass and considered as protein synthesis.
[3H]-2-deoxyglucose uptake
Glucose uptake was determined by [3H]-2-deoxy-glucose uptake with modification 28. In brief, the [3H]-2-deoxy-glucose uptake assay was performed with six-well plates. C2C12 myotubes were washed twice with DMEM, starved for 1 h in serum-free DMEM at 37 °C, and then treated with α-LA at 0 ~ 2 mM. Cells were washed twice with KRH buffer (136 mM NaCl, 4.7 mM KCl, 1.25 mM MgSO4, 1.25 mM CaCl2, 20 mM HEPES [pH 7.4]) without glucose, and then assayed for the glucose uptake for 20 min at 0.5 mM 2-deoxy-glucose containing 1 μCi/ml [3H]-2-deoxy-glucose (Amersham Biosciences). Nonspecific uptake was determined with the presence or absence of 5 μM cytochalasin B. The cells were then solubilised in 1 ml of 0.3 N NaOH for 30 min before aliquots were taken for liquid scintillation counting. Measurements for glucose uptake were corrected for nonspecific uptake. Glucose uptake was expressed as DPM/μg protein mass.
Fatty acid β-oxidation
Fatty acid β-oxidation was determined by [14C]-palmitate assay with modification 29, 30. C2C12 myotubes were grown in T-25 cm2 flasks, starved for 1 h in serum-free DMEM, treated with α-LA at 1 mM for 6 h, and then incubated with 2% BSA and 0.4 mM palmitate (plus 0.25 μCi/ml [14C]-palmitate (Amersham Biosciences)) for 1 h, and finally quenched by adding 2 ml of 6 M HCl. The release of 14CO2 was trapped with 300 μl of 1 N NaOH. The trapped 14CO2 were determined by liquid scintillation counting.
Statistical analysis
Data (body composition, metabolic action, relative abundance of PGC-1α and GLUT-4 mRNA, relative abundance of mtDNA, and phosphorylation of proteins (AMPK, mTOR, P70S6, and 4E-BP1)) were analyzed by ANOVA. Data (glucose concentration, O2 consumption, CO2 production, energy expenditure, and respiratory quotient) were analyzed by repeated measures ANOVA using the MIXED Procedure (SAS Version 9.1, SAS Institute Inc., Cary, NC), in which time of sampling was considered as the repeated effect. The relationship between α-LA dose and glucose uptake was fitted by an exponential regression model. Data were expressed as means ± SEM. P values < 0.05 or 0.01 were considered as statistical significance.
Results
Food intake and body composition
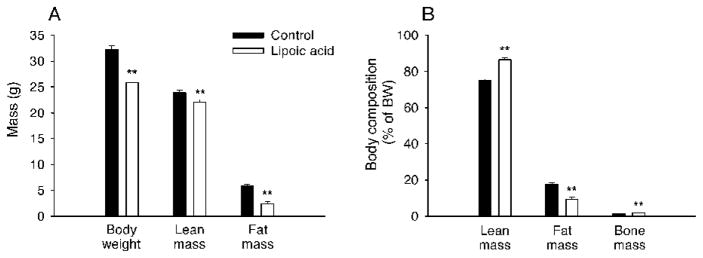
Daily intake of food was decreased (P < 0.05) by 18% in LA-treated mice (4.50 ± 0.30 g/d) compared with that (5.50 ± 0.30 g/d) in control mice. Over the period of four weeks, LA-treated mice lost (P < 0.05) body weight of 5.27 ± 0.62 g (an equivalent to 15.8% of initial body weight) while the control mice maintained their body weight. The LA-treated mice had less adipose as well as lean mass (Fig. 1A). Therefore, the LA-treated mice had lower percentage of fat mass (Fig. 1B), but higher percentage of lean and bone mass.
FIG. 1.
Lipoic acid improved body composition in the aged mice. A: Lipoic acid-treated mice had significantly lower final body weight, fat mass, and lean mass than the control mice. B: lipoic acid decreased body proportion of fat mass, but increased those of lean mass and bone mass. The data were analyzed by ANOVA and expressed as mean ± SEM (n = 10 per group). *P < 0.05; **P < 0.01.
Energy expenditure
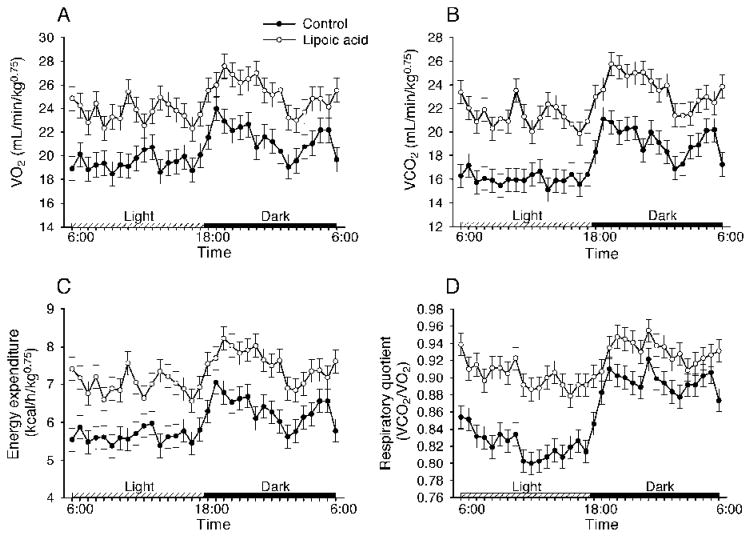
LA-treated mice had higher oxygen consumption (25.7 ± 0.35 mL/kg0.75/min) and CO2 production (23.9 ± 0.37 mL/kg0.75/min) than control mice (20.2 ± 0.35 and 17.2 ± 0.37 for O2 consumption and CO2 production, respectively)(Fig. 2A and 2B). Note that the difference between two groups was significant (P < 0.01) at individual time points in Fig. 2. Calculated energy expenditure was higher (7.64 ± 0.10 kcal/kg0.75/h) in the LA-treated mice than that (5.90 ± 0.10 kcal/kg0.75/h) in the control mice (Fig. 2C). Calculated respiratory quotient (i.e., ratio of VCO2 to VO2) was higher (0.93 ± 0.01) in the LA-treated mice than that (0.85 ± 0.01) in the control mice. However, the difference for respiratory quotient was even more profound during the inactive (light) phase (Fig. 2D), indicating that LA stimulated more glucose utilization.
FIG. 2.
Lipoic acid increased energy expenditure in the aged mice. Energy expenditure was measured with a 30-min interval for 48 h during the 4th week of lipoic acid treatment. A: Lipoic acid treatment increased O2 consumption compared with the control. B: Lipoic acid treatment increased CO2 production compared with the control. C and D: Energy expenditure and respiratory quotient were significantly higher in the lipoic acid-treated mice than those in the control mice during the light and dark cycles and at each sampling time point. Data were analyzed by repeated measures ANOVA using the MIXED Procedure, and expressed as means ± SEM (n = 10 per group). Note that significance symbols (** for P < 0.01) are not shown.
Glucose tolerance
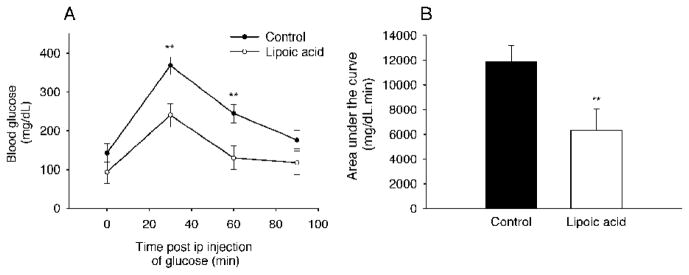
After glucose challenge in overnight fasted mice, blood glucose concentrations were lower at 30 and 60 min (P < 0.01) during a GTT in the LA-treated group than those in the control group (Fig. 3A). Moreover, the area under curve was dramatically reduced by 47% (P < 0.01) in the LA-treated mice (Fig. 3B). These results suggest that LA not only stimulated glucose utilization, but also increased insulin sensitivity.
FIG. 3.
Lipoic acid increased glucose tolerance in the aged mice. Glucose tolerance test was performed in the aged mice fasted for 16 h. D-glucose was administered intraperitoneally at 2 g/kg BW. A: After glucose load, the lipoic acid-treated mice had faster clearance than the control mice. However, no significant difference in basal levels of blood glucose was observed between the groups. B: There was a significant reduction in area under the curve in the lipoic acid-treated mice compared with that in the control mice. Data are presented as mean ± SEM (n = 6 per group). *P < 0.05; **P < 0.01.
LA stimulates PGC-1α and GLUT-4 gene expression in skeletal muscle
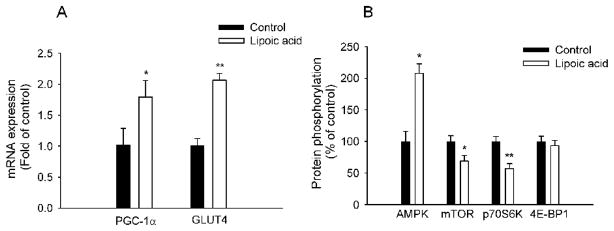
The mRNA abundance for PGC-1α in the skeletal muscle was increased (P < 0.05) by 80.0% in the LA-treated mice compared to that in the control mice (Fig. 4A). Interestingly, the mRNA abundance for GLUT-4 in the skeletal muscle was increased (P < 0.01) by 105.0% in the LA-treated mice as well (Fig. 4A).
FIG. 4.
Lipoic acid altered gene and protein expression in the skeletal muscle. A: Gene expression was measured by real-time qRT-PCR and expressed in terms of mRNA levels relative to 18S rRNA. The lipoic acid-treated mice had significantly elevated mRNA levels of PGC-1α and GLUT-4 in the skeletal muscle. B: Lipoic acid increased AMPK activation with decreased phosphorylation of mTOR and p70S6K; however, it did not significantly alter 4E-BP1 phosphorylation. Data are presented as mean ± SEM (n = 6 per group). *P < 0.05; **P < 0.01.
AMPK and mTOR signalling in the skeletal muscle
To determine whether AMPK accounts for LA-induced metabolic changes, we measured AMPK activation. AMPK phosphorylation at Thr172 increased (P < 0.05) by 107.5 % in response to LA treatment (Fig. 4B). In contrast to AMPK, mTOR phosphorylation at Ser2448 was decreased by 31.5 % and followed by a reduction of p70S6 kinase phosphorylation at Thr389 by 43.0 %. However, there was no difference (P > 0.05) in 4E-BP1 phosphorylation at Thr37/46 between the two groups.
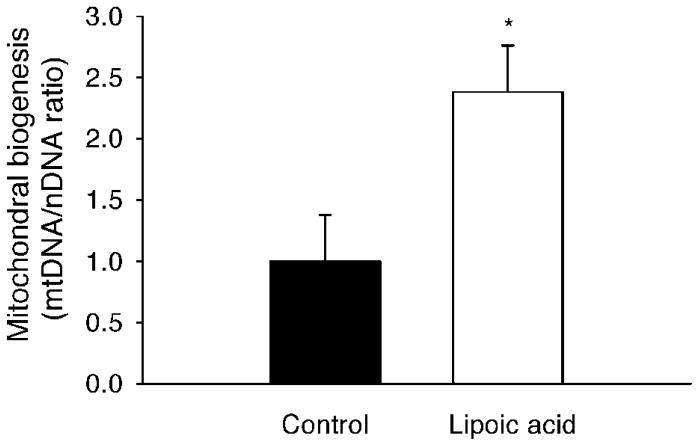
Mitochondrial biogenesis in the skeletal muscle
The relative abundance of mtDNA content (indicated by the mitochondrial gene cytochrome c oxidase subunit I) in the skeletal muscle was increased (P < 0.05) by 138% in the LA-treated mice compared to that in the control mice (Fig. 5).
FIG. 5.
Lipoic acid improved mitochondrial biogenesis in the skeletal muscle. Mitochondrial biogenesis was determined by the ratio between mitochondrial and nuclear DNA contents. The abundance of mitochondrial gene cytochrome c oxidase subunit I (CoxI) was quantified by real-time qPCR and expressed in relative to that of the nuclear gene ribosomal protein large p0. Data are presented as mean ± SEM (n = 6 per group). *P < 0.05.
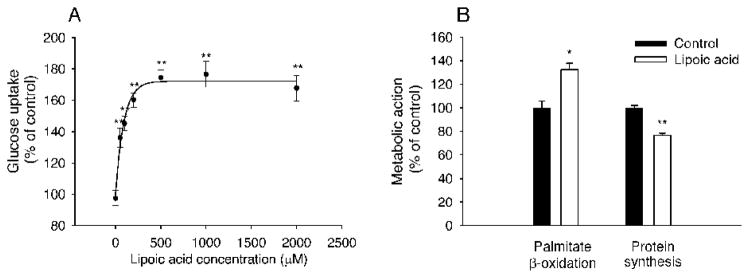
Glucose uptake in C2C12 myotubes
We next evaluated LA-stimulated glucose uptake in C2C12 myotubes using [3H]-deoxy-glucose assay (Fig. 6A). LA dose-dependently stimulated glucose uptake (P < 0.01). Significant stimulation was observed at 50 μM (P < 0.01), and a maximal stimulation was achieved at 1,000 μM (P < 0.01). The LA dosage was estimated at 512.4 μM for glucose uptake plateau.
FIG. 6.
Lipoic acid altered energy metabolism and protein synthesis in differentiated C2C12 cells. A: Lipoic acid dose-dependently increased glucose uptake compared with the control. B: Lipoic acid at 1000 μM increased fatty acid oxidation and decreased protein synthesis compared with the control. Each treatment was repeated at least three times. Data are presented as mean ± SEM. *P < 0.05; **P < 0.01.
Fatty acid β-oxidation in C2C12 myotubes
To further establish whether LA affects fatty acid β-oxidation, we quantified 14C-CO2 released from [14C]-palmitate oxidation upon LA stimulation in C2C12 myotubes. LA at 1000 μM for 6 h increased (P < 0.05) fatty acid β-oxidation by 33.2% compared with the control (Fig. 6B).
Protein synthesis in C2C12 myotubes
Lean mass was decreased in the LA-treated mice, which might be attributed to decreased protein synthesis and/or increased protein degradation. Therefore, we determined whether LA affected protein synthesis in C2C12 myotubes. The time course indicated that at least 24-h time of LA treatment (1 mM) was needed to suppress protein synthesis (data not shown). LA at 1,000 μM for 24 h significantly inhibited (by 24%, P < 0.01) [3H]-phenylalanine incorporation into total protein in C2C12 myotubes (Fig. 6B).
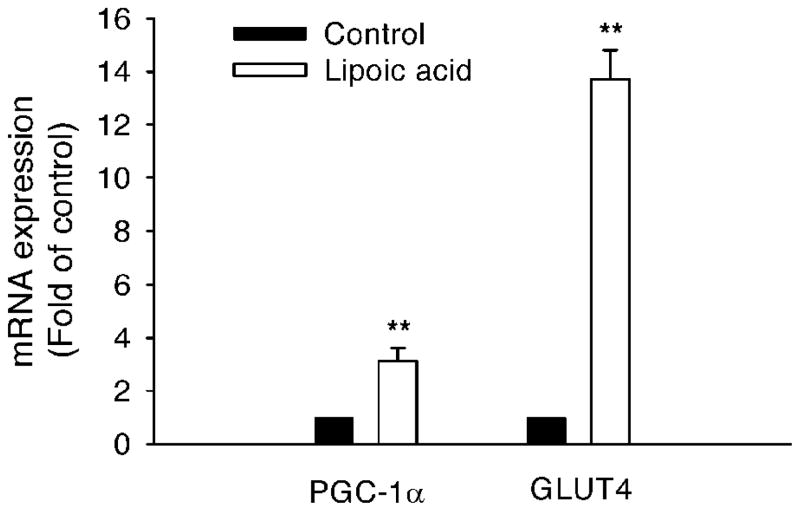
Gene expression in C2C12 myotubes
The mRNA abundance for PGC-1α was increased (P < 0.01) by 2.1 folds in the LA-treated C2C12 myotubes compared to that in the control (Fig. 7). Furthermore, the mRNA abundance for GLUT-4 was increased (P < 0.01) by 13.7 folds in the LA-treated C2C12 myotubes as well (Fig. 7).
FIG. 7.
Lipoic acid altered gene expression in differentiated C2C12 cells. Lipoic acid at 1000 μM for 16 h induced gene expressions in differentiated C2C12 cells. Gene expression was measured by real-time qRT-PCR and expressed in terms of mRNA levels relative to 18S rRNA. Lipoic acid stimulation had significantly elevated mRNA levels of PGC-1α and GLUT-4. Each treatment was repeated at least three times. Data are presented as mean ± SEM. **P < 0.01.
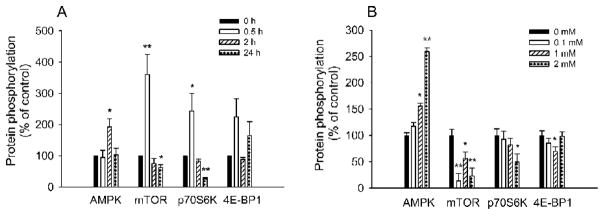
AMPK and mTOR signalling in C2C12 myotubes
To further investigate LA-mediated metabolic action and cellular signalling, we explored LA-mediated effects in C2C12 myotubes. LA treatment (100 ~ 2000 μM for 2 h; or 1,000 μM for 0.5, 2, and 24 h) stimulated phosphorylation of AMPKα at Thr172 in a dose- and time-dependent manner in C2C12 myotubes (Fig. 8). AMPK activation reached its maximum at 2 h post LA treatment. Phosphorylation of mTOR at Ser2448 and its downstream target P70S6 kinase at Thr389 was stimulated at 0.5 h post LA treatment, but was inhibited later on. This inhibited phosphorylation of mTOR and p70S6 kinase was correlated in a time-dependent manner with increased AMPK activity (Fig. 8). Prior to activation of AMPK, phosphorylation of mTOR and p70S6 kinase was increased. After activation of AMPK, however, time- and dose-dependent decreases in mTOR and p70S6 kinase phosphorylation were observed in the LA-treated C2C12 myotubes. However, 4E-BP1 phosphorylation was not altered by time or dosage.
FIG. 8.
Lipoic acid altered protein phosphorylation in a time- and dose-dependent manner in differentiated C2C12 cells. A: Lipoic acid at 1000 μM increased AMPK activation after 2 h treatment; increased phosphorylation of mTOR and p70S6K at 0.5 h; but decreased them after 24-h treatment. However, it did not significantly alter 4E-BP1 phosphorylation. B: Lipoic acid dose-dependently increased AMPK activation with decreased phosphorylation of mTOR and p70S6K; however, it did not significantly alter 4E-BP1 phosphorylation. Each treatment was repeated at least three times. Data are presented as mean ± SEM. *P < 0.05; **P < 0.01.
Discussion
In the present study, we demonstrated that oral intake of α-LA improved body composition, glucose tolerance, and energy expenditure of the aged mice, which was associated with increased mitochondrial biogenesis in the skeletal muscle. It is well known that the aging in humans is associated with increased fat mass, declined lean mass 31, 32, and decreased basal metabolic rate 33. Previous studies have demonstrated that dietary LA increases whole-body energy expenditure in adult mice 34; however, our data are the first to show that LA had the same metabolic action in aged mice. Moreover, this LA-mediated effect on energy metabolism appears to be mediated partially by increased mitochondrial biogenesis in the skeletal muscle. As a result, oral intake of LA might decrease body fat accumulation and increase glucose utilization in the aging mice. However, it should be pointed out that oral intake of LA also increased body protein loss possibly by decreasing protein synthesis. Overall, LA might facilitate the whole-body energy catabolism and glucose utilization in the aged mice.
One of our major findings in the present study is that oral intake of LA increased mitochondrial biogenesis in the skeletal muscle. Brown adipose tissue in small mammals makes the largest contribution towards adaptive thermogenesis. Since humans have relatively little brown fat, however, this effect might thus have limited clinical relevance. In contrast, the skeletal muscle in large adult mammals is a major determinant of energy expenditure 35. Thus, it is critical to establish whether LA has any beneficial outcomes in the skeletal muscle. In agreement with previous reports 36, our data showed that LA activated AMPK in the skeletal muscle. As a cellular energy sensor, activation of AMPK in peripheral tissues stimulates glucose uptake through initiated GLUT-4 translocation and enhanced GLUT-4 expression 37, 38; and increases fatty acid oxidation by inhibiting the activity of acetyl coenzyme A carboxylase 39, 40. We note that peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) is not only a key transcriptional coactivator, but also a major regulator of energy metabolism 41, 42. Forced over-expression of PGC-1α in the skeletal muscle dramatically increases mitochondrial respiration and elevates GLUT-4 transcript levels 43. In contrast, PGC-1α deficient mice have diminished mitochondrial number and respiratory capacity in slow-twitch skeletal muscle 44. Notably, PGC-1α expression is decreased in the skeletal muscle of aged mice 45, 46. In the present study, we show that LA increases mRNA expression of PGC-1α and GLUT-4 in the skeletal muscle of aged mice, which might account for improved glucose homeostasis. Moreover, LA increases the skeletal muscle mitochondrial respiration in aged rats 47. We note that caloric restriction has shown to increase longevity and mitochondrial biogenesis 48. Thus, increased biogenesis of mitochondria could be attributed to LA-specific metabolic action and decreased intake of energy as well. In addition to suppression of AMPK activity in the hypothalamus, however, LA-increased energy expenditure is partially through augmentation of AMPK activity in the skeletal muscle, which increases energy fuel (substrate) oxidation and results in reduced whole-body adiposity. Our data on cultured C2C12 cells not only support this notion, but also agree with those published previously 49. Most recently, AMPK has been shown to stimulate mitochondrial biogenesis through up-regulating PGC-1α 50. Therefore, it appears that LA-mediated metabolic phenomenon is mediated partially through activation of AMPK-PGC-1α signalling in the skeletal muscle.
Lipoic acid-mediated loss of body weight and improvement in glucose tolerance might be related to suppression of food intake. The evidence that LA stimulated energy expenditure even under suppression of food intake, however, indicates LA-mediated metabolic effects probably independent of reduced food intake in the present study. It should be pointed out that LA-mediated metabolic effects might be dose dependent. As demonstrated previously 51, daily intake of dietary LA (0.5~1%, equivalently to ~500 mg/kg BW0.75) not only inhibits food intake, but also stimulates energy expenditure in pair-fed, three-week treatments. In the present study, daily intake of water was estimated at 5.5 mL/d (=BW0.667 × 60 mL/kg BW0.667/d)52. Thus, the daily intake of LA through 0.75% in drinking water would be approximate at 550 mg/kg BW0.75. AMPK, as a metabolic “fuel gauge” that oscillates between anabolic and catabolic metabolism, integrates nutritional and hormonal signals to regulate food intake and energy metabolism. Dominant negative AMPK expression in the hypothalamus is sufficient to reduce food intake and body weight, whereas constitutively active AMPK increases both 53. Importantly, energy expenditure is increased not only by stimulation of AMPK activity in peripheral tissues but also suppression of it in the hypothalamus. Note that the hypothalamus coordinates signals from peripheral tissues to control energy homeostasis. It seems that LA-mediated increase in the whole-body energy expenditure might be mainly due to its modulation of the hypothalamic function. In addition to direct effects on the muscle, LA inhibited the hypothalamic activity of APMK to promote negative energy balance 54. However, further studies are warranted to define LA-mediated central vs. peripheral actions using tissue-specific AMPK deficiency mouse models. Therefore, LA-mediated metabolic effects in the present study might not only result from suppression of food intake, but also stimulation of energy expenditure, which is regulated by LA-mediated AMPK signaling in the hypothalamus and peripheral tissues (such as muscle, adipose tissue, and liver). Notably, there was a reduction in lean mass in LA-treated mice. Due to a greater loss in fat mass, however, lean mass expressed as the percentage of body composition actually increased in the LA-treated mice. It is unclear whether LA-mediated loss of lean mass resulted from increased rates of protein degradation and/or decreased rates of protein synthesis. The mammalian target of rapamycin (mTOR, a nutrient sensor) regulates cell growth and survival and controls protein synthesis. Phosphorylation of both mTOR and p70S6 kinase decreased in the skeletal muscle of the LA-treated mice, probably indicating decreased protein synthesis. In consistent with this, protein synthesis was suppressed in LA-treated C2C12 cells. Importantly, activation of AMPK by AICAR appears to suppress protein synthesis in rat skeletal muscle through down-regulating mTOR signalling 55, 56. Thus, LA might suppress protein synthesis in the skeletal muscle, probably resulting from activated AMPK-mediated suppression of the mTOR signalling pathway. Further studies are warranted to establish how LA down-regulates the mTOR signalling involved in protein and energy metabolism to optimise a LA-based treatment for the management of the metabolic syndrome, which might offer the maximum benefit on energy expenditure and glucose metabolism with the minimum loss of lean body mass.
Body fat mass (especially visceral fat) tends to increase with increasing age, while physical activity energy expenditure tends to decrease. Reduced levels of physical activity energy expenditure might be attributed to obesity in the older persons. LA improved glucose tolerance and energy expenditure (possibly through increasing mitochondrial biogenesis in the skeletal muscle), suggesting that oral intake of LA be able to restore reduced oxidative phosphorylation in the older persons. Thus, the results here may provide further insight into LA-mediated metabolic benefits and potential treatment in older patients who suffer from obesity and/or insulin resistance. However, it would be better to compare LA-mediated beneficial, metabolic effects on aged animals with those on young ones. Thus, a lack of this comparison is a pitfall in the present study. A complete factorial design, i.e., 2 ages (young vs. old) × 2 treatments (vehicle vs. LA) should be employed in future studies to address LA-mediated age-specific beneficial effects if any.
In summary, our present study has demonstrated that LA increased energy expenditure in the skeletal muscle of aged mice partially through enhancing AMPK-PGC-1α-mediated mitochondrial biogenesis and function. Moreover, LA increases lean mass loss possibly by suppressing protein synthesis in the skeletal muscle, which might be attributed to LA-activated AMPK-PGC-1α-mediated down-regulation of the mTOR signalling pathway. With beneficial metabolic actions, LA may be a promising supplement for treatment of obesity and/or insulin resistance in older patients.
Acknowledgments
The authors thank Qiang Tong, Douglas Burrin, Nancy Butte, and Adam Gillum at Baylor College of Medicine for scientific and technical support. This work is supported by the USDA/ARS under Cooperative Agreement No. 6250-51000-043 and NIH Grants 5K01DK75489 (X. G) and HL51586 (L. C). This work is a publication of the United States Department of Agriculture, Agricultural Research Service (USDA/ARS) Children’s Nutrition Research Centre, Department of Paediatrics, Baylor College of Medicine and Texas Children’s Hospital, Houston, TX. The contents of this publication do not necessarily reflect the views or policies of the U.S. Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Abbreviations used: AICAR, 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside; AMPK, AMP-activated protein kinase; DXA, dual-energy X-ray absorptiometry; 4E-BP1, eukaryotic initiation factor 4E-binding protein 1; GLUT-4, glucose transporter-4; GTT, glucose tolerance test; LA, lipoic acid; mtDNA, mitochondrial DNA; mTOR, mammalian target of rapamycin; nDNA, nuclear DNA; PGC-1α, peroxisome proliferator–activated receptor-γ coactivator-1α
USDA/ARS Children’s Nutrition Research Center where the work was done.
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Civitarese AE, Ravussin E. Mitochondrial energetics and insulin resistance. Endocrinology. 2008;149:950–954. doi: 10.1210/en.2007-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang YC, Colditz GA, Kuntz KM. Forecasting the obesity epidemic in the aging U.S. population. Obesity (Silver Spring) 2007;15:2855–2865. doi: 10.1038/oby.2007.339. [DOI] [PubMed] [Google Scholar]
- 4.Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair KS. Aging muscle. Am J Clin Nutr. 2005;81:953–963. doi: 10.1093/ajcn/81.5.953. [DOI] [PubMed] [Google Scholar]
- 6.Attie AD, Kendziorski CM. PGC-1alpha at the crossroads of type 2 diabetes. Nat Genet. 2003;34:244–245. doi: 10.1038/ng0703-244. [DOI] [PubMed] [Google Scholar]
- 7.Liu J. The effects and mechanisms of mitochondrial nutrient alpha-lipoic acid on improving age-associated mitochondrial and cognitive dysfunction: an overview. Neurochem Res. 2008;33:194–203. doi: 10.1007/s11064-007-9403-0. [DOI] [PubMed] [Google Scholar]
- 8.Palaniappan AR, Dai A. Mitochondrial ageing and the beneficial role of alpha-lipoic acid. Neurochem Res. 2007;32:1552–1558. doi: 10.1007/s11064-007-9355-4. [DOI] [PubMed] [Google Scholar]
- 9.Konrad T, Vicini P, Kusterer K, Hoflich A, Assadkhani A, Bohles HJ, Sewell A, Tritschler HJ, Cobelli C, Usadel KH. alpha-Lipoic acid treatment decreases serum lactate and pyruvate concentrations and improves glucose effectiveness in lean and obese patients with type 2 diabetes. Diabetes Care. 1999;22:280–287. doi: 10.2337/diacare.22.2.280. [DOI] [PubMed] [Google Scholar]
- 10.Jacob S, Ruus P, Hermann R, Tritschler HJ, Maerker E, Renn W, Augustin HJ, Dietze GJ, Rett K. Oral administration of RAC-alpha-lipoic acid modulates insulin sensitivity in patients with type-2 diabetes mellitus: a placebo-controlled pilot trial. Free Radic Biol Med. 1999;27:309–314. doi: 10.1016/s0891-5849(99)00089-1. [DOI] [PubMed] [Google Scholar]
- 11.Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, Yun JY, Namgoong IS, Ha J, Park IS, Lee IK, Viollet B, Youn JH, Lee HK, Lee KU. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med. 2004;10:727–733. doi: 10.1038/nm1061. [DOI] [PubMed] [Google Scholar]
- 12.Hagen TM, Liu J, Lykkesfeldt J, Wehr CM, Ingersoll RT, Vinarsky V, Bartholomew JC, Ames BN. Feeding acetyl-L-carnitine and lipoic acid to old rats significantly improves metabolic function while decreasing oxidative stress. Proc Natl Acad Sci U S A. 2002;99:1870–1875. doi: 10.1073/pnas.261708898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Head E, Gharib AM, Yuan W, Ingersoll RT, Hagen TM, Cotman CW, Ames BN. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha-lipoic acid. Proc Natl Acad Sci U S A. 2002;99:2356–2361. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 15.Hardie DG, Sakamoto K. AMPK: a key sensor of fuel and energy status in skeletal muscle. Physiology (Bethesda ) 2006;21:48–60. doi: 10.1152/physiol.00044.2005. [DOI] [PubMed] [Google Scholar]
- 16.Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol. 1999;277:E1–10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- 18.Jessen N, Pold R, Buhl ES, Jensen LS, Schmitz O, Lund S. Effects of AICAR and exercise on insulin-stimulated glucose uptake, signaling, and GLUT-4 content in rat muscles. J Appl Physiol. 2003;94:1373–1379. doi: 10.1152/japplphysiol.00250.2002. [DOI] [PubMed] [Google Scholar]
- 19.Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, Moller DE, Thorell A, Goodyear LJ. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- 20.Lee WJ, Song KH, Koh EH, Won JC, Kim HS, Park HS, Kim MS, Kim SW, Lee KU, Park JY. Alpha-lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochem Biophys Res Commun. 2005;332:885–891. doi: 10.1016/j.bbrc.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 21.Shen QW, Zhu MJ, Tong J, Ren J, Du M. Ca2+/calmodulin-dependent protein kinase kinase is involved in AMP-activated protein kinase activation by alpha-lipoic acid in C2C12 myotubes. Am J Physiol Cell Physiol. 2007;293:C1395–C1403. doi: 10.1152/ajpcell.00115.2007. [DOI] [PubMed] [Google Scholar]
- 22.Sethumadhavan S, Chinnakannu P. Carnitine and lipoic acid alleviates protein oxidation in heart mitochondria during aging process. Biogerontology. 2006;7:101–109. doi: 10.1007/s10522-006-6497-8. [DOI] [PubMed] [Google Scholar]
- 23.Suh JH, Shigeno ET, Morrow JD, Cox B, Rocha AE, Frei B, Hagen TM. Oxidative stress in the aging rat heart is reversed by dietary supplementation with (R)-(alpha)-lipoic acid. FASEB J. 2001;15:700–706. doi: 10.1096/fj.00-0176com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee WJ, Song KH, Koh EH, Won JC, Kim HS, Park HS, Kim MS, Kim SW, Lee KU, Park JY. Alpha-lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochem Biophys Res Commun. 2005;332:885–891. doi: 10.1016/j.bbrc.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 25.Song KH, Lee WJ, Koh JM, Kim HS, Youn JY, Park HS, Koh EH, Kim MS, Youn JH, Lee KU, Park JY. alpha-Lipoic acid prevents diabetes mellitus in diabetes-prone obese rats. Biochem Biophys Res Commun. 2005;326:197–202. doi: 10.1016/j.bbrc.2004.10.213. [DOI] [PubMed] [Google Scholar]
- 26.Schild L, Dombrowski F, Lendeckel U, Schulz C, Gardemann A, Keilhoff G. Impairment of endothelial nitric oxide synthase causes abnormal fat and glycogen deposition in liver. Biochim Biophys Acta. 2008;1782:180–187. doi: 10.1016/j.bbadis.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Reiter AK, Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMPK represses TOP mRNA translation but not global protein synthesis in liver. Biochem Biophys Res Commun. 2008;374:345–350. doi: 10.1016/j.bbrc.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di SN, Di NF, Marzioni D, Castellucci M, Sanguinetti M, D’Ippolito S, Caruso A. RESISTIN MODULATES GLUCOSE UPTAKE AND GLUCOSE TRANSPORTER-1 (GLUT-1) EXPRESSION IN TROPHOBLAST CELLS. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di SN, Di NF, Marzioni D, Castellucci M, Sanguinetti M, D’Ippolito S, Caruso A. RESISTIN MODULATES GLUCOSE UPTAKE AND GLUCOSE TRANSPORTER-1 (GLUT-1) EXPRESSION IN TROPHOBLAST CELLS. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen QW, Zhu MJ, Tong J, Ren J, Du M. Ca2+/calmodulin-dependent protein kinase kinase is involved in AMP-activated protein kinase activation by alpha-lipoic acid in C2C12 myotubes. Am J Physiol Cell Physiol. 2007;293:C1395–C1403. doi: 10.1152/ajpcell.00115.2007. [DOI] [PubMed] [Google Scholar]
- 31.Lee WJ, Song KH, Koh EH, Won JC, Kim HS, Park HS, Kim MS, Kim SW, Lee KU, Park JY. Alpha-lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochem Biophys Res Commun. 2005;332:885–891. doi: 10.1016/j.bbrc.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 32.Novak LP. Aging, total body potassium, fat-free mass, and cell mass in males and females between ages 18 and 85 years. J Gerontol. 1972;27:438–443. doi: 10.1093/geronj/27.4.438. [DOI] [PubMed] [Google Scholar]
- 33.Vaughan L, Zurlo F, Ravussin E. Aging and energy expenditure. Am J Clin Nutr. 1991;53:821–825. doi: 10.1093/ajcn/53.4.821. [DOI] [PubMed] [Google Scholar]
- 34.Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, Yun JY, Namgoong IS, Ha J, Park IS, Lee IK, Viollet B, Youn JH, Lee HK, Lee KU. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med. 2004;10:727–733. doi: 10.1038/nm1061. [DOI] [PubMed] [Google Scholar]
- 35.Zurlo F, Larson K, Bogardus C, Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest. 1990;86:1423–1427. doi: 10.1172/JCI114857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee WJ, Song KH, Koh EH, Won JC, Kim HS, Park HS, Kim MS, Kim SW, Lee KU, Park JY. Alpha-lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochem Biophys Res Commun. 2005;332:885–891. doi: 10.1016/j.bbrc.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 37.Ojuka EO, Jones TE, Nolte LA, Chen M, Wamhoff BR, Sturek M, Holloszy JO. Regulation of GLUT4 biogenesis in muscle: evidence for involvement of AMPK and Ca(2+) Am J Physiol Endocrinol Metab. 2002;282:E1008–E1013. doi: 10.1152/ajpendo.00512.2001. [DOI] [PubMed] [Google Scholar]
- 38.Jorgensen SB, Treebak JT, Viollet B, Schjerling P, Vaulont S, Wojtaszewski JF, Richter EA. Role of AMPKalpha2 in basal, training-, and AICAR-induced GLUT4, hexokinase II, and mitochondrial protein expression in mouse muscle. Am J Physiol Endocrinol Metab. 2007;292:E331–E339. doi: 10.1152/ajpendo.00243.2006. [DOI] [PubMed] [Google Scholar]
- 39.Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol. 1997;273:E1107–E1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- 40.Kurth-Kraczek EJ, Hirshman MF, Goodyear LJ, Winder WW. 5′ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes. 1999;48:1667–1671. doi: 10.2337/diabetes.48.8.1667. [DOI] [PubMed] [Google Scholar]
- 41.Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michael LF, Wu Z, Cheatham RB, Puigserver P, Adelmant G, Lehman JJ, Kelly DP, Spiegelman BM. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci U S A. 2001;98:3820–3825. doi: 10.1073/pnas.061035098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodriguez-Calvo R, Jove M, Coll T, Camins A, Sanchez RM, Alegret M, Merlos M, Pallas M, Laguna JC, Vazquez-Carrera M. PGC-1beta down-regulation is associated with reduced ERRalpha activity and MCAD expression in skeletal muscle of senescence-accelerated mice. J Gerontol A Biol Sci Med Sci. 2006;61:773–780. doi: 10.1093/gerona/61.8.773. [DOI] [PubMed] [Google Scholar]
- 46.Hepple RT, Baker DJ, McConkey M, Murynka T, Norris R. Caloric restriction protects mitochondrial function with aging in skeletal and cardiac muscles. Rejuvenation Res. 2006;9:219–222. doi: 10.1089/rej.2006.9.219. [DOI] [PubMed] [Google Scholar]
- 47.Kumaran S, Panneerselvam KS, Shila S, Sivarajan K, Panneerselvam C. Age-associated deficit of mitochondrial oxidative phosphorylation in skeletal muscle: role of carnitine and lipoic acid. Mol Cell Biochem. 2005;280:83–89. doi: 10.1007/s11010-005-8234-z. [DOI] [PubMed] [Google Scholar]
- 48.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 49.Shen QW, Zhu MJ, Tong J, Ren J, Du M. Ca2+/calmodulin-dependent protein kinase kinase is involved in AMP-activated protein kinase activation by alpha-lipoic acid in C2C12 myotubes. Am J Physiol Cell Physiol. 2007;293:C1395–C1403. doi: 10.1152/ajpcell.00115.2007. [DOI] [PubMed] [Google Scholar]
- 50.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, Yun JY, Namgoong IS, Ha J, Park IS, Lee IK, Viollet B, Youn JH, Lee HK, Lee KU. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med. 2004;10:727–733. doi: 10.1038/nm1061. [DOI] [PubMed] [Google Scholar]
- 52.Tordoff MG, Bachmanov AA, Reed DR. Forty mouse strain survey of water and sodium intake. Physiol Behav. 2007;91:620–631. doi: 10.1016/j.physbeh.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 54.Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, Yun JY, Namgoong IS, Ha J, Park IS, Lee IK, Viollet B, Youn JH, Lee HK, Lee KU. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med. 2004;10:727–733. doi: 10.1038/nm1061. [DOI] [PubMed] [Google Scholar]
- 55.Pruznak AM, Kazi AA, Frost RA, Vary TC, Lang CH. Activation of AMP-activated protein kinase by 5-aminoimidazole-4-carboxamide-1-beta-D-ribonucleoside prevents leucine-stimulated protein synthesis in rat skeletal muscle. J Nutr. 2008;138:1887–1894. doi: 10.1093/jn/138.10.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]