Abstract
Objective
This study investigated whether high central adiposity was associated with pre-diabetes and decreased insulin sensitivity (IS) in both normal weight (BMI < 23 kg/m2) and overweight (BMI ≥ 23) rural Chinese women.
Methods
Adipose variables measured by dual energy X-ray absorptiometry (DEXA) (Percent body fat -%BF, percent lower body fat -%LF and percent trunk fat -%TF) and general adipose variables (BMI and waist circumference - WC) were used for examining the association of adiposity with pre-diabetes among 4,071 rural Chinese females aged 20–60 years. Also, the association of adiposity with IS was tested in both normal and overweight women with normal glucose tolerance (NGT).
Results
BMI was highly correlated with %BF and WC, but weakly correlated with %LF and %TF. Both high %TF (top quartile of %TF) and low %LF (lower three quartiles of %LF) were associated with higher prevalence of pre-diabetes in both normal and overweight women. Compared to normal weight women in low %TF, the odds of pre-diabetes were similarly increased for women with high %TF regardless of whether they were overweight (OR (95% CI) = 1.6 (1.3–2.0)) or not (OR (95% CI) = 1.5 (1.2–2.0)). Similarly, among 3,280 women with NGT, high %TF was associated with increased fasting insulin, 2H-OGTT insulin and HOMA-IR regardless of normal or overweight.
Conclusions
Among relatively lean, rural Chinese women, high %TF was associated with increased odds of pre-diabetes and lower IS regardless of normal or overweight.
Keywords: body fat, adiposity, pre-diabetes, insulin sensitivity, Chinese female
INTRODUCTION
Many studies indicated that individuals with pre-diabetes have higher lifetime risks of developing type 2 diabetes or cardiovascular disease (CVD).[1,2] Furthermore, preventive interventions are able to prevent diabetes or CVD development from the pre-diabetes in non-Asian [3–6], and Asian populations.[7] It is thus very crucial to explore the potential risk factors for pre-diabetes. Obesity is a serious public health problem worldwide. Obesity increases the risks of metabolic syndrome (MS), type II diabetes, hypertension, dyslipidemia and cardiovascular disease.[8–12] A key link between obesity and metabolic diseases may be that obesity increases insulin resistance (IR) [13], particularly when adiposity is centrally located. Visceral fat accumulation increased the risk of MS, HOMA-IR and insulin concentrations in Japanese men with pre-diabetes.[14] Even in 20 normal weight (BMI<25 kg/m2) Japanese individuals with normal glucose tolerance (NGT), increased visceral fat was associated with IR.[15] It is noted that Asian females have higher amounts of visceral fat than Caucasians for a given BMI.[16,17] Such ethnic differences in fat distribution warrant further investigation since the relationships between adiposity and IR observed in non-Asian populations may or may not apply to Asians.
To date, there are a few published studies on MS in Chinese populations [12,18], but none of them has evaluated total and central adiposity in relation to pre-diabetes or insulin sensitivity (IS). To our knowledge, only one study explored the relationship of central adiposity with IS in Asian populations with BMI < 25 and NGT [15]. This is the first study that simultaneously evaluated surrogate adiposity measurements (BMI, waist circumference (WC)) and direct adiposity measures derived from dual-energy X-ray absorptiometry (DEXA) in relation to pre-diabetes or IS in both normal weight (BMI < 23 kg/m2) and overweight (BMI ≥ 23 kg/m2) rural Chinese women.
The primary aim of this cross-sectional study was to examine whether adiposity, particularly central adiposity, was independently associated with pre-diabetes and decreased IS among both normal weight (BMI < 23 kg/m2) and overweight (BMI ≥ 23 kg/m2) women. The second aim focused on if DEXA measures of adiposity could better predict the risk assessment of pre-diabetes and IS than BMI and WC.
RESEARCH DESIGN AND METHODS
Study Population
The study population was described in detail elsewhere [19]. Briefly, this study was conducted in Anhui Province, China from September 1998 to May 2000. The study population was derived from a large, community-based twin study to examine environmental and genetic risk factors of chronic diseases. Twins were chosen based on the following criteria: 1. age 6 years or older; 2. both twins were available for the survey; 3. both twins (or parents/guardians of children) consented to participate in the survey; 4. no history of stroke or cardiovascular, renal, hepatic or malignant diseases; and 5. not breastfeeding or pregnant. Written informed consent was obtained from each subject or parents/guardians of children prior to any data collection. This study was reviewed and approved by the Institutional Review Boards of Children’s Memorial Hospital and the Biomedical Institute of Anhui Medical University.
Eligible twins were invited to a central office to complete a questionnaire interview, oral glucose tolerance testing (OGTT), DEXA scan, and physical exam including anthropometric measures. Subjects were included in this analysis if they met the following criteria: 1. females aged 20–60 years; 2. completion of both OGTT and DEXA scan; 3. absence of diabetes (defined as 2 hr OGTT ≥ 11.1 mmol/L or fasting glucose ≥ 7.0 mmol/L) and other severe diseases (stroke or cardiovascular, renal, hepatic or malignant diseases); 4. did not previously or currently smoke cigarettes; 5. BMI was equal or greater than 18.5 kg/m2.
Anthropometric and DEXA measures of adiposity
Body mass, standing height and WC were measured according to standard protocols. Body fat was measured using DEXA (GE-lunar Prodigy, Waukesha, WI, USA) according to the standard operating protocol. BMI was calculated as the ratio of body mass (kg) divided by the square of height (m). Percentage body fat (%BF) was calculated as 100 times whole body fat mass divided by whole body mass. Percentage truncal-to-total fat (%TF) was calculated as 100 times truncal fat mass divided by whole body fat mass. Percentage lower body-to-total fat (%LF) was calculated as whole body fat mass minus head, two upper extremities and truncal fat mass, and then divided by whole body fat mass and times 100.
Plasma insulin and glucose assays and HOMA-IR calculation
Plasma glucose concentrations were measured by the glucose oxidase method with an automated biochemical analyzer (Model 7020, Hitachi Company, Japan). Plasma insulin concentrations were determined with electro-chemiluminescence immunoassay (Model 2010, Roche), for which insulin mean CV% of intra- and inter-assays were 2.0 and 5.96%, respectively. HOMA-IR, a marker of IS, was calculated as fasting insulin concentration (mU/L) times fasting glucose concentration (mmol/L) divided by 22.5 [20,21].
Definition for NGT, impaired fasting glucose (IFG), impaired glucose tolerance (IGT) and pre-diabetes
NGT was defined as fasting glucose: <5.6 mmol/L and 2H-OGTT glucose: <7.8 mmol/L [22]. Pre-diabetes was defined as IFG - fasting glucose: 5.6~7.0 mmol/L [22] and/or IGT - 2H-OGTT glucose: 7.8~11.1 mmol/L [23].
Statistical analysis
For descriptive statistics, discrete and continuous variables were expressed as frequencies and percentages, and means and standard deviations, respectively. T tests and chi squared tests were used to investigate differences in means and prevalence of continuous and categorical variables respectively among women with NGT and pre-diabetes. Plasma insulin concentrations at fasting and 2H-OGTT were transformed to the natural log (ln) scale because they had skewed distributions. Because BMI≥23 kg/m2 is the World Health Organization definition of overweight for Chinese adults and WC≥80 cm increases the risk of metabolic syndrome in Asian women [24], the cut points of BMI≥23 kg/m2 and WC≥80 cm were used to define binary categories of high BMI and high WC, respectively. High %LF and high %TF were defined as the upper quartile of each while the bottom three quartiles of each were defined as “low.” The binary variables of BMI, WC, %LF and %TF were used in further analyses.
We conducted the analysis in the following sequential steps to examine if central adiposity was independently associated with pre-diabetes and decreased insulin sensitivity (IS) in normal and overweight women. First, we used LOESS smoothing to plot %BF, %TF, %LF and WC against BMI to examine their correlations. We then plotted the prevalence of pre-diabetes against BMI, stratified by low versus high %TF and %LF, respectively to examine whether high %TF or %LF were associated with the prevalence of pre-diabetes for a given BMI. Furthermore, we used logistic regression to examine how the odds of pre-diabetes compared in four groups of women defined by the combination of their BMI (normal or overweight) and central adiposity (low or high %TF).
Second, we explored the associations of central adiposity with IS among women with NGT. We plotted HOMA-IR against BMI stratified by low versus high %TF and %LF, respectively, to examine if high %TF and/or high %LF were associated with IS for a given BMI. Furthermore, we used multiple linear regression to examine how mean levels of ln(fasting insulin), HOMA-IR, 2H-OGTT ln(insulin) and 2H-OGTT glucose pre-diabetes compared in four groups of women defined by the combination of their BMI (normal or overweight) and central adiposity (low or high %TF).
All regression models described above were adjusted for age (continuous variable), age2, education (primary school, middle school, greater than middle school versus illiterate), occupation (farmer versus not farmer), previous alcohol use (yes versus no), and current alcohol use (yes versus no). For all analyses, twins were treated as individual observations rather than twin pairs. To account for the correlation within-twin pair measures, we calculated robust estimates of variances with generalized estimating equations (GEE) using SAS procedure GENMOD (SAS Institute, Cary, NC, USA) [25]. We also repeated the analyses mentioned above, using only one randomly selected subject per family.
RESULTS
Population characteristics by pre-diabetes status
4,071 women (3,280 with NGT and 791 with pre-diabetes) were included in this study. The distributions of important variables by pre-diabetes status were shown in Table 1. Women with pre-diabetes were older, had higher %BF, WC, %TF, ln(fasting insulin), fasting glucose, HOMA-IR, 2H-OGTT ln(insulin) and 2H-OGTT glucose, and lower %LF than those with NGT. Women with pre-diabetes had slightly less education and were slightly more likely to be farmers. Prevalence of alcohol use was low and not different between the two groups.
Table 1.
The general characteristics among 4071 Chinese women twins aged 20~60 years by normal glucose tolerance (NGT) and pre-diabetes.
| Variables | NGT (n=3280) | Pre-diabetes a (n=791) | P |
|---|---|---|---|
| Mean ± S.D. | |||
| Age, year | 31.7 ± 7.5 | 33.7 ± 8.6 | <0.0001 |
| Height, m | 1.5 ± 0.1 | 1.5 ± 0.1 | 0.0920 |
| Weight, kg | 52.5 ± 6.9 | 53.4 ± 7.5 | 0.0014 |
| BMI, kg/m2 | 22.2 ± 2.5 | 22.7 ± 2.8 | <0.0001 |
| Whole body fat, kg | 13.9 ± 4.7 | 14.6 ± 5.2 | 0.0002 |
| %whole body fat b | 25.9 ± 6.0 | 26.8 ± 6.3 | 0.0002 |
| Truncal fat, kg | 7.0 ± 2.7 | 7.6 ± 3.0 | <0.0001 |
| %Truncal fat c | 49.8 ± 4.4 | 50.9 ± 4.3 | <0.0001 |
| Lower body fat, kg | 4.5 ± 1.4 | 4.6 ± 1.4 | 0.0981 |
| %Lower body fat d | 33.3 ± 5.0 | 32.3 ± 4.9 | <0.0001 |
| Waist circumference (WC), cm | 72.2 ± 7.3 | 74.3 ± 8.1 | <0.0001 |
| Fasting insulin, mU/L | 6.7 ± 6.2 | 7.6 ± 9.1 | 0.0097 |
| Fasting glucose, mmol/L | 4.4 ± 0.6 | 5.7 ± 0.8 | <0.0001 |
| HOMA-1e | 1.3 ± 1.2 | 1.9 ± 2.5 | <0.0001 |
| 2H-OGTT insulin, mU/L | 24.2 ± 23.3 | 28.1 ± 26.9 | 0.0001 |
| 2H-OGTT glucose, mmol/L | 5.2 ± 1.1 | 7.0 ± 1.9 | <0.0001 |
| N (%) | |||
| Education | |||
| Illiterate | 1256(38.3) | 343(43.4) | 0.0050 |
| Primary School | 1067(32.5) | 265(33.5) | |
| Middle School | 745(22.7) | 140(17.7) | |
| Higher Middle School | 212(6.5) | 43(5.4) | |
| Farmer | 2505(76.4) | 639(80.8) | 0.0080 |
| Previous Alcohol Use | 36(1.1) | 12(1.5) | 0.3270 |
| Current Alcohol Use | 79(2.4) | 23(2.9) | 0.4200 |
NGT, normal glucose tolerance; pre-diabetes: fasting glucose: 5.6~7.0 mmol/L and/or 2H-OGTT glucose: 7.8~11.1 mmol/L;
% whole body fat (%BF) = 100 × (whole body fat) ÷ Weight;
%Truncal fat (%TF)= 100 × (Truncal fat) ÷ Whole body fat;
%Lower body fat (%LF)=100 × (whole body fat-truncal fat-upper extremities fat-head fat ) ÷ whole body fat;
HOMA-1 = fast insulin × fast glucose ÷ 22.5, where the unit of fasting glucose and fasting insulin is mmol/L and mU/L, respectively;
P value was generated by t-test for continuous variables and Chi square test for categorical variables.
Associations of central and lower adiposity with pre-diabetes
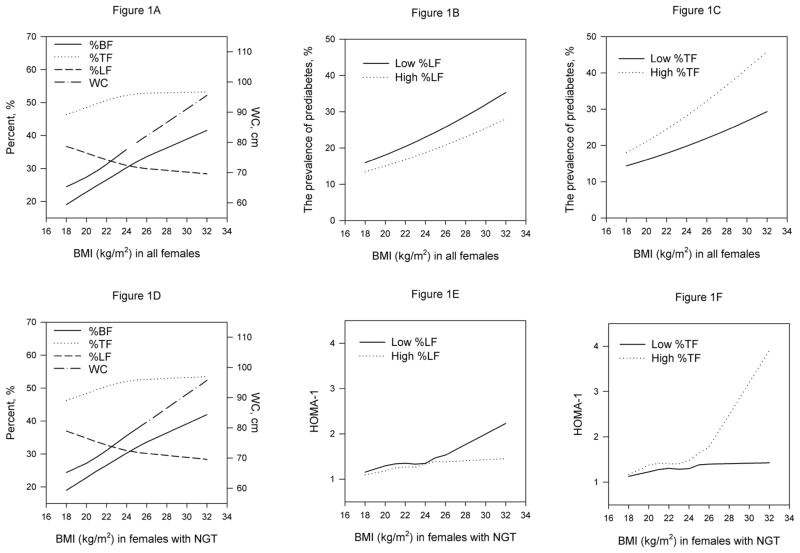
Seventeen percent of 2,739 women with normal weight (BMI < 23kg/m2) had pre-diabetes compared to 23% of 1,332 overweight women (data not shown). Sixteen percent of normal weight women versus 43% of overweight women had high %TF (data not shown). Figure 1A plotted the means of %BF, %TF, %LF and WC against BMI in 4071 females. Overall, BMI was positively associated with %BF, %TF and WC, negatively with %LF. However, BMI was more highly correlated with %BF (correlation coefficient r=0.75) and WC (r=0.77), but weakly with %TF (r=0.43) and %LF (r=−0.41) in this population. The correlation of WC and %TF with %BF (correlation coefficient r=0.67 for WC and 0.47 for %TF) were similar to those of %TF and WC with BMI.
Figure 1.
%BF, %TF, %LF and WC plotted against BMI among 4071 women (A) and 3280 women with normal glucose tolerance (NGT) (D); prevalence of pre-diabetes plotted against BMI stratified by %LF (B) and %TF (C) among all women; and HOMA-1 plotted against BMI stratified by %LF (E) and %TF (F) among women with NGT.
Figure 1B shows that given BMI, women who had high %LF had lower prevalence of pre-diabetes. Conversely, women with high %TF had higher prevalence of pre-diabetes, given BMI (figure 1C). Furthermore, the linear correlation coefficients of BMI and pre-diabetes among females with low and high %LF were similar (0.011 and 0.010, respectively); those among females with low and high %TF were also similar (0.010 and 0.007, respectively). Table 2 shows that compared to normal weight women with low %TF, the odds of pre-diabetes in normal weight women with high %TF (OR (95%CI) = 1.5 (1.2–2.0)) were similar to those in overweight women with high %TF (OR (95%CI) = 1.6 (1.3–2.0)). While similar patterns of association were found for WC, they were not as consistent or significant as those for %TF.
Table 2.
Relative odds of pre-diabetes a by BMI and central adiposity (%TF and WC) groups among 4071 Chinese women twins aged 20–60 years.
| Adiposity | Total | Pre-diabetes | Joint associationb |
Stratified by BMIc |
|||
|---|---|---|---|---|---|---|---|
| N | (%) | OR (95%CI) | P | OR (95%CI) | P | ||
| BMI | %TF d | ||||||
| <23 | Low | 2295 | 377(16.4) | 1.0 | -- | 1.0 | -- |
| High | 444 | 107(24.1) | 1.5(1.2–2.0) | 0.0012 | 1.5(1.2–2.0) | 0.0011 | |
| ≥23 | Low | 758 | 159(21.0) | 1.3(1.0–1.6) | 0.0198 | 1.0 | -- |
| High | 574 | 148(25.8) | 1.6(1.3–2.0) | 0.0001 | 1.2(0.9–1.6) | 0.1656 | |
| BMI | WC | ||||||
| <23 | <80 | 2656 | 461(17.4) | 1.0 | -- | 1.0 | -- |
| ≥80 | 83 | 23(27.7) | 1.5(0.9–2.6) | 0.1211 | 1.5(0.9–2.5) | 0.1294 | |
| ≥23 | <80 | 732 | 142(19.4) | 1.1(0.9–1.4) | 0.2986 | 1.0 | -- |
| ≥80 | 600 | 165(27.5) | 1.6(1.3–2.0) | <0.0001 | 1.4(1.1–1.9) | 0.0115 | |
Adjusted for age, age2, previous and current alcohol drinking, education, occupation and correlations among twin pairs.
Pre-diabetes was defined as fasting glucose: 5.6~7.0 mmol/L and/or 2H-OGTT glucose: 7.8~11.1 mmol/L.
Joint associations of BMI and central adiposity (%TF and WC) on the risk of pre-diabetes.
The associations between central adiposity (%TF and WC) and pre-diabetes stratified by BMI.
High %TF defined as highest quartile for entire study population.
Associations of central and lower adiposity with IS among women with NGT
Figure 1D shows that the correlation patterns of BMI with %BF, %TF, %LF and WC in 3,280 women with NGT were similar to those in all women (Figure 1A). Figures 1E and 1F show that for a given BMI, both low %LF and high %TF were associated with elevated HOMA-IR. The associations were stronger for higher BMI. Tables 3 presents the associations of central (high %TF and high WC) adiposity with ln(fasting insulin), HOMA-IR, and 2h-OGTT ln(insulin) among normal and overweight women with NGT. Compared with women with low %TF, those with high %TF had elevated fasting insulin, HOMA-IR, and 2H-OGTT insulin in both normal and overweight women. High WC was associated with elevated fasting insulin and 2H-OGTT insulin for normal weight women, however the associations were not as strong as those for high %TF.
Table 3.
Mean fasting insulin, 2H-OGTT insulin and HOMA-1 by BMI and central adiposity (%TF and WC) groups in 3280 Chinese women aged 20–60 years with NGT.
| Adiposity | N | Fasting ln(insulin) | 2H-OGTT ln(insulin) | HOMA-1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | β(se) | p | Mean ± SD | β(se) | p | Mean ± SD | β(se) | p | |||
| BMI | %TF a | ||||||||||
| <23 | Low | 1882 | 1.6 ± 0.8 | Ref | -- | 2.8 ± 0.9 | Ref | -- | 1.3 ± 1.2 | Ref | -- |
| High | 373 | 1.7 ± 0.8 | 0.13(0.05) | 0.0045 | 2.9 ± 0.9 | 0.12(0.05) | 0.0308 | 1.4 ± 1.0 | 0.13(0.06) | 0.0353 | |
| ≥23 | Low | 578 | 1.6 ± 0.7 | Ref | -- | 2.8 ± 0.9 | Ref | -- | 1.3 ± 1.2 | Ref | -- |
| High | 447 | 1.8 ± 0.8 | 0.14(0.05) | 0.0058 | 3.0 ± 0.9 | 0.17(0.06) | 0.0059 | 1.6 ± 1.6 | 0.24(0.09) | 0.0109 | |
| BMI | WC | ||||||||||
| <23 | <80 | 2195 | 1.6 ± 0.8 | Ref | -- | 2.8 ± 0.9 | Ref | -- | 1.3 ± 1.2 | Ref | -- |
| ≥80 | 60 | 1.7 ± 0.7 | 0.19(0.09) | 0.0418 | 2.9 ± 0.8 | 0.24(0.1) | 0.0235 | 1.4 ± 1.0 | 0.14(0.13) | 0.2639 | |
| ≥23 | <80 | 590 | 1.7 ± 0.8 | Ref | -- | 2.9 ± 1.0 | Ref | -- | 1.4 ± 1.3 | Ref | -- |
| ≥80 | 435 | 1.7 ± 0.7 | 0.05(0.05) | 0.3429 | 2.9 ± 0.9 | 0.04(0.06) | 0.5739 | 1.4 ± 1.5 | 0.07(0.09) | 0.4666 | |
Adjusted for age, age2, previous and current alcohol drinking, education, occupation and correlations among twin pairs.
High %TF defined as highest quartile for entire study population.
In addition, the analyses of Table 2 and 3 were repeated by limiting to only one randomly-selected subject per family. The results were very similar to those in the whole sample (data not shown).
DISCUSSION
Several important findings were observed in this study. Although this was a relatively lean and low risk rural Chinese population, 17% of the normal weight women had pre-diabetes compared to 23% of overweight women. Remarkably, 16% of normal weight women had high central adiposity as measured by %TF. Both high %TF and low %LF (lower three quartiles of %LF) were associated with higher prevalence of pre-diabetes in both normal and overweight women. Similarly, high %TF was associated with increased fasting insulin, 2H-OGTT insulin and HOMA-IR among normal or overweight women with NGT.
The prevalence of pre-diabetes in the Philippines is 31.3% in adults aged 20 years and older [26]. Although the prevalence of pre-diabetes in Chinese rural women was relatively lower than US and Philippines adults, the prevalence of pre-diabetes in rural, Chinese women with normal weight was higher (17% vs. 11%) than that in rural adults of [27]. Many studies indicated that individuals with pre-diabetes have higher lifetime risks of developing type 2 diabetes or cardiovascular disease (CVD) [1,2]. Therefore, it is clinically important to investigate the risk factors for pre-diabetes in the normal weight population. Our data demonstrated that high %TF was independently associated with increased risk of pre-diabetes after accounting for BMI and other covariates. Consistently, both central adiposity (%TF) and low %LF were associated with lower IS in both normal and overweight women with NGT, but these associations were stronger for overweight women. Most importantly, we found that normal weight women with high %TF had 50% greater odds of pre-diabetes compared to normal weight women without high %TF. The relative odds of pre-diabetes in normal weight women with high %TF were similar to those in overweight women with high %TF. Similar to our study, Yu et al [28] found that abdominal obesity was associated with higher risks of metabolic abnormalities than higher BMI and that central obesity conferred a greater risk of diabetes than obesity defined by BMI. However, their study population (aged 50–70) was older than ours and they did not specifically focus on the association of central obesity with pre-diabetes among normal and overweight individuals.
Our findings indicate that in addition to BMI, %TF and %LF could further improve risk assessments with regard to pre-diabetes and IS in this relatively lean, rural Chinese population. Our results are consistent with previous studies among adult men [14,29] and adolescents [30,31]. For example, Weiss et al [30] found intra-abdominal lipid accumulation was closely linked to the development of severe peripheral IR in obese adolescents with pre-diabetes. Also, previous findings from other groups provide strong evidence that onset of pre-diabetes or diabetes was highly associated with body adiposity (especially central body adiposity), which lead to reduced IS [15,32–35]. Caucasian women develop gynoid (predominantly lower-body) adiposity distribution, which is associated with increased circulating adiponectin levels that can effectively counter the adverse effects of visceral fat mass on IS. For this reason even obese women can have better IS and lower risk of diabetes than those with lower BMI, but predominant central adiposity [36]. Of particular clinical relevance is our finding that among females with clinically normal weight (BMI<23kg/m2), 16% of them had elevated %TF, which was significantly and independently associated with increased odds of pre-diabetes and decreased IS. Our finding was very similar to the data reported in 20 Japanese adults with BMI < 25 kg/m2 and NGT [15]. Despite the stringent criteria for the definition of normal weight that was used in this study, high central adiposity was still related with a higher prevalence of pre-diabetes and decreased IS in normal weight women. However, although a significant difference in HOMA-IR between normal weight women with low and high %TF was observed, this finding needs to confirmed in another study. While previous studies found that Asians have higher amounts of visceral adipose tissue for a given BMI [16,17], this is the first study to document a substantial proportion of normal weight women with elevated %TF in a rural Chinese population. Furthermore, this is the first study to demonstrate that this group of women had odds of pre-diabetes that were similar to those in overweight women with high %TF (the highest risk group). Thus, in assessing individual risks of pre-diabetes and decreased IS among rural Chinese women, both BMI and degree of central adiposity need to be considered.
In this study, while similar associations were found for %TF and WC, the associations for WC were not as consistent and significant as for %TF. Further, WC was more highly correlated with BMI (r=0.77) than %TF (r=0.50) in this study population. Although Feng et al [12] showed that WC was a good surrogate for abdominal fat among a study sample recruited from the same area, they did not examine %TF, and the study subjects were older (mean age: 45 years) than those in this study (mean age: 32 years). Sierra-Johnson et al [37] also reported that WC was a robust predictor of reduced IS among 256 healthy adults (mean age: 40 years) and the predictive information provided by adiposity measured using DEXA was approximately equal to that provided by WC. The different observations between our and other groups may result from changes in correlations of WC and %TF with increasing age [38]. Recently, data from Shanghai [39] and Hong Kong [40] indicated that the optimal WC cutoffs were 82 cm for abdominal obesity and 75 cm for mesenteric fat thickness among Chinese women, respectively. In this study, the WC cutoff was 80 cm for central obesity, which is similar. Our data also showed high correlations of WC and %TF with age (data not shown). Therefore, WC may be a better surrogate for central body fat in older adults than in younger adults.
When interpreting our findings, one should be cautious. This is a population-based twin cohort. However, their demographic and clinical characteristics were similar to those of the local general population (data not shown). Furthermore, the results of our analyses were similar whether we included both twins or if we analyzed only one randomly selected twin from each pair. We previously found that lung function in twins did not differ from that in singleton children in the same region where we conducted this twin study. [41] Additionally, the findings of this study were consistent to those from similar studies. Thus, we believe that our findings can be generalized to the local rural Chinese women. Our findings remain to be confirmed independently in other populations. This is a population of relatively lean, healthy, and relatively younger age women. Nevertheless, we found associations between %TF and pre-diabetes and markers of insulin sensitivity. Such findings raise the possibility that women at high-risk of pre-diabetes or insulin resistance can be identified at early stage and effective early intervention can be developed. As we continue to follow-up this cohort of women, we will be better able to address our findings in predicting the risk of diabetes and MS in the future.
In summary, in this cross-sectional study of relatively lean, rural Chinese women, we found that 16% of normal weight women versus 45% of overweight women had high %TF; and high %TF was independently associated with increased odds of pre-diabetes and decreased IS, even after accounting for BMI and other covariates. Of particular clinical importance is that normal weight women with high %TF had odds of pre-diabetes that were similar to those in overweight women with high %TF (the highest risk group). While similar associations were found for high WC, they were not as consistent or significant as those for high %TF. Our study underscores that when assessing individual risk of pre-diabetes and decreased IS in rural Chinese women, both BMI and the degree of central adiposity need to be carefully considered.
Acknowledgments
This study is supported in part by grant R01 HD049059, R01 HL066385, and RO1AG032221 from National Institute of Health and by the Food Allergy Project. Scott A. Venners supported by K01 ES12052 from the National Institute of Environmental Health Sciences. There is not any conflict-of-interest/financial disclosure statement about this study.
We gratefully acknowledge the assistance and cooperation of the faculty and staff of Anhui Medical University. We thank all the participants in the study for their time and support. We greatly thank Hui-Ju Tsai and Amy P. Mucha for editing the manuscript. This study is supported in part by grant R01 HD049059, R01 HL066385, and RO1AG032221 from National Institute of Health and by the Food Allergy Project. Scott A. Venners supported by K01 ES12052 from the National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weyer C, Tataranni PA, Bogardus C, et al. Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Diabetes Care. 2001;24:89–94. doi: 10.2337/diacare.24.1.89. [DOI] [PubMed] [Google Scholar]
- 2.Burchfiel CM, Hamman RF, Marshall JA, et al. Cardiovascular risk factors and impaired glucose tolerance: the San Luis Valley Diabetes Study. Am J Epidemiol. 1990;131:57–70. doi: 10.1093/oxfordjournals.aje.a115485. [DOI] [PubMed] [Google Scholar]
- 3.Maji D, Roy RU, Das S. Prevention of type 2 diabetes in the prediabetic population. J Indian Med Assoc. 2005;103:609–611. [PubMed] [Google Scholar]
- 4.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 5.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson KF, Lindgarde F. Prevention of type 2 (non-insulin-dependent) diabetes mellitus by diet and physical exercise. The 6-year Malmo feasibility study. Diabetologia. 1991;34:891–898. doi: 10.1007/BF00400196. [DOI] [PubMed] [Google Scholar]
- 7.Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract. 2005;67:152–162. doi: 10.1016/j.diabres.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Manson JE, Colditz GA, Stampfer MJ, et al. A prospective study of obesity and risk of coronary heart disease in women. N Engl J Med. 1990;322:882–889. doi: 10.1056/NEJM199003293221303. [DOI] [PubMed] [Google Scholar]
- 9.Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities--the role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996;334:374–381. doi: 10.1056/NEJM199602083340607. [DOI] [PubMed] [Google Scholar]
- 10.Bjorntorp P. Metabolic implications of body fat distribution. Diabetes Care. 1991;14:1132–1143. doi: 10.2337/diacare.14.12.1132. [DOI] [PubMed] [Google Scholar]
- 11.Sironi AM, Gastaldelli A, Mari A, et al. Visceral fat in hypertension: influence on insulin resistance and beta-cell function. Hypertension. 2004;44:127–133. doi: 10.1161/01.HYP.0000137982.10191.0a. [DOI] [PubMed] [Google Scholar]
- 12.Feng Y, Hong X, Li Z, et al. Prevalence of metabolic syndrome and its relation to body composition in a Chinese rural population. Obesity (Silver Spring) 2006;14:2089–2098. doi: 10.1038/oby.2006.244. [DOI] [PubMed] [Google Scholar]
- 13.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori Y, Hoshino K, Yokota K, et al. Differences in the pathology of the metabolic syndrome with or without visceral fat accumulation: a study in pre-diabetic Japanese middle-aged men. Endocrine. 2006;29:149–153. doi: 10.1385/endo:29:1:149. [DOI] [PubMed] [Google Scholar]
- 15.Katsuki A, Sumida Y, Urakawa H, et al. Increased visceral fat and serum levels of triglyceride are associated with insulin resistance in Japanese metabolically obese, normal weight subjects with normal glucose tolerance. Diabetes Care. 2003;26:2341–2344. doi: 10.2337/diacare.26.8.2341. [DOI] [PubMed] [Google Scholar]
- 16.Park YW, Allison DB, Heymsfield SB, et al. Larger amounts of visceral adipose tissue in Asian Americans. Obes Res. 2001;9:381–387. doi: 10.1038/oby.2001.49. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka S, Horimai C, Katsukawa F. Ethnic differences in abdominal visceral fat accumulation between Japanese, African-Americans, and Caucasians: a meta-analysis. Acta Diabetol. 2003;40 (Suppl 1):S302–304. doi: 10.1007/s00592-003-0093-z. [DOI] [PubMed] [Google Scholar]
- 18.Gu D, Reynolds K, Wu X, et al. Prevalence of the metabolic syndrome and overweight among adults in China. Lancet. 2005;365:1398–1405. doi: 10.1016/S0140-6736(05)66375-1. [DOI] [PubMed] [Google Scholar]
- 19.Wang B, Necheles J, Ouyang F, et al. Monozygotic co-twin analyses of body composition measurements and serum lipids. Prev Med. 2007;45:358–365. doi: 10.1016/j.ypmed.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 21.Chang AM, Smith MJ, Bloem CJ, et al. Limitation of the homeostasis model assessment to predict insulin resistance and beta-cell dysfunction in older people. J Clin Endocrinol Metab. 2006;91:629–634. doi: 10.1210/jc.2005-1803. [DOI] [PubMed] [Google Scholar]
- 22.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27 (Suppl 1):S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 23.WHO Expert Committee on Diabetes Mellitus: second report. World Health Organ Tech Rep Ser. 1980;646:1–80. [PubMed] [Google Scholar]
- 24.Misra A, Wasir JS, Pandey RM. An evaluation of candidate definitions of the metabolic syndrome in adult Asian Indians. Diabetes Care. 2005;28:398–403. doi: 10.2337/diacare.28.2.398. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez RN, Strokes ME. Recent enhancements and new directions in SAS/STAT Software, Part I. Nonparametric modeling procedure. Proceedings of the 23rd SAS Users Group International Conference; 1998. pp. 1262–1270. [Google Scholar]
- 26.Soria ML, Sy RG, Vega BS, et al. The incidence of type 2 diabetes mellitus in the Philippines: A 9-year cohort study. Diabetes Res Clin Pract. 2009 doi: 10.1016/j.diabres.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Katulanda P, Constantine GR, Mahesh JG, et al. Prevalence and projections of diabetes and pre-diabetes in adults in Sri Lanka--Sri Lanka Diabetes, Cardiovascular Study (SLDCS) Diabet Med. 2008;25:1062–1069. doi: 10.1111/j.1464-5491.2008.02523.x. [DOI] [PubMed] [Google Scholar]
- 28.Yu Z, Lin X, Haas JD, et al. Obesity related metabolic abnormalities: distribution and geographic differences among middle-aged and older Chinese populations. Prev Med. 2009;48:272–278. doi: 10.1016/j.ypmed.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Orisaka M, Nakai K, Tominaga M, et al. Risk factors for development of pre-diabetic state from normal glucose regulation. Tohoku J Exp Med. 2006;210:279–283. doi: 10.1620/tjem.210.279. [DOI] [PubMed] [Google Scholar]
- 30.Weiss R, Dufour S, Taksali SE, et al. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet. 2003;362:951–957. doi: 10.1016/S0140-6736(03)14364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S, Gungor N, Bacha F, et al. Insulin resistance: link to the components of the metabolic syndrome and biomarkers of endothelial dysfunction in youth. Diabetes Care. 2007;30:2091–2097. doi: 10.2337/dc07-0203. [DOI] [PubMed] [Google Scholar]
- 32.Lillioja S, Mott DM, Spraul M, et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med. 1993;329:1988–1992. doi: 10.1056/NEJM199312303292703. [DOI] [PubMed] [Google Scholar]
- 33.Haffner SM, Kennedy E, Gonzalez C, et al. A prospective analysis of the HOMA model. The Mexico City Diabetes Study. Diabetes Care. 1996;19:1138–1141. doi: 10.2337/diacare.19.10.1138. [DOI] [PubMed] [Google Scholar]
- 34.Lyssenko V, Almgren P, Anevski D, et al. Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes. 2005;54:166–174. doi: 10.2337/diabetes.54.1.166. [DOI] [PubMed] [Google Scholar]
- 35.Klein DJ, Aronson Friedman L, Harlan WR, et al. Obesity and the development of insulin resistance and impaired fasting glucose in black and white adolescent girls: a longitudinal study. Diabetes Care. 2004;27:378–383. doi: 10.2337/diacare.27.2.378. [DOI] [PubMed] [Google Scholar]
- 36.Tanko LB, Bagger YZ, Alexandersen P, et al. Peripheral adiposity exhibits an independent dominant antiatherogenic effect in elderly women. Circulation. 2003;107:1626–1631. doi: 10.1161/01.CIR.0000057974.74060.68. [DOI] [PubMed] [Google Scholar]
- 37.Sierra-Johnson J, Johnson BD, Bailey KR, et al. Relationships between insulin sensitivity and measures of body fat in asymptomatic men and women. Obes Res. 2004;12:2070–2077. doi: 10.1038/oby.2004.258. [DOI] [PubMed] [Google Scholar]
- 38.Xiao SM, Lei SF, Chen XD, et al. Correlation and prediction of trunk fat mass with four anthropometric indices in Chinese males. Br J Nutr. 2006;96:949–955. doi: 10.1017/bjn20061820. [DOI] [PubMed] [Google Scholar]
- 39.Ye Y, Bao Y, Hou X, et al. Identification of waist circumference cutoffs for abdominal obesity in the Chinese population: a 7.8-year follow-up study in the Shanghai urban area. Int J Obes (Lond) 2009;33:1058–1062. doi: 10.1038/ijo.2009.134. [DOI] [PubMed] [Google Scholar]
- 40.Ko GT, Liu KH, So WY, et al. Cutoff values for central obesity in Chinese based on mesenteric fat thickness. Clin Nutr. 2009 doi: 10.1016/j.clnu.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 41.Yu Y, Kumar R, Venners S, et al. Age and gender specific lung function predictive equations provide similar predictions for both a twin population and a general population from age 6 through adolescence. Pediatr Pulmonol. 2007;42:631–639. doi: 10.1002/ppul.20631. [DOI] [PubMed] [Google Scholar]