Abstract
Dietary lipid absorption is dependent on chylomicron production whose rate-limiting step across the intestinal absorptive cell is the exit of chylomicrons from the endoplasmic reticulum (ER) in its ER-to-Golgi transport vesicle, the prechylomicron transport vesicle (PCTV). This study addresses the composition of the budding complex for PCTV. Immunoprecipitation (IP) studies from rat intestinal ER solubilized in Triton X-100 suggested that vesicle-associated membrane protein 7 (VAMP7), apolipoprotein B48 (apoB48), liver fatty acid-binding protein (L-FABP), CD36, and the COPII proteins were associated on incubation of the ER with cytosol and ATP. This association was confirmed by chromatography of the solubilized ER over Sephacryl S400-HR in which these constituents cochromatographed with an apparent kDa of 630. No multiprotein complex was detected when the ER was chromatographed in the absence of PCTV budding activity (resting ER or PKCζ depletion of ER and cytosol). Treatment of the ER with anti-apoB48 or anti-VAMP7 antibodies or using gene disrupted L-FABP or CD36 mice all significantly inhibited PCTV generation. A smaller complex (no COPII proteins) was formed when only rL-FABP was used to bud PCTV. The data support the conclusion that the PCTV budding complex in intestinal ER is composed of VAMP7, apoB48, CD36, and L-FABP, plus the COPII proteins.
Keywords: chylomicron, lipid absorption, vesicle budding complex, CD36, L-FABP
Considerable information has been acquired on the machinery necessary for the generation of the transport vesicle that takes newly synthesized proteins from the endoplasmic reticulum (ER) to the Golgi, referred to as protein vesicles (1–4), and the order of their accretion to the ER exit sites (ERES) (5). The initial observations described uniform vesicles of ∼55–70 nm. Although it was proposed that changing cage protein angles could enlarge these protein vesicles to accommodate up to 100-nm-sized cargo (2), this still would not be large enough to transport chylomicrons whose diameter averages 250 nm. These considerations led to the proposal that alternative budding machinery was needed for the prechylomicron transport vesicle (PCTV) whose diameter is 350–500 nm (6).
With respect to PCTV, besides the requirement for an expanded vesicle, another unique feature is its need for large-scale expansion and contraction of either the number or the size of the vesicles produced. During fasting in the rat, for example, only 4 μmol/h triacylglycerol (TAG) is exported into the lymph (7). By contrast, during near-maximal intraduodenal infusions of glyceryltrioleate, up to 110 μmol/h of TAG are exported into the lymph in chylomicrons (8). Thus during the fasting state, either a presumed minimal number of vesicles would be needed to transport the small amount of TAG, which would be greatly increased after a fatty meal, or, alternatively, using the same number of vesicles as present in the fasting state, a 3-fold increase in the radius of the vesicles on fat feeding would accommodate transporting the increased TAG (9). In either case, a large vesicle would be needed for chylomicron transport. The advantage of separate machinery for PCTV transport, in particular, is that it would avoid potential competition for components that could become limiting under maximal PCTV budding conditions, in addition to providing a large trafficking vesicle.
In all cells there is continuous protein traffic between the ER and Golgi. The majority of these protein cargoes use the COPII budding machinery, consisting of an inner layer of Sar1 and Sec23/24 and an outer layer of Sec13/31 to produce protein vesicles. The minimal machinery necessary for the generation of cargo carrying protein vesicles has been established using COPII proteins and liposomes (10, 11). Certain cargoes, such as the amyloid precursor protein, require cytosolic proteins in addition to COPII (11), signifying an expanded budding complex requirement. In the case of PCTV, although COPII proteins are required for fusion with the Golgi, specifically Sec24C (12), they are not necessary for budding a cargo-containing vesicle from the ER membrane (6), suggesting a different budding mechanism. Indeed we showed that the small Mr protein, liver fatty acid-binding protein (L-FABP) (14.1 kDa), can generate cargo-carrying PCTV, although the vesicle produced is incomplete in that it cannot fuse with the Golgi (13). It seemed highly unlikely that L-FABP alone could select cargo for inclusion in PCTV, deform the ER membrane, and enable fission from the membrane to produce a cargo-carrying transport vesicle because of its small size compared with the COPII proteins (total, 369 kDa) required for the initiation of the vesicle that transports newly synthesized proteins from the ER to the Golgi (14). In the protein transport system, differing members of the five-protein COPII complex are each assigned specific functions in vesicle assembly (15–17), supporting the thesis that L-FABP would be unlikely to perform these multiple functions on its own. In sum, the data suggested that ER and/or cytosolic proteins other than the COPII proteins are involved in the complex series of steps required for the formation of PCTV.
This article describes the identification of several proteins used for PCTV budding from intestinal ER. The experiments were based on using three known components of PCTV budding; namely, the unique cargo protein, apolipoprotein B48 (apoB48), which is present in all chylomicrons (18); vesicle-associated membrane protein 7 (VAMP7); the vesicle-associated soluble N-ethylmaleimide-sensitive factor attachment protein receptor (v-SNARE) for PCTV (19); and L-FABP, which as we showed, is able to initiate PCTV budding (13). The results indicate that apoB48, L-FABP, VAMP7, and the FA transporter CD36 are essential components of the PCTV vesicle complex and that formation of the complex requires PKCζ.
METHODS
Materials
3H Oleic acid (9.2 Ci/mM) was procured from Perkin Elmer Life Sciences. Sephacryl S-400 High Resolution gel filtration medium was purchased from GE Healthcare (Piscataway, NJ). Immunoblot reagents were purchased from Bio-Rad. Enhanced chemiluminescence (ECL) reagents were procured from GE Healthcare. Protease inhibitor cocktail tablets were obtained from Roche Applied Science. Other biochemicals used were of analytical grade and purchased from Sigma (St. Louis, MO) or local companies. Male Sprague Dawley rats, 150–200 g were purchased from Harlan (Indianapolis, IN).
Antibodies and proteins
Rabbit anti-rat apolipoproteinB48 (apoB48) antibodies were made from amino acid sequence 2055-2067 and rabbit anti-Sar1 antibodies were raised commercially (Protein Tech Group, Chicago, IL) using recombinant Sar1 protein (6). Polyclonal antibodies against rat VAMP7 were raised in rabbits commercially (Genemed Synthesis, San Francisco, CA) using a synthetic 19-mer peptide corresponding to amino acids 105-123 of rat VAMP7. The antibody was purified against its immunogenic peptide (20). Titration of the anti-VAMP7 antibody against PCTV budding is shown in supplementary Fig. I. Antibody against purified rat intestinal fatty acid-binding protein (I-FABP) and rat liver fatty acid-binding protein (L-FABP) were raised in rabbits commercially (Affinity Bioreagents, Golden, CO) and used at 1: 30,000 or 1:20,000 dilution for immunoblotting, respectively. Recombinant L-FABP (rL-FABP) was produced in Escherichia coli without tags and was isolated and delipidated (21) (11). Affinity-purified rabbit polyclonal anti-Sec24C antibodies were a gift of J. P. Paccaud (University of Geneva, Geneva, Switzerland). Goat anti-Sec 23, rabbit anti-CD36, and rabbit polyclonal anti-PKCζ were procured from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-mouse monoclonal antibodies to rBet1and GOS28 were purchased from Stressgen (Ann Arbor, MI). Goat anti-rabbit IgG conjugated with agarose beads was purchased from Sigma. Goat anti-rabbit IgG and goat anti-mouse IgG conjugated with horseradish peroxidase (HRP) were procured from Sigma Chemical Co. (St. Louis, Mo).
Gene-disrupted mice
L-FABP−/−C57BL/6J congenic mice (backcrossed more than 10 generations to C57BL/6J animals) were generated (22). CD36-null mice were backcrossed to C57BL/6J background mice (23, 24). Both gene-disrupted mice were compared with age-matched C57BL/6J mice (Jackson Laboratory).
Preparation of ER, Golgi, cytosol, and labeling of enterocytes
Enterocytes from the proximal half of the male Sprague Dawley rats (or mice) small intestine were isolated and radiolabeled with 3H-TAG as described (25). In brief, enterocytes were isolated from intestinal villi, collected, incubated with albumin bound 3H-oleate for 30 min at 35°C and washed with 2% BSA in PBS to remove the excess 3H-oleate. The labeled enterocytes were homogenized using a Parr bomb, and the ER was isolated using a sucrose density step gradient, which was repeated to purify the ER that contained calnexin and calreticulin but neither GOS28 nor rab11 (6, 20). By TLC,(8) 4% of the radiolabel was in the phospholipid fraction. In PCTV, isolated in prior studies (26), 3% of the radiolabel by TLC was in the phospholipid fraction. The cis Golgi was isolated from nonradiolabeled enterocytes, and marker proteins determined its purity. The preparation of cytosol was essentially the same as previously described (6).
Isolation of the PCTV budding complex
ER (1 mg) was incubated at 4°C for 1 h with native cytosol (2 mg) and an ATP-regenerating system (6). 4°C was chosen to prevent active PCTV budding and consequent escape of PCTV component proteins (6). The ER was washed twice with cold PBS and solubilized with 1% Triton X-100 in PBS (pH 7.2). The ER membranes, pre- or post-incubation with cytosol and an ATP regenerating system as indicated in the figure legends, were loaded on a Sephacryl S-400 HR column (45 cm × 1.5 cm) previously equilibrated with 1% Triton X-100 in PBS (pH 7.2) with a 0.5 ml/min flow rate and eluted with 1% Triton X-100 in PBS (pH 7.2) at 4°C. 1 ml fractions were collected, and the proteins from each fraction were precipitated with trichloroacetic acid (TCA) and immunoblotted with specific antibodies as indicated. Band density was measured using a GelDoc XR (Bio-Rad, Hercules, CA). When PCTV budding using rL-FABP was required, 80 µg of rL-FABP was used instead of native cytosol (13). In other studies as shown in the figure legends, PKCζ depletion of ER and cytosol was accomplished as described below, and the depleted ER and cytosol incubated at 37°C for 30 min. The column was characterized by determining the elution volume of Dextran blue (taken as the void volume, 0 ml), thyroglobulin (10 ml), apoB48 from rat mesenteric lymph chylomicrons (8) (21 ml), conalbumin (27 ml), ovalbumin (33 ml), L-FABP (38 ml), and glucose (40 ml). 3H-oleate, chromatographed over the column in 1% Triton X-100, eluted at 14 ml.
In vitro PCTV formation
PCTV containing 3H -TAG were formed from 3H-TAG-loaded intestinal ER (6). In brief, ER (500 µg protein) was incubated at 37°C for 30 min with cytosol (1 mg protein) and an ATP-regenerating system. The incubation mixture was resolved on a continuous sucrose gradient, and PCTV was isolated from the light portions of the gradient. The PCTV was concentrated using a Centricon-10 filter (Millipore Corp., Bedford, MA).
Effect of antibody treatment on PCTV budding
Intestinal 3H-TAG-loaded ER (500 µg protein) was treated with 10 µl of specific antibodies as required or IgG for 60 min at 4°C. The ER was washed with PBS to remove excess antibody and then used in a PCTV budding assay as described above. The PCTV was collected from the top of a continuous sucrose gradient, and its 3H-TAG-dpm was determined. In the case of VAMP7, antibody titration was performed with 2.5–10 µl anti-VAMP7 antibody incubated with ER (supplementary Fig. I). 10 µl was used in the reported experiments.
Immunodepletion of PKCζ from cytosol
Removal of PKCζ from cytosol was performed by immunodepletion (26). Cytosol (1 mg) was incubated with 20 µl of rabbit polyclonal anti-PKCζ antibodies at 4°C for 4 h. Anti-rabbit IgG conjugated with agarose beads was added, and the mixture was stirred slowly at 4°C overnight .The antibody-protein complexes were removed by centrifugation. Successful depletion PKCζ from cytosol was obtained by three rounds of immunodepletion and confirmed by immunoblot.
Removal of PKCζ from the ER
To remove PKCζ from ER membranes, ER was incubated with 2 M urea for 15 min at 4°C (26). ER membranes were washed twice with cold PBS. The removal of PKCζ confirmed by immunoblotting.
SDS-PAGE and immunoblot
Proteins were separated by SDS-PAGE and then transblotted to nitrocellulose membranes (Bio-Rad). After incubation with specific primary antibodies and peroxidase-conjugated secondary antibodies, labeled proteins were detected using ECL (Amersham Biosciences) and Biomax film (Eastman Kodak, Rochester, NY).
Immunoprecipitation of proteins from ER membranes
Intestinal ER (200 µg protein) was solubilized in 1% Triton X-100 and then centrifuged in a bench-top microcentrifuge. The supernatant was incubated with specific bead-bound primary antibodies (10–20 µl) with gentle rocking for 4 hrs at 4°C. 40 µl of protein A or protein G agarose beads (Sigma) were added depending on the primary antibody and incubated for another 4 h. The beads were collected by centrifugation, washed eight times with PBS, and resuspended in SDS-PAGE loading buffer for immunoblot analysis.
Coimmunoprecipitation of proteins from the multiprotein complex or monomer species as eluted from the Sephacryl S-400 HR column
Peak column fractions (3 fractions) from the multiprotein fractions and peak monomer fractions of apoB48, VAMP7, and L-FABP from chromatography on the Sephacryl S-400 HR column as previously described were collected, and the proteins precipitated using TCA. The TCA was removed by washing with cold acetone. The proteins were solubilized with 0.25 M sucrose in PBS. Specific bead-bound antibodies to apoB48, VAMP7, or L-FABP were incubated with the solubilized proteins for 4 h at 4°C. The beads were washed in PBS eight times to remove unattached proteins. The bead-bound proteins were separated by SDS-PAGE after boiling in Laemmli's buffer. Immunoblots for apoB48, VAMP7, and L-FABP were performed on the transblotted proteins as indicated.
Measurement of TAG radioactivity
TAG radioactivity was determined by liquid scintillation as described (25).
RESULTS
Isolation of the PCTV budding complex and identification of its components
The ability to isolate the PCTV budding complex is critically dependent on its stability so that its protein components remain interactive during the column isolation procedures. We have previously shown that ER and cytosol depleted of PKCζ cannot bud PCTV (26). That the components would likely remain together was suggested by our prior data showing that once exposed to ATP and cytosol, intestinal ER membranes were able to bud PCTV 30 min after stopping further phosphorylation by replacing the cytosol with PKCζ-depleted cytosol (26). PKCζ is necessary for PCTV budding (26).
Our first approach to the isolation of the complex was to narrow the focus of potential proteins composing the complex by a series of immunoprecipitation (IP) experiments beginning with four proteins that were known to be required for PCTV generation. ApoB48, the nonexchangeable apolipoprotein of chylomicrons present in PCTV (6), is the quintessential apolipoprotein of chylomicrons (27). VAMP7 is the v-SNARE for PCTV (19). L-FABP initiates PCTV budding (13). Sec24C, while not required for budding, is required for PCTV docking and fusion with the Golgi (12). Further, Sec24 has been shown to function in cargo selection (17, 28). The results of using bead-bound antibodies to these four proteins in IP experiments are shown in Table 1. Intestinal ER was incubated with cytosol and ATP at 4°C and probed with antibodies to specific proteins as shown. The data show that VAMP7 interacts with Sec24C, L-FABP, apoB48, and Sar1. ApoB48 is shown to interact with Sec23, Sec24C, L-FABP, I-FABP, VAMP7, and Sar1. L-FABP was shown to interact with VAMP7, apoB48, and Sar1. Sec24C is shown to interact with Sec23, VAMP7, apoB48, and Sar1. In addition, we used antibodies to I-FABP, which gave 21% the budding activity of L-FABP (13). It interacted with apoB48 but not Sar1 or VAMP7, to which L-FABP reacted. Unexpectedly, using antibodies to L-FABP, we coimmunoprecipitated an 88 kDa protein that on immunoblot was the fully glycosylated form of CD36 (29), which has a variety of lipid-related functions (23, 30, 31). CD36 was found to interact with L-FABP, aopB48, and VAMP7. In sum, these data gave us information on the potential composition of the PCTV budding complex. In addition to the data suggesting the interactivity of the various proteins, these IP studies support the thesis that the proteins composing the PCTV budding complex as isolated from the ER would remain together during subsequent chromatographic procedures. Supplementary Fig. II shows the immunoblots on which the data in Table 1 are based.
TABLE 1.
Immunoprecipitation of proteins associated with PCTV budding using proteins required for PCTV budding as bait
| Interactive Proteins | |||||||
|---|---|---|---|---|---|---|---|
| Bead-Bound Antibodies | Sec23 | Sec24C | L-FABP | I-FABP | VAMP-7 | ApoB48 | Sar1 |
| VAMP-7 | N | Y | Y | N | # | Y | Y |
| ApoB48 | Y | Y | Y | Y | Y | # | Y |
| Sec24C | Y | # | N | N | Y | Y | Y |
| L-FABP | N | N | # | N | Y | Y | Y |
| I-FABP | N | N | N | # | N | Y | N |
| CD36 | ND | ND | Y | ND | Y | Y | ND |
Immunoprecipitation studies of ER membrane proteins related to PCTV budding. Specific antibodies to the proteins listed in the left column (bead-bound antibodies) were used as bait in immunoprecipitation studies (see “Methods”). The precipitated proteins for each bait protein were identified by immunoblot using the specific antibodies shown in each column (interactive proteins). Proteins associated with the bait protein are identified as follows: Y = interactivity; N = no interaction; # = identical proteins; ND = not done. Abbreviations: Apo, apolipoprotein; I-FABP, intestinal fatty acid-binding protein; L-FABP, liver fatty acid-binding protein; VAMP7, vesicle-associated membrane protein 7.
Since the protein-protein interactions identified in Table 1 were performed in the cold, we proposed that this should be reflected in their continued interactivity when intestinal ER was solubilized by Triton X-100 after incubation with intestinal cytosol and ATP at 4°C or when the ER was incubated with L-FABP alone. We further proposed that the proteins would remain as monomers in the absence of cytosol or in the absence of PKCζ where no PCTV budding occurs (6, 26), and therefore, these conditions could serve as controls. If our assumptions were correct, each of the proteins proposed to compose the PCTV budding complex should elute from the Sephacryl S-400 HR column at the same, small elution volume, indicating a large complex under conditions appropriate for budding. Under conditions where no budding would be expected, the proteins should elute at larger volumes consistent with their specific, smaller monomer Mr. Further, by incubating the ER at 4°C, we hoped that in the absence of PCTV budding, the budding complex proteins would be maximized in the ER rather than removed by active PCTV budding because these proteins are concentrated in PCTV versus the ER (6). Supplementary Figs. III–VI show the immunoblots on which the subsequent data are based.
ApoB48, VAMP7, L-FABP, and CD36 are PCTV budding complex components
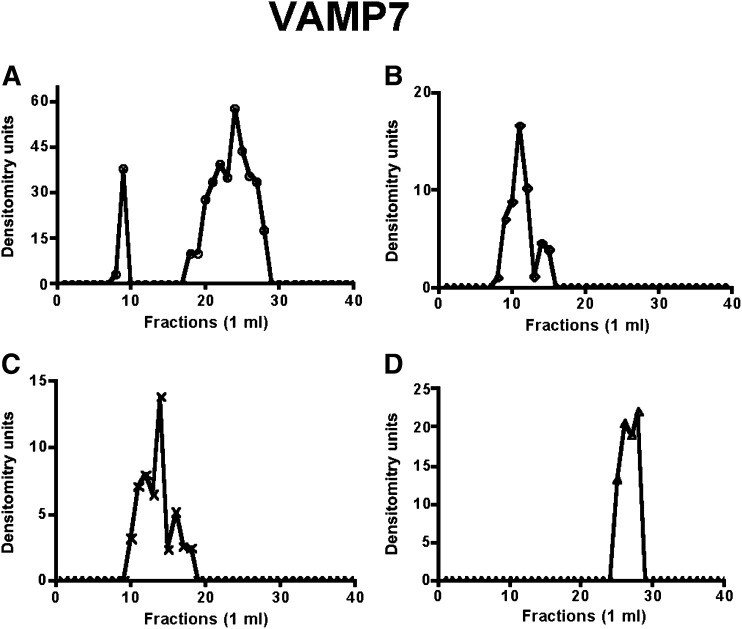
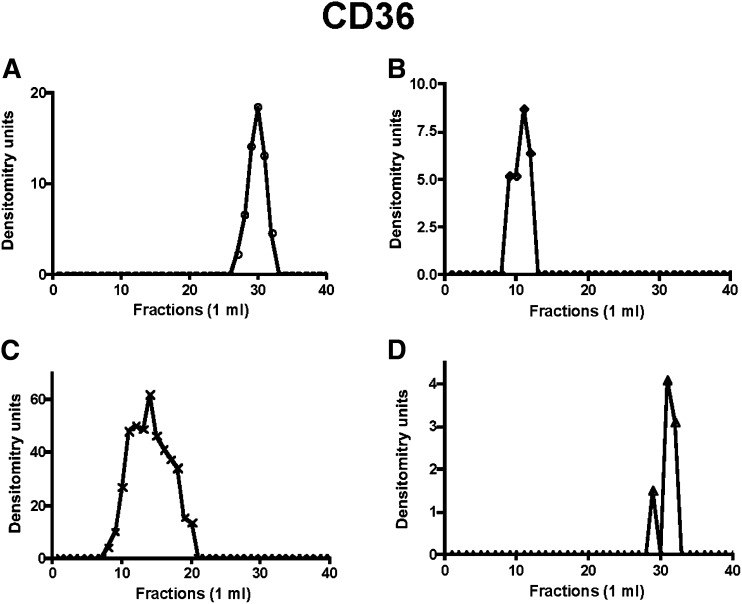
When ER was solubilized without exposure to cytosol or ATP, apoB48 eluted from the column in the fractions expected for its monomer species (Fig. 1A), indicating that fully translated apoB48 exists in ER unlinked to other proteins in the basal state. Similarly, L-FABP (Fig. 2A) eluted in a volume expected for its monomer species in resting ER. VAMP7 in basal ER mostly eluted as a larger protein than the expected 25 kDa (Fig. 3A). The likely explanation for this is that VAMP7 is present either as a homodimer or a hexamer on isolation from the ER and requires reducing conditions associated with the performance of 2D gels to disassociate the VAMP7 aggregates (20). Like L-FABP, CD36 (Fig. 4A) eluted in a volume expected for its monomer species in resting ER. L-FABP and CD36’s lack of association in a protein complex was also true for the COPII proteins Sar1, Sec24C, and Sec31 (supplementary Figs. VII-A, VIII-A, and IX-A).
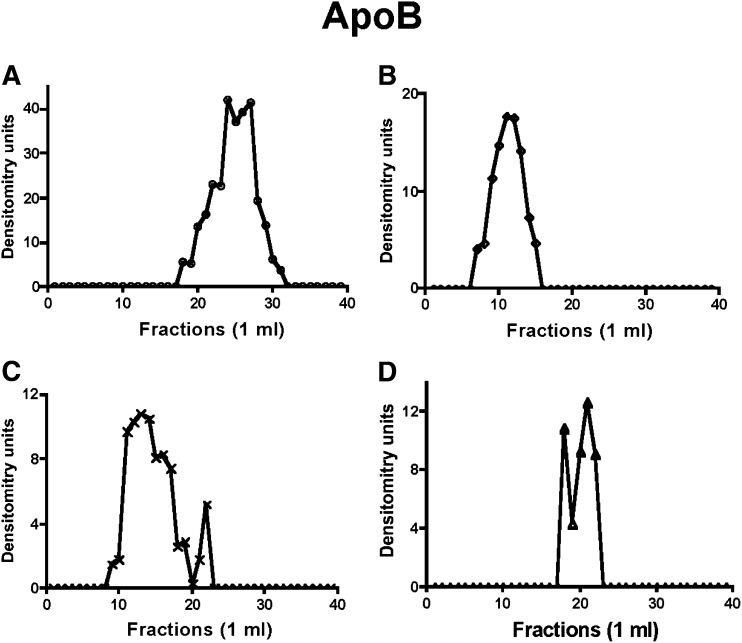
Fig. 1.
Distribution of ER-apoB48 across a Sephacryl S-400 HR column under differing conditions. In each case (A–D), ER membranes (1 mg protein) were treated as indicated, collected by centrifugation, washed with PBS, and solubilized in 1% Triton X-100. The ER protein was chromatographed over a 1% Triton X-100 equilibrated Sephacryl S-400 HR column (see “Methods”), and 1-ml fractions were collected. Each fraction was tested for apoB48 by immunoblot. The band densities were quantitated by the GelDoc XR imaging system. The resulting arbitrary density units are reported. (A) Native ER membranes. (B) ER incubated with native cytosol and ATP. (C) ER membranes incubated with rL-FABP without cytosol. (D) Urea-washed ER incubated with PKCζ immunodepleted cytosol and ATP (see “Methods”). In A–C, incubations were at 4°C; in D, the incubation was at 37°C (see “Methods”). Apo, apolipoprotein; ER, endoplasmic reticulum; L-FABP, liver fatty acid-binding protein; PKC, protein kinase C.
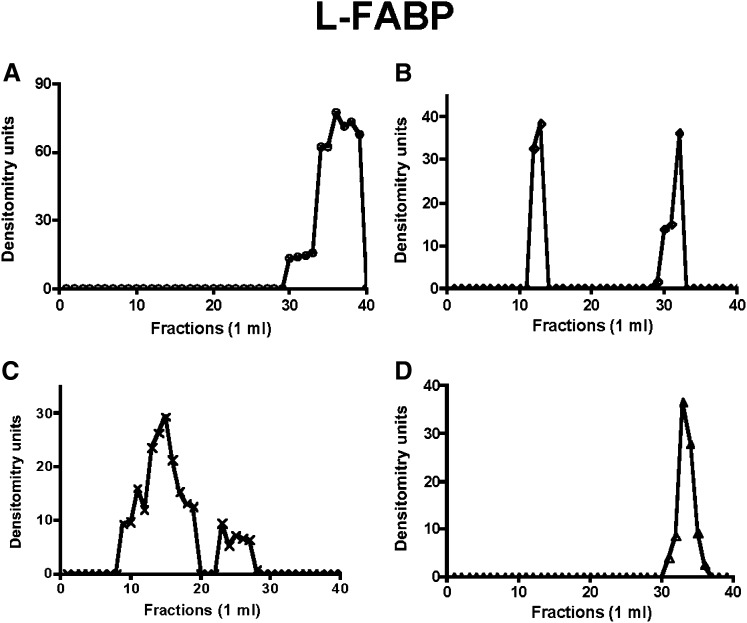
Fig. 2.
ER-L-FABP distributes differently across a Sephacryl S-400 HR column under various conditions. Native ER membranes (1 mg protein) were incubated with no cytosol (A), cytosol plus ATP (B), rL-FABP (C), PKCζ-depleted cytosol, and urea-washed ER (D). The treated ER was chromatographed, immunoblotted for L-FABP, and band densities were determined as in Fig. 1. ER, endoplasmic reticulum; L-FABP, liver fatty acid-binding protein; PKC, protein kinase C.
Fig. 3.
ER-VAMP7’s distribution across a Sephacryl S-400 HR column under various conditions. Incubation conditions and chromatography were as in Fig. 1. Solubilized ER membranes after treatment as indicated were chromatographed and fractions immunoblotted for VAMP7. (A) Native ER, (B) ER after incubation with cytosol and ATP, (C) ER after incubation with rL-FABP, and (D) ER after incubation with urea-washed ER and PKCζ-depleted cytosol. The band densities were determined as in Fig. 1. ER, endoplasmic reticulum; L-FABP, liver fatty acid-binding protein; PKC, protein kinase C; VAMP7, vesicle-associated membrane protein 7.
Fig. 4.
The distribution of CD36 in a Sephacryl S-400 HR column eluent of solubilized intestinal ER treated as indicated in Fig. 1. Native ER membranes (A). ER membranes after incubation with native cytosol and ATP (B). ER membranes incubated with L-FABP alone (C). Urea-washed ER membranes incubated with PKCζ-depleted cytosol (D). The band densities were determined as in Fig. 1. ER, endoplasmic reticulum; L-FABP, liver fatty acid-binding protein; PKC, protein kinase C.
By contrast, when ER was incubated with native cytosol and ATP (6), apoB48 eluted in earlier fractions (Fig. 1B) suggestive of a protein complex of ∼630 kDa compared with its monomer Mr of ∼250 kDa (27). In concert with the apoB48 data, L-FABP (Fig. 2B), VAMP7 (Fig. 3B), and CD36 (Fig. 4B) all eluted in nearly the same volume as apoB48, suggesting the possibility that each was associated in a multiprotein complex of 630 kDa. This was also true for Sar1, Sec24C, and Sec31 (supplementary Figs. VII-B, VIII-B, and IX-B).
Since L-FABP is able to generate PCTV from ER membranes as a recombinant protein (13), we predicted that it would also be able to generate a budding complex. To test this, we incubated intestinal ER with rL-FABP, solubilized the ER in 1% Triton X-100, chromatographed it on Sephacryl S400-HR, and then immunoblotted the eluate for apoB48 (Fig. 1C). ApoB48 had a broad elution volume whose peak suggested a complex of ∼500 Mr (Fig. 1C), smaller than when native cytosol was used. A smaller budding complex is the expected result as no COPII protein is present on PCTV in the absence of cytosol and ATP. These proteins, Sar1, Sec24C, and Sec31, presumably originating from that already bound to ER membranes (6), were present only as monomers under these conditions (supplementary Figs. VII-C, VIII-C, and IX-C). By contrast to the COPII proteins, L-FABP (Fig. 2C), VAMP7 (Fig. 3C), and CD36 (Fig. 4C) all eluted in a volume similar to that of apoB48 (Fig. 1C), suggesting that these proteins associated in a multiprotein complex when L-FABP was the initiating protein, supporting the data obtained in Figs. 1B, 2B, and 3B.
Formation of the PCTV budding complex requires PKCζ
In the last series of experiments, we wished to know if a budding complex formed in the absence of PKCζ, a condition where no PCTV budding occurs (26). For these studies, PKCζ was immunodepleted from cytosol, and the ER was washed with urea to remove PKCζ (26). As shown in Fig. 1D, when the washed ER was incubated with the depleted cytosol, no budding complex was detected, and apoB migrated near its monomer Mr as in Fig. 1A. Urea-washed ER can generate both PCTV and protein vesicles when incubated with cytosol and ATP (6, 26), suggesting that urea treatment of the ER does not impair the generation of PCTV. In sum, these data suggest that in the absence of phosphorylation by PKCζ, no budding complex occurs, providing a mechanism for the lack of PCTV budding activity under this condition (26). In concert with the data for apoB48, L-FABP (Fig. 2D), VAMP7 (Fig. 3D), and CD36 (Fig. 4D), all eluted in a volume of eluent consistent with their respective monomer Mr. Similarly, Sar1, Sec24C, and Sec31 all eluted in the volume expected for their monomer species (supplementary Figs. VII-D, VIII-D, and IX-D).
The COPII proteins are part of the budding complex
We tested for other proposed components of the budding complex (Sar1, Sec24C, and Sec31) using the same paradigm, expecting results as in the data above, except that the COPII proteins were present only at their monomer elution volumes when L-FABP was used to initiate the budding complex. These data are shown as supplementary Figs. III–V. The other COPII proteins, Sec23 and Sec13, were not tested.
In sum, the data support the thesis that in the presence of cytosol and an ATP-generating system, proteins associated with PCTV budding are maintained in an interactive complex. Further, L-FABP alone generates a slightly smaller complex in the absence of the COPII proteins. Under basal conditions, the ER contains the interactive proteins in their monomer state. Similarly, under conditions where no PCTV budding is expected in the absence of PKCζ, the components elute as monomers.
Immunoprecipitation studies of the budding complex
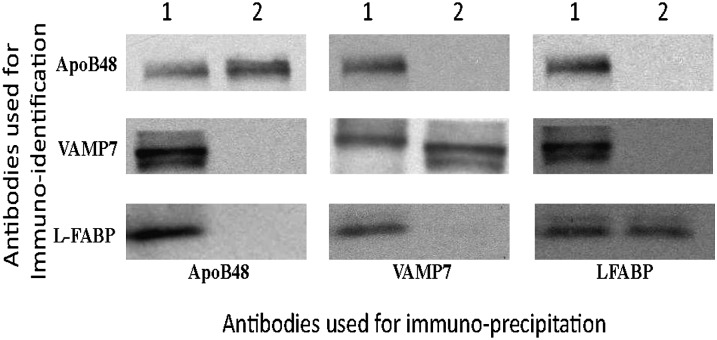
We wished to confirm the interactivity of the proteins comprising the budding complex by an alternative method to coelution on column chromatography. For this purpose, we performed a series of immunoprecipitation studies using bead-bound antibodies to apoB48, VAMP7, and L-FABP, and we identified the proteins that were coprecipitated. As shown in Fig. 5, when antibodies to apoB48 were used to precipitate proteins from the multiprotein complex, VAMP7 and L-FABP were also precipitated, indicating their interactivity with apoB48 in the complex. By contrast, when the anti-apoB48 antibodies were used to immunoprecipitate apoB48 from fractions consistent with the elution volume of its monomer species, only apoB48 was precipitated; neither VAMP7 nor L-FABP were precipitated. Similarly, antibodies to VAMP7 immunoprecipitated not only VAMP7 but also ApoB48 and L-FABP from the multiprotein complex; however, only VAMP7 was precipitated from the fractions where its monomer species eluted. Finally, antibodies to L-FABP immunoprecipitated not only L-FABP but also ApoB48 and VAMP7 from column fractions composing the protein complex; however, only L-FABP was immunoprecipitated from fractions where its monomer eluted. The protein-protein interactions from the column eluates (Fig. 5) are supported by the interactivity of these proteins as isolated from ER membranes as shown in Table 1. An interpretation of these data is that these three proteins not only coeluted from the column but also that they interacted as judged by their ability to be coimmunoprecipitated.
Fig. 5.
Coimmunoprecipitation studies from column fractions containing the multiprotein complex and from fractions containing monomer species of the proteins. Immunoblots that bead-bound antibodies to apoB48, VAMP7, or L-FABP were used in immunoprecipitation studies of Sephacryl S-400 HR column fractions, suggesting either a multiprotein complex (1, above the immunoblots) or, from fractions of the column eluent, the monomer species of the indicated protein (2, above the immunoblots), as judged from Figs 1–3. The immunoprecipitated proteins in each case were separated by SDS-PAGE and immunoblotted for apoB48, VAMP7, or L-FABP as indicated on the left side of the immunoblots. Apo, apolipoprotein; ER, endoplasmic reticulum; L-FABP, liver fatty acid-binding protein; VAMP7, vesicle-associated membrane protein 7.
Functionality of PCTV budding complex components
To support the composition of the PCTV budding complex, we sought to block the function of the components of the complex either by immunologic or genetic means to test their effect on PCTV budding activity. We reasoned that if inhibiting the budding complex component resulted in reduced budding activity, the protein in question could be considered important for complex formation and consequent budding activity.
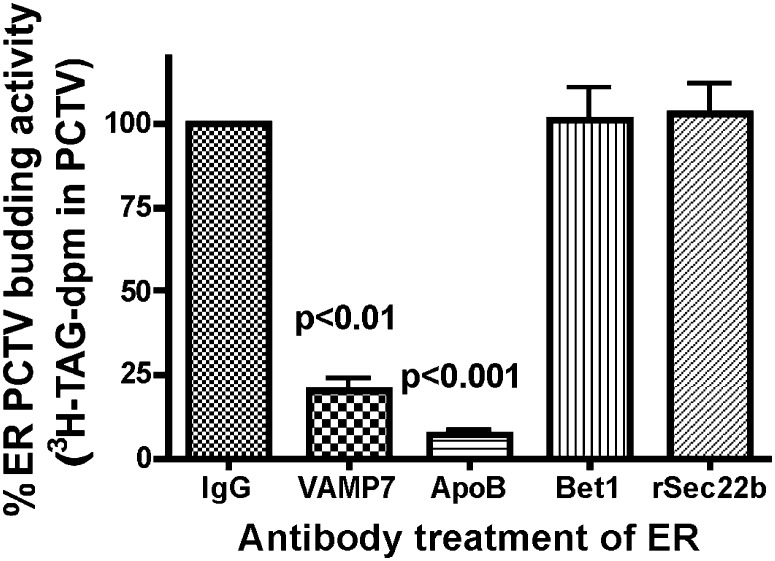
In the first series of experiments (Fig. 6), antibodies to budding components VAMP7 and apoB48 were incubated with intestinal ER, the excess antibodies removed by washing, and the ER used in a budding assay. The results were compared with ER budding activity where the ER was incubated with either IgG or antibodies to ER components Bet1 and Sec22b, which are v-SNAREs for ER-to-Golgi transport vesicles other than PCTV (32). When the ER was incubated with antibodies to apoB48 or VAMP7, PCTV production was severely attenuated (Fig. 6). By contrast, when antibodies to Bet1, a protein concentrated from ER in PCTV (6), were used, no decrease in budding activity was found compared with ER incubated with IgG (Fig. 6). Similarly, antibodies to Sec22b, a potential v-SNARE not present in PCTV (20), did not affect budding activity (Fig. 6).
Fig. 6.
The effect of various antibody treatments on PCTV generation from rat intestinal ER membranes. 3H-TAG loaded intestinal ER was incubated with IgG. Excess IgG was removed by washing, and the resulting ER incubated with intestinal cytosol and ATP. Other ER membranes were incubated with anti-VAMP7, anti-apoB48, anti-Bet1, or anti-rSec22b antibodies as indicated, the excess antibodies removed by washing, and the resulting ER incubated with cytosol and ATP. The amount of PCTV budding activity was determined (see “Methods”). The amount of PCTV budding activity using IgG was set to 100%. The percentage PCTV budding activity compared with ER incubated with IgG is reported on the ordinate for each antibody used. The data are the mean ± SEM (N = 4). The P values test the difference between the indicated bars and ER treated with IgG. ER, endoplasmic reticulum; PCTV, prechylomicron transport vesicle; TAG, triacylglycerol; VAMP7, vesicle-associated membrane protein 7.
The results of apoB48 antibody inhibition suggest that a portion of the apoB48 was on the cytosolic side of the ER membrane. Consistent with this thesis, exposure of the ER to proteinase K resulted in partial degradation of apoB48 in which a 70-kDa portion of the apoB48 was cleaved from apoB48 (Fig. 7, No Prot K, 240 kDa), resulting in a 170-kDa fragment (Fig. 7, Prot K). We have shown that the ER-resident protein calreticulin is not attacked by proteinase K using the same conditions (data not shown). By contrast, the apoB48 signal was completely extinguished when Triton X-100-disrupted ER was treated with proteinase K (Fig. 7, Triton + Prot K). One interpretation of these data is that a 70-kDa portion of apoB48 is exposed to the cytosol and thus susceptible to proteinase K proteolysis. This data is in contrast with prior data in liver that showed that apoB in ER was resistant to treatment with proteinase K (33). Also in contrast to our data in ER showing partial degradation of apoB48, apoB48, when present on chylomicrons in PCTV, is resistant to proteinase K treatment suggesting that it is protected by the vesicular membrane (6). Further, because our anti-apoB48 antibody is polyclonal, it is not surprising that it can identify both the full-length protein suggested by the data shown in Fig. 5 and the truncated one shown in Fig. 7.
Fig. 7.
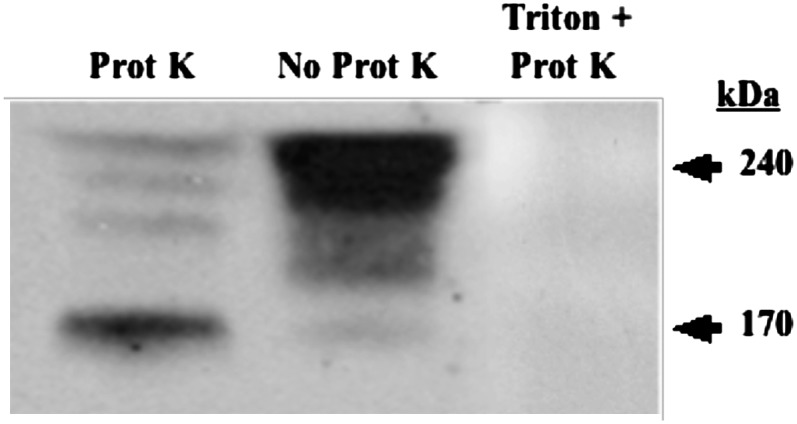
Proteinase K digests a portion of apoB48 in intestinal ER membranes. ER membranes, 150 µg protein, were incubated either with proteinase K (0.5 mg/ml) for 30 min at 4°C (Prot K), without proteinase K (No Prot K), or with proteinase K and ER membranes solubilized in 1% Triton X-100 (Triton + Prot K) as indicated. All incubations with proteinase K were washed with 3 M excess PMSF after the proteinase K incubations to stop further proteolysis. The ER was washed, and 30 µg of ER protein was separated by SDS-PAGE, transblotted to a nitrocellulose membrane, and immunoblotted for apoB48. The arrows mark proteins migrating at 240 and 170 kDa. In other experiments performed in the same manner, calreticulin was shown not to be attacked by proteinase K in the absence of Triton X-100 (data not shown). Apo, apolipoprotein; ER, endoplasmic reticulum.
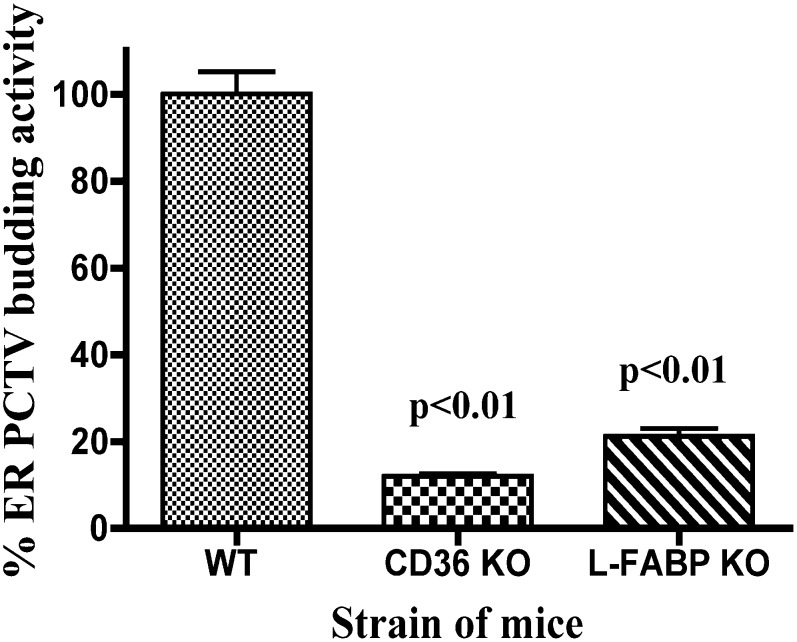
L-FABP can initiate PCTV budding (13) and is part of the budding complex identified (Figs. 2–4). To test the importance of L-FABP in the generation of PCTV, we tested the L-FABP gene-disrupted mouse model for its ability to generate PCTV (Fig. 8). Only 21% of the activity was found in the L-FABP knockout (KO) mouse compared with the wild type (WT) controls. This amount of budding activity is consistent with the known activity of I-FABP present in the cytosol of L-FABP KO mice; I-FABP has 22% of the ability of L-FABP to bud PCTV from the ER (13).
Fig. 8.
CD36 and L-FABP gene-disrupted mice have impaired generation of PCTV from intestinal ER. Mice whose CD36 or L-FABP genes had been disrupted as indicated were used in a PCTV-generation assay. 3H-TAG loaded ER from wild-type (WT) mice was incubated with WT cytosol and the amount of PCTV budding activity determined by 3H-TAG-dpm quantitation (see “Methods”). This activity was set to 100% (ordinate). CD36 knockout (KO) mice ER and cytosol were used in a similar PCTV budding assay as shown. L-FABP KO mice ER and cytosol were also tested for PCTV budding activity. The results are shown as a percentage of activity of the WT mice. The data are the mean ± 1 SEM (n = 4). The P values test the differences between the KO mice and the WT mice. ER, endoplasmic reticulum; L-FABP, liver fatty acid-binding protein; PCTV, prechylomicron transport vesicle; TAG, triacylglycerol.
We considered CD36 to play a potential role in PCTV budding because CD36 gene-disrupted mice are less able to export dietary lipid into the lymph than WT mice, and they have an increase in mucosal TAG on dietary fat loading, suggesting a TAG transport defect (24, 34). Further, CD36 was part of the PCTV budding complex (Fig. 4). We tested CD36 KO mice for their ability to generate PCTV. Consistent with the presence of CD36 in the PCTV budding complex (Fig. 4), ER and cytosol from CD36 KO mice had only of 12% the PCTV budding activity of WT mice (Fig. 8). We considered the possibility that in both CD36 and L-FABP KO mice, the ER was loaded with a reduced amount of 3H-TAG compared with the WT mice as an explanation for the reduced PCTV production. However, we found that the gene-disrupted mice had over 90% of the amount of 3H-TAG loading as did the WT mice in both cases (data not shown).
DISCUSSION
The PCTV budding complex from intestinal ER membranes that we describe is a composite of nine proteins. Despite the numerous proteins involved, eight are associated with PCTV either as cargo (apoB48) (6), v-SNARE (VAMP7) (19), Golgi-targeting proteins (COPII proteins) (6), or a budding initiator protein (L-FABP) for PCTV (13). The 9th protein, CD36, has been described as a fatty acid translocase (23), a processor of dietary fat in intestinal ER (30), and a lipoprotein binder (31). The formation of these nine proteins into a budding complex requires cytosol and phosphorylation of a 9-kDa protein by PKCζ (26). When L-FABP is used to generate the budding complex, it is smaller, consistent with the absence of the cytosolic components of the complex, the COPII proteins. The removal or functional blockade of any component of the complex, except for the COPII proteins, either by antibody treatment or genetic disruption significantly attenuates the ability of the ER membrane to generate PCTV, suggesting that each component of the complex needs to function together to produce PCTV. The L-FABP and CD36 KO mouse both result in nearly complete cessation of PCTV budding activity. Specific antibodies to VAMP7 and apoB48 all significantly attenuate PCTV budding.
Because of the specialized nature of the cargo for PCTV and the intermittency of its generation in relation to meals, it is not surprising that its components are different from other ER-to-Golgi transport vesicles whose cargo is constantly required either for intracellular utilization or for export. First, because of size constraints, the Sec13/31 cage that encompasses typical protein transport vesicles cannot contain chylomicrons, PCTV's cargo (2). Chylomicrons are large, averaging 250 nm, significantly larger than the typical ER-to-Golgi transport vesicle, ∼55–70 nm. The proposed Sec13/31 cage can easily contain these smaller vesicles, and the cage can even be enlarged to ∼100 nm but not more, making them unable to accommodate chylomicrons (2).
Second, newly synthesized protein vesicles require the COPII proteins for their generation, whereas PCTV do not (6). Interestingly, increasing numbers of cargo proteins are being recognized that either require cytosolic proteins other than COPII to maximize vesicle generation (11) or do not require COPII proteins at all for transport vesicle formation (35–40). The specific cargoes transported by COPII-independent mechanisms are diverse, encompassing both soluble and integral membrane proteins. The lack of COPII proteins in these systems is associated with vesicles that may either bypass the Golgi all together, indicating a loss of a Golgi targeting signal (36), or the vesicles may be targeted to the Golgi despite a lack of the COPII proteins (35, 37). In the case of PCTV, the COPII proteins are required for PCTV-Golgi fusion (6, 12).
Another unique feature of intestinal ER is the presence of VAMP7, a VAMP usually found in the post-Golgi fraction (41). With respect to PCTV, VAMP7 functions as the v-SNARE (19) for fusion with the Golgi. It is not clear what specific mechanism maintains VAMP7 in the ER in the intestine, although the N-terminal longin domain usually determines its subcellular location (42, 43). A functional role for VAMP7 in prechylomicron export in PCTV is suggested by an antibody blockade that significantly reduces PCTV output by the ER as we show. Additionally, Caco2 cells that do not express VAMP7 in their ER (44) are poor exporters of chylomicrons, although they synthesize TAG (45) and the apolipoproteins associated with chylomicrons (46).
This report adds a fourth novel feature of PCTV budding compared with the proteins that are transported from the ER to the Golgi using the COPII protein system. Whereas the only ER membrane protein required in the COPII protein vesicle system is Sec12, which is the GDP-GTP exchanger, Sec12 is not required with PCTV because Sar1 does not need to be loaded with GTP (6). However, in the case of PCTV, the transmembrane proteins VAMP7 and CD36 are needed as we show. A further distinction is that Sec12 remains on the ER membrane after protein vesicle budding, but with PCTV, VAMP7 and CD36 do not; they are components of PCTV.
A fifth novel feature of PCTV is the mechanism of cargo capture and required chaperones. As shown in Table 1, five different proteins important for PCTV budding interact with apoB48, the major cargo protein. Although the COPII proteins are important for Golgi targeting by PCTV (12, 13), they are not required for cargo capture or budding from intestinal ER membranes (6). By contrast, both the inner layer (Sar1, Sec23/24) and outer layers (Sec13/31) of COPII are important for both protein cargo and collagen transport from the ER (28, 47, 48). One possibility for the multiple differing proteins utilized for apoB48 capture for PCTV is the size of apoB48 (250,000 kDa) and its hydrophobic nature. These parameters may require these four proteins not only to capture cargo but also to serve in part as chaperones. Typical of transport vesicles, PCTV concentrate specific cargo proteins, such as apoB48 and apoAIV (6), but exclude others; the ER-resident proteins calnexin and calreticulin (6), Syn8, the ER-to-Golgi vSNARE, Ykt6 (20), and apolipoprotein apoAI (6).
The calculated kDa of the PCTV budding complex (766 kDa) is more than that predicted from the standardization of the column by the elution volumes of proteins of known kDa (∼630 kDa). This could be due to binding of the complex to the column matrix. In the case of Sec23A/24C, this led to a suggested kDa of 50 as judged by the elution volume from a size exclusion column, whereas the total kDa of the proteins as suggested by their DNA was 210 (11).
The demonstration that CD36 is part of the PCTV budding complex provides a mechanistic explanation for the defect in chylomicron production described in the CD36 null mouse (24, 34). CD36 has been shown to be important for uptake of fatty acids (23, 49) and cholesterol (49) and may contribute to delivering the lipid to ER sites relevant for budding complex formation. However, its presence in the budding complex and the finding that PCTV budding is almost completely eliminated in the absence of CD36 suggests that it plays additional roles related to complex assembly or interaction with the ER membrane.
The most likely protein to initiate complex assembly is L-FABP, which is present only in small amounts in native ER but is abundantly present in cytosol (50). On incubation with cytosol and ATP, L-FABP (but not I-FABP) readily binds to ER membranes (13). More specifically, when rL-FABP is incubated with ER, increased L-FABP binding occurs (13). As we show, under both conditions large protein complexes form. It is of interest that our budding complex assembled in the cold, confirming our pull-down assays, suggesting that multiple protein-protein interactions occur in the absence of energy. Since PCTV budding is not expected in the cold (6), the current data suggests that ER membrane deformation and vesicle fission are events that require incubation at 37°C. In this context, L-FABP gene-disrupted KO mice have a significant but only modest reduction in chylomicron output compared with WT mice (51). This may be due in part to the ability of I-FABP to generate PCTV, albeit at only a 22% rate of L-FABP (13), or other unrecognized compensatory factors.
CONCLUSION
PCTV budding and cargo capture require proteins that differ from those used for protein vesicles or other specialized vesicles, such as those that transport collagen from the ER to the Golgi. The rationale for these differences may lie in the intermittent tempo of PCTV production. PCTV generation is only minimally needed during fasting (7), but it is required in abundance after a fatty meal (8). In addition, the major cargo protein apoB48 is likely to need specialized proteins for inclusion in PCTV due to its size and hydrophobic nature. Finally, the very large cargo to be carried in PCTV is larger than can be accommodated in the Sec13/31 cage.
Supplementary Material
Footnotes
Abbreviations:
- Apo
- apolipoprotein
- ER
- endoplasmic reticulum
- ERES
- ER exit site
- I-FABP
- intestinal fatty acid-binding protein
- immunoprecipitation
- IP
- L-FABP
- liver fatty acid-binding protein
- PCTV
- prechylomicron transport vesicle
- PKC
- protein kinase C
- TAG
- triacylglycerol
- VAMP7
- vesicle-associated membrane protein 7
- v-SNARE
- vesicle-associated soluble N-ethylmaleimide-sensitive factor attachment protein receptor
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health Grants DK-60022 and DK-33301 (N.A.); DK-56260, DK-52574, and HL-38180 (N.O.D.); DK-038389 (J.S.); DK-3202019 (U.S.); DK-81413 (S.A.S.); and DK-38760 and DK-074565 (C.M.M.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of nine figures.
REFERENCES
- 1.Stagg S. M., LaPointe P., Balch W. E. 2007. Structural design of cage and coat scaffolds that direct membrane traffic. Curr. Opin. Struct. Biol. 17: 221–228. [DOI] [PubMed] [Google Scholar]
- 2.Stagg S. M., LaPointe P., Razvi A., Gurkan C., Potter C. S., Carragher B., Balch W. E. 2008. Structural basis for cargo regulation of COPII coat assembly. Cell. 134: 474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fromme J. C., Ravazzola M., Hamamoto S., Al-Balwi M., Eyaid W., Boyadjiev S. A., Cosson P., Schekman R., Orci L. 2007. The genetic basis of a craniofacial disease provides insight into COPII coat assembly. Dev. Cell. 13: 623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fromme J. C., Orci L., Schekman R. 2008. Coordination of COPII vesicle trafficking by Sec23. Trends Cell Biol. 18: 330–336. [DOI] [PubMed] [Google Scholar]
- 5.Aridor M., Fish K. N., Bannykh S., Weisman J., Roberts T. H., Lippincott-Schwartz J., Balch W. E. 2001. The Sar1 GTPase coordinates biosynthetic cargo selection with endoplasmic reticulum export site assembly. J. Cell Biol. 152: 213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siddiqi S. A., Gorelick F. S., Mahan J. T., Mansbach C. M., 2nd 2003. COPII proteins are required for Golgi fusion but not for endoplasmic reticulum budding of the pre-chylomicron transport vesicle. J. Cell Sci. 116: 415–427. [DOI] [PubMed] [Google Scholar]
- 7.Ockner R. K., Hughes F. B., Isselbacher K. J. 1969. Very low density lipoproteins in intestinal lymph: origin, composition, and role in lipid transport in the fasting state. J. Clin. Invest. 48: 2079–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mansbach C. M., 2nd, Arnold A. 1986. Steady-state kinetic analysis of triacylglycerol delivery into mesenteric lymph. Am. J. Physiol. 251: G263–G269. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi H., Fujimoto K., Cardelli J. A., Nutting D. F., Bergstedt S., Tso P. 1990. Fat feeding increases size, but not number, of chylomicrons produced by small intestine. Am. J. Physiol. 259: G709–G719. [DOI] [PubMed] [Google Scholar]
- 10.Matsuoka K., Orci L., Amherdt M., Bednarek S. Y., Hamamoto S., Schekman R., Yeung T. 1998. COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell. 93: 263–275. [DOI] [PubMed] [Google Scholar]
- 11.Kim J., Hamamoto S., Ravazzola M., Orci L., Schekman R. 2005. Uncoupled packaging of amyloid precursor protein and presenilin 1 into coat protein complex II vesicles. J. Biol. Chem. 280: 7758–7768. [DOI] [PubMed] [Google Scholar]
- 12.Siddiqi S., Siddiqi S. A., Mansbach C. M., II 2010. Sec24C is required for docking the prechylomicron transport vesicle with the Golgi. J Lipid Res. 51: 1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neeli I., Siddiqi S. A., Siddiqi S., Mahan J., Lagakos W. S., Binas B., Gheyi T., Storch J., Mansbach C. M., II 2007. Liver fatty acid-binding protein initiates budding of pre-chylomicron transport vesicles from intestinal endoplasmic reticulum. J. Biol. Chem. 282: 17974–17984. [DOI] [PubMed] [Google Scholar]
- 14.Bannykh S. I., Rowe T., Balch W. E. 1996. The organization of endoplasmic reticulum export complexes. J. Cell Biol. 135: 19–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuge O., Dascher C., Orci L., Rowe T., Amherdt M., Plutner H., Ravazzola M., Tanigawa G., Rothman J. E., Balch W. E. 1994. Sar1 promotes vesicle budding from the endoplasmic reticulum but not Golgi compartments. J. Cell Biol. 125: 51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stagg S. M., Gurkan C., Fowler D. M., LaPointe P., Foss T. R., Potter C. S., Carragher B., Balch W. E. 2006. Structure of the Sec13/31 COPII coat cage. Nature. 439: 234–238. [DOI] [PubMed] [Google Scholar]
- 17.Miller E., Antonny B., Hamamoto S., Schekman R. 2002. Cargo selection into COPII vesicles is driven by the Sec24p subunit. EMBO J. 21: 6105–6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips M. L., Pullinger C., Kroes I., Kroes J., Hardman D. A., Chen G., Curtiss L. K., Gutierrez M. M., Kane J. P., Schumaker V. N. 1997. A single copy of apolipoprotein B-48 is present on the human chylomicron remnant. J. Lipid Res. 38: 1170–1177. [PubMed] [Google Scholar]
- 19.Siddiqi S. A., Siddiqi S., Mahan J., Peggs K., Gorelick F. S., Mansbach C. M., II 2006. The identification of a novel endoplasmic reticulum to Golgi SNARE complex used by the prechylomicron transport vesicle. J. Biol. Chem. 281: 20974–20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siddiqi S. A., Mahan J., Siddiqi S., Gorelick F. S., Mansbach C. M., 2nd 2006. Vesicle-associated membrane protein 7 is expressed in intestinal ER. J. Cell Sci. 119: 943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Storch J., Bass N. M. 1990. Transfer of fluorescent fatty acids from liver and heart FABP to model membranes. J. Biol. Chem. 265: 7827–7831. [PubMed] [Google Scholar]
- 22.Newberry E. P., Xie Y., Kennedy S., Han X., Buhman K. K., Luo J., Gross R. W., Davidson N. O. 2003. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid-binding protein gene. J. Biol. Chem. 278: 51664–51672. [DOI] [PubMed] [Google Scholar]
- 23.Coburn C. T., Knapp F. F., Febbraio M., Beets A. L., Silverstein R. L., Abumrad N. A. 2000. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissue of CD36 knockout mice. J. Biol. Chem. 275: 32523–32529. [DOI] [PubMed] [Google Scholar]
- 24.Drover V. A., Ajmal M., Nassir F., Davidson N. O., Nauli A. M., Sahoo D., Tso P., Abumrad N. A. 2005. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J. Clin. Invest. 115: 1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar N. S., Mansbach C. M. 1997. Determinants of triacylglycerol transport from the endoplasmic reticulum to the Golgi in intestine. Am. J. Physiol. 273: G18–G30. [DOI] [PubMed] [Google Scholar]
- 26.Siddiqi S. A., Mansbach C. M., II 2008. PKC zeta-mediated phosphorylation controls budding of the pre-chylomicron transport vesicle. J. Cell Sci. 121: 2327–2338. [DOI] [PubMed] [Google Scholar]
- 27.Kane J. P., Hardman D. A., Paulus H. E. 1980. Heterogeneity of apolipoprotein B: isolation of a new species from human chylomicrons. Proc. Natl. Acad. Sci. USA. 77: 2465–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller E. A., Beilharz T. H., Malkus P. N., Lee M. C. S., Hamamoto S., Orci L., Schekman R. 2003. Multiple cargo binding sites on the COPII subunit Sec24p ensure capture of diverse membrane proteins into transport vesicles. Cell. 114: 497–509. [DOI] [PubMed] [Google Scholar]
- 29.Greenwalt D. E., Watt K. W., So O. Y., Jiwani N. 1990. PAS IV, an integral membrane protein of mammary epithelial cells, is related to platelet and endothelial cell CD36 (GP IV). Biochemistry. 29: 7054–7059. [DOI] [PubMed] [Google Scholar]
- 30.Drover V. A., Nguyen D. V., Bastie C. C., Darlington Y. F., Abumrad N. A., Pessin J. E., London E., Sahoo D., Phillips M. C. 2008. CD36 mediates both cellular uptake of very long chain fatty acids and their intestinal absorption in mice. J. Biol. Chem. 283: 13108–13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calvo D., Gomez-Coronado D., Suarez Y., Lasuncion M. A., Vega M. A. 1998. Human CD36 is a high affinity receptor for the native lipoproteins HDL, LDL, and VLDL. J. Lipid Res. 39: 777–788. [PubMed] [Google Scholar]
- 32.Joglekar A. P., Xu D., Rigotti D. J., Fairman R., Hay J. C. 2003. The SNARE motif contributes to rbet1 intracellular targeting and dynamics independently of SNARE interactions. J. Biol. Chem. 278: 14121–14133. [DOI] [PubMed] [Google Scholar]
- 33.Rusinol A. E., Jamil H., Vance J. E. 1997. In vitro reconstitution of assembly of apolipoprotein B48-containing lipoproteins. J. Biol. Chem. 272: 8019–8025. [DOI] [PubMed] [Google Scholar]
- 34.Nauli A. M., Nassir F., Zheng S., Yang Q., Lo C. M., Vonlehmden S. B., Lee D., Jandacek R. J., Abumrad N. A., Tso P. 2006. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology. 131: 1197–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasdemir B., Fitzgerald D. J., Prior I. A., Tepikin A. V., Burgoyne R. D. 2005. Traffic of Kv4 K+ channels mediated by KChIP1 is via a novel post-ER vesicular pathway. J. Cell Biol. 171: 459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Juschke C., Wachter A., Schwappach B., Seedorf M. 2005. SEC18/NSF-independent, protein-sorting pathway from the yeast cortical ER to the plasma membrane. J. Cell Biol. 169: 613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schepetilnikov M. V., Manske U., Solovyev A. G., Zamyatnin A. A., Jr., Schiemann J., Morozov S. Y. 2005. The hydrophobic segment of Potato virus X TGBp3 is a major determinant of the protein intracellular trafficking. J. Gen. Virol. 86: 2379–2391. [DOI] [PubMed] [Google Scholar]
- 38.Zuber C., Cormier J. H., Guhl B., Santimaria R., Hebert D. N., Roth J. 2007. EDEM1 reveals a quality control vesicular transport pathway out of the endoplasmic reticulum not involving the COPII exit sites. Proc. Natl. Acad. Sci. USA. 104: 4407–4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fatal N., Karhinen L., Jokitalo E., Makarow M. 2004. Active and specific recruitment of a soluble cargo protein for endoplasmic reticulum exit in the absence of functional COPII component Sec24p. J. Cell Sci. 117: 1665–1673. [DOI] [PubMed] [Google Scholar]
- 40.Fatal N., Suntio T., Makarow M. 2002. Selecive protein exit from yeast endoplasmic reticulum in absence of functional GOPII coat component Sec13p. Mol. Biol. Cell. 13: 4130–4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Advani R., Prekeris R., Lee K., Klumperman J., Scheller R. 1999. VAMP-7 mediates vesicular transport from endosomes to lysosomes. J. Cell Biol. 146: 765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng Q., Tran T. T., Tan H. X., Hong W. 2003. The cytoplasmic domain of Vamp4 and Vamp5 is responsible for their correct subcellular targeting: the N-terminal extension of VAMP4 contains a dominant autonomous targeting signal for the trans-Golgi network. J. Biol. Chem. 278: 23046–23054. [DOI] [PubMed] [Google Scholar]
- 43.Uemura T., Sato M. H., Takeyasu K. 2005. The longin domain regulates subcellular targeting of VAMP7 in Arabidopsis thaliana. FEBS Lett. 579: 2842–2846. [DOI] [PubMed] [Google Scholar]
- 44.Galli T. 1998. A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells. Mol. Biol. Cell. 9: 1437–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trotter P. J., Storch J. 1991. Fatty acid uptake and metabolism in a human intestinal cell line (Caco-2): comparison of apical and basolateral incubation. J. Lipid Res. 32: 293–304. [PubMed] [Google Scholar]
- 46.Traber M. G., Kayden H. J., Rindler M. J. 1987. Polarized secretion of newly synthesized lipoproteins by the Caco-2 human intestinal cell line. J. Lipid Res. 28: 1350–1363. [PubMed] [Google Scholar]
- 47.Townley A. K., Feng Y., Schmidt K., Carter D. A., Porter R., Verkade P., Stephens D. J. 2008. Efficient coupling of Sec23-Sec24 to Sec13-Sec31 drives COPII-dependent collagen secretion and is essential for normal craniofacial development. J. Cell Sci. 121: 3025–3034. [DOI] [PubMed] [Google Scholar]
- 48.Duden R. 2003. ER-to-Golgi transport: COP I and COP II function. [Review] Mol. Membr. Biol. 20: 197–207. [DOI] [PubMed] [Google Scholar]
- 49.Nassir F., Wilson B., Han X., Gross R. W., Abumrad N. A. 2007. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J. Biol. Chem. 282: 19493–19501. [DOI] [PubMed] [Google Scholar]
- 50.Bass N. M., Manning J. A., Ockner R. K., Gordon J. I., Seetharam S., Alpers D. H. 1985. Regulation of the biosynthesis of two distinct fatty acid-binding proteins in rat liver and intestine. J. Biol. Chem. 260: 1432–1436. [PubMed] [Google Scholar]
- 51.Newberry E. P., Xie Y., Kennedy S. M., Davidson N. O. 2006. Protection against Western diet-induced obesity and hepatic steatosis in liver fatty acid-binding protein knockout mice. Hepatology. 44: 1191–1205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.