Abstract
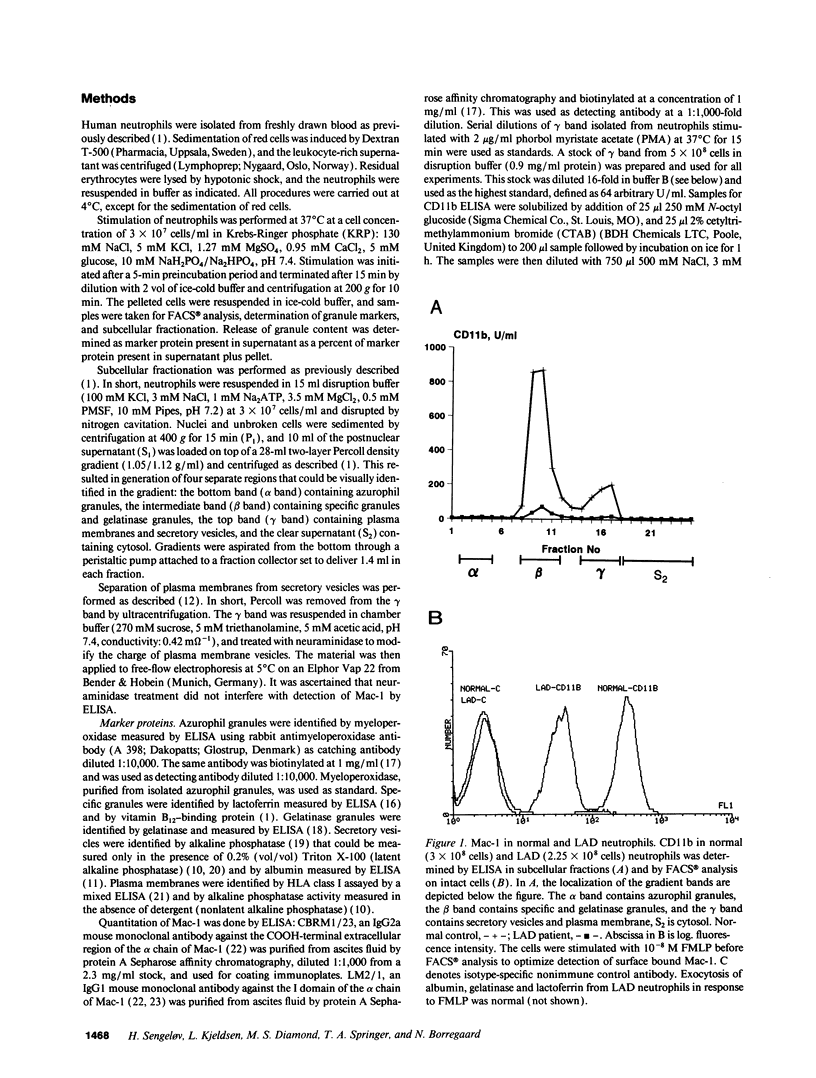
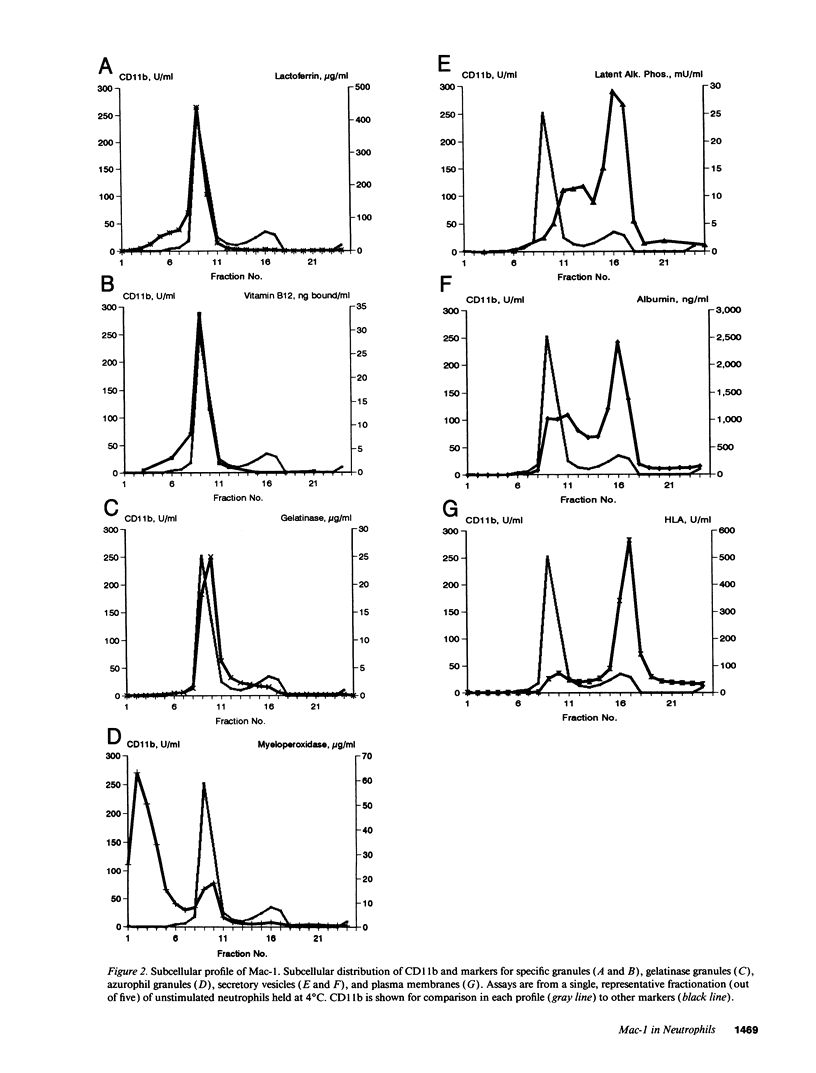
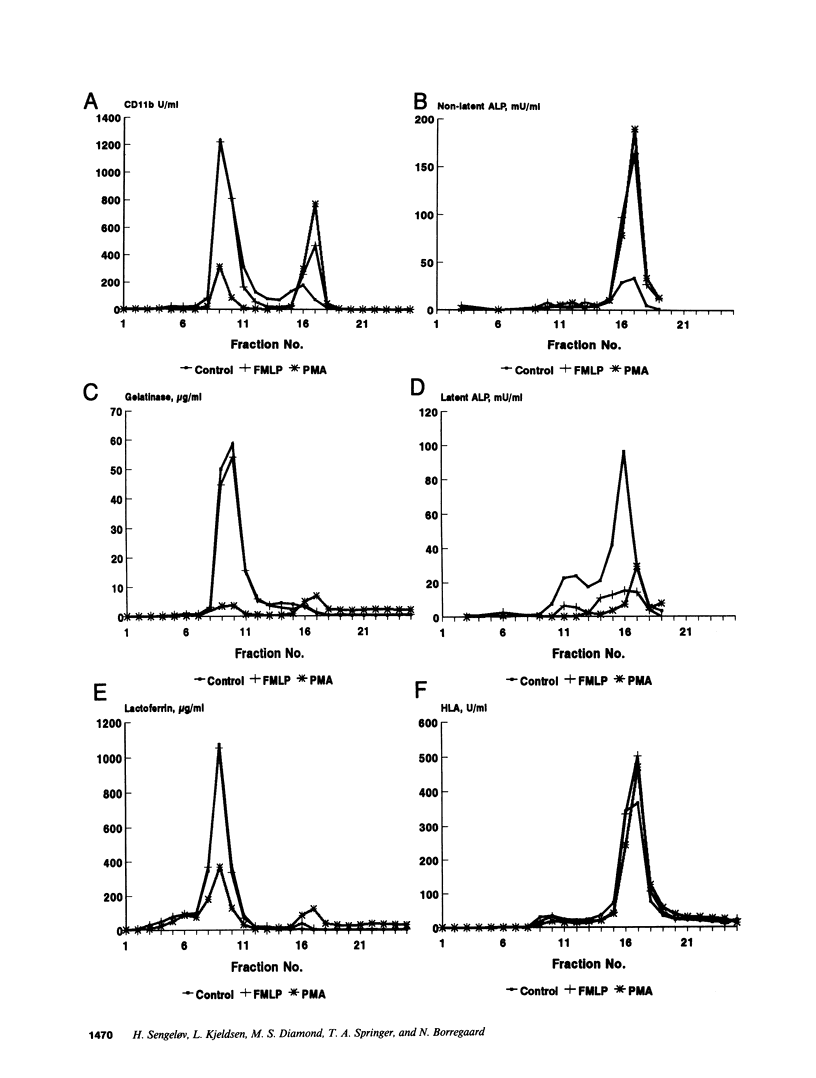
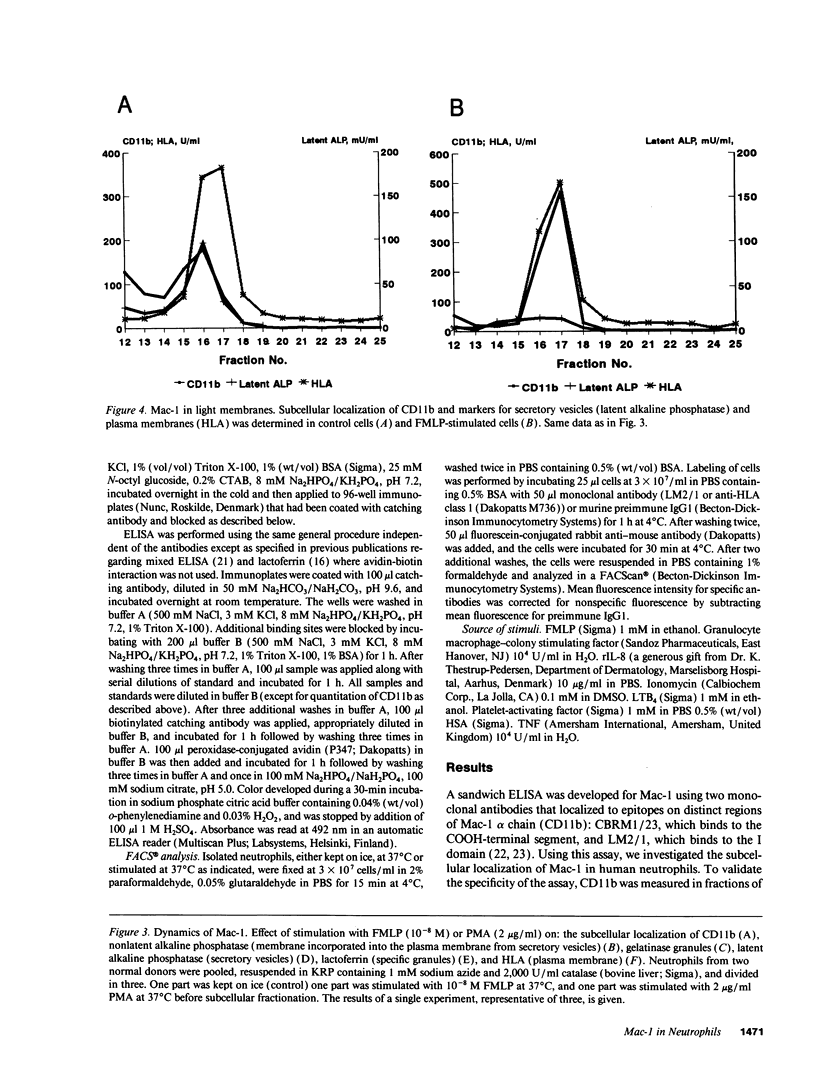
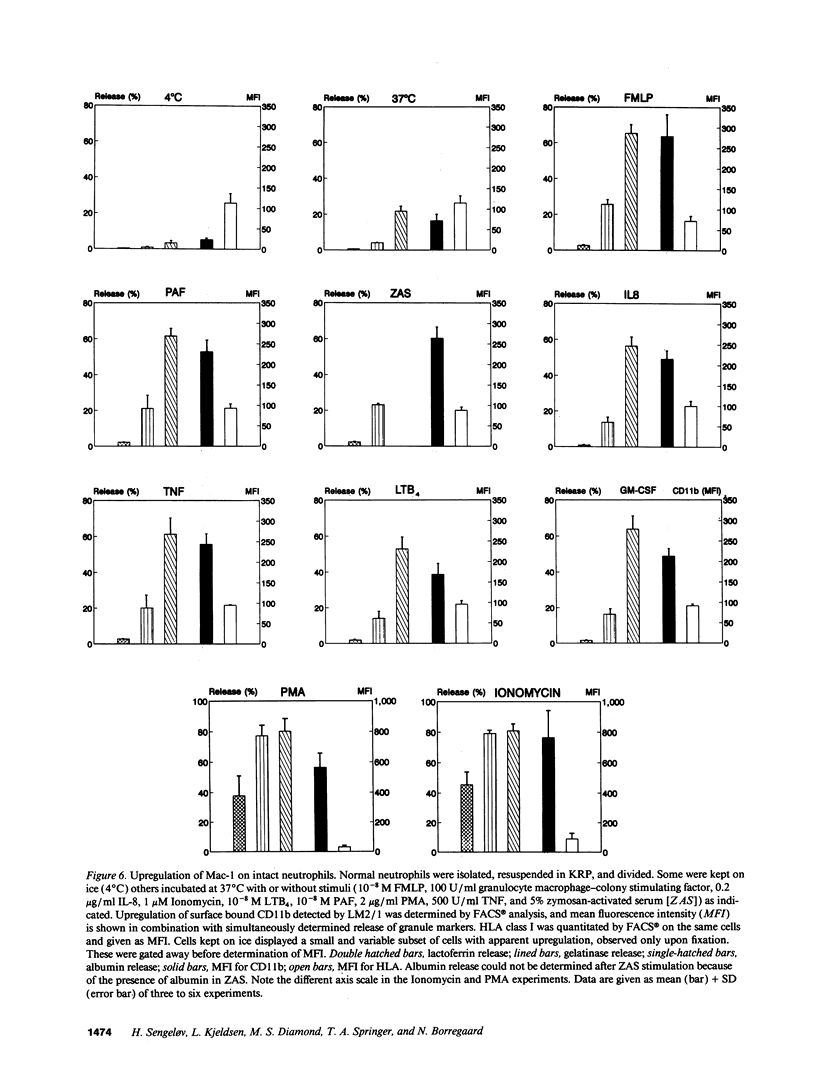
The subcellular localization of Mac-1 was determined in resting and stimulated human neutrophils after disruption by nitrogen cavitation and fractionation on two-layer Percoll density gradients. Light membranes were further separated by high voltage free flow electrophoresis. Mac-1 was determined by an ELISA with monoclonal antibodies that were specific for the alpha-chain (CD11b). In unstimulated neutrophils, 75% of Mac-1 colocalized with specific granules including gelatinase granules, 20% with secretory vesicles and the rest with plasma membranes. Stimulation with nanomolar concentrations of FMLP resulted in the translocation of Mac-1 from secretory vesicles to the plasma membrane, and only minimal translocation from specific granules and gelatinase granules. Stimulation with PMA or Ionomycin resulted in full translocation of Mac-1 from secretory vesicles and gelatinase granules to the plasma membrane, and partial translocation of Mac-1 from specific granules. These findings were corroborated by flow cytometry, which demonstrated a 6-10-fold increase in the surface membrane content of Mac-1 in response to stimulation with FMLP, granulocyte-macrophage colony stimulating factor, IL-8, leukotriene B4, platelet-activating factor, TNF-alpha, and zymosan-activated serum, and a 25-fold increase in response to Ionomycin. Thus, secretory vesicles constitute the most important reservoir of Mac-1 that is incorporated into the plasma membrane during stimulation with inflammatory mediators.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Miller L. J., Schmalstieg F. C., Rothlein R., Springer T. A. Contributions of the Mac-1 glycoprotein family to adherence-dependent granulocyte functions: structure-function assessments employing subunit-specific monoclonal antibodies. J Immunol. 1986 Jul 1;137(1):15–27. [PubMed] [Google Scholar]
- Anderson D. C., Schmalsteig F. C., Finegold M. J., Hughes B. J., Rothlein R., Miller L. J., Kohl S., Tosi M. F., Jacobs R. L., Waldrop T. C. The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency: their quantitative definition and relation to leukocyte dysfunction and clinical features. J Infect Dis. 1985 Oct;152(4):668–689. doi: 10.1093/infdis/152.4.668. [DOI] [PubMed] [Google Scholar]
- Bainton D. F., Miller L. J., Kishimoto T. K., Springer T. A. Leukocyte adhesion receptors are stored in peroxidase-negative granules of human neutrophils. J Exp Med. 1987 Dec 1;166(6):1641–1653. doi: 10.1084/jem.166.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beller D. I., Springer T. A., Schreiber R. D. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J Exp Med. 1982 Oct 1;156(4):1000–1009. doi: 10.1084/jem.156.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M., Wetzler E. M., Wallis R. S. Tumor necrosis factor is the major monocyte product that increases complement receptor expression on mature human neutrophils. Blood. 1988 Jan;71(1):151–158. [PubMed] [Google Scholar]
- Bjerrum O. W., Borregaard N. Mixed enzyme-linked immunosorbent assay (MELISA) for HLA class I antigen: a plasma membrane marker. Scand J Immunol. 1990 Mar;31(3):305–313. doi: 10.1111/j.1365-3083.1990.tb02773.x. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Christensen L., Bejerrum O. W., Birgens H. S., Clemmensen I. Identification of a highly mobilizable subset of human neutrophil intracellular vesicles that contains tetranectin and latent alkaline phosphatase. J Clin Invest. 1990 Feb;85(2):408–416. doi: 10.1172/JCI114453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Kjeldsen L., Rygaard K., Bastholm L., Nielsen M. H., Sengeløv H., Bjerrum O. W., Johnsen A. H. Stimulus-dependent secretion of plasma proteins from human neutrophils. J Clin Invest. 1992 Jul;90(1):86–96. doi: 10.1172/JCI115860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Miller L. J., Springer T. A. Chemoattractant-regulated mobilization of a novel intracellular compartment in human neutrophils. Science. 1987 Sep 4;237(4819):1204–1206. doi: 10.1126/science.3629236. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Tauber A. I. Subcellular localization of the human neutrophil NADPH oxidase. b-Cytochrome and associated flavoprotein. J Biol Chem. 1984 Jan 10;259(1):47–52. [PubMed] [Google Scholar]
- Dahlgren C. Difference in extracellular radical release after chemotactic factor and calcium ionophore activation of the oxygen radical-generating system in human neutrophils. Biochim Biophys Acta. 1987 Aug 19;930(1):33–38. doi: 10.1016/0167-4889(87)90152-2. [DOI] [PubMed] [Google Scholar]
- DeChatelet L. R., Cooper M. R. A modified procedure for the determination of leukocyte alkaline phosphatase. Biochem Med. 1970 Aug;4(1):61–68. doi: 10.1016/0006-2944(70)90103-1. [DOI] [PubMed] [Google Scholar]
- Dewald B., Bretz U., Baggiolini M. Release of gelatinase from a novel secretory compartment of human neutrophils. J Clin Invest. 1982 Sep;70(3):518–525. doi: 10.1172/JCI110643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M. S., Garcia-Aguilar J., Bickford J. K., Corbi A. L., Springer T. A. The I domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J Cell Biol. 1993 Feb;120(4):1031–1043. doi: 10.1083/jcb.120.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogstad G. O., Hagen I., Korsmo R., Solum N. O. Characterization of the proteins of isolated human platelet alpha-granules. Evidence for a separate alpha-granule-pool of the glycoproteins IIb and IIIa. Biochim Biophys Acta. 1981 Sep 29;670(2):150–162. doi: 10.1016/0005-2795(81)90003-9. [DOI] [PubMed] [Google Scholar]
- Graves V., Gabig T., McCarthy L., Strour E. F., Leemhuis T., English D. Simultaneous mobilization of Mac-1 (CD11b/CD18) and formyl peptide chemoattractant receptors in human neutrophils. Blood. 1992 Aug 1;80(3):776–787. [PubMed] [Google Scholar]
- Hibbs M. S., Bainton D. F. Human neutrophil gelatinase is a component of specific granules. J Clin Invest. 1989 Nov;84(5):1395–1402. doi: 10.1172/JCI114312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickstein D. D., Ozols J., Williams S. A., Baenziger J. U., Locksley R. M., Roth G. J. Isolation and characterization of the receptor on human neutrophils that mediates cellular adherence. J Biol Chem. 1987 Apr 25;262(12):5576–5580. [PubMed] [Google Scholar]
- Jones D. H., Anderson D. C., Burr B. L., Rudloff H. E., Smith C. W., Krater S. S., Schmalstieg F. C. Quantitation of intracellular Mac-1 (CD11b/CD18) pools in human neutrophils. J Leukoc Biol. 1988 Dec;44(6):535–544. doi: 10.1002/jlb.44.6.535. [DOI] [PubMed] [Google Scholar]
- Jones D. H., Schmalstieg F. C., Dempsey K., Krater S. S., Nannen D. D., Smith C. W., Anderson D. C. Subcellular distribution and mobilization of MAC-1 (CD11b/CD18) in neonatal neutrophils. Blood. 1990 Jan 15;75(2):488–498. [PubMed] [Google Scholar]
- Kjeldsen L., Bjerrum O. W., Askaa J., Borregaard N. Subcellular localization and release of human neutrophil gelatinase, confirming the existence of separate gelatinase-containing granules. Biochem J. 1992 Oct 15;287(Pt 2):603–610. doi: 10.1042/bj2870603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjeldsen L., Bjerrum O. W., Hovgaard D., Johnsen A. H., Sehested M., Borregaard N. Human neutrophil gelatinase: a marker for circulating blood neutrophils. Purification and quantitation by enzyme linked immunosorbent assay. Eur J Haematol. 1992 Oct;49(4):180–191. doi: 10.1111/j.1600-0609.1992.tb00045.x. [DOI] [PubMed] [Google Scholar]
- Kjeldsen L., Johnsen A. H., Sengeløv H., Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993 May 15;268(14):10425–10432. [PubMed] [Google Scholar]
- Lacal P., Pulido R., Sánchez-Madrid F., Mollinedo F. Intracellular location of T200 and Mo1 glycoproteins in human neutrophils. J Biol Chem. 1988 Jul 15;263(20):9946–9951. [PubMed] [Google Scholar]
- Lewis J. C., Hantgan R. R., Stevenson S. C., Thornburg T., Kieffer N., Guichard J., Breton-Gorius J. Fibrinogen and glycoprotein IIb/IIIa localization during platelet adhesion. Localization to the granulomere and at sites of platelet interaction. Am J Pathol. 1990 Jan;136(1):239–252. [PMC free article] [PubMed] [Google Scholar]
- Miller L. J., Bainton D. F., Borregaard N., Springer T. A. Stimulated mobilization of monocyte Mac-1 and p150,95 adhesion proteins from an intracellular vesicular compartment to the cell surface. J Clin Invest. 1987 Aug;80(2):535–544. doi: 10.1172/JCI113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. J., Schwarting R., Springer T. A. Regulated expression of the Mac-1, LFA-1, p150,95 glycoprotein family during leukocyte differentiation. J Immunol. 1986 Nov 1;137(9):2891–2900. [PubMed] [Google Scholar]
- Nitsch M., Gabrijelcic D., Tschesche H. Separation of granule subpopulations in human polymorphonuclear leukocytes. Biol Chem Hoppe Seyler. 1990 Jul;371(7):611–615. doi: 10.1515/bchm3.1990.371.2.611. [DOI] [PubMed] [Google Scholar]
- Petrequin P. R., Todd R. F., 3rd, Devall L. J., Boxer L. A., Curnutte J. T., 3rd Association between gelatinase release and increased plasma membrane expression of the Mo1 glycoprotein. Blood. 1987 Feb;69(2):605–610. [PubMed] [Google Scholar]
- Sengeløv H., Kjeldsen L., Borregaard N. Control of exocytosis in early neutrophil activation. J Immunol. 1993 Feb 15;150(4):1535–1543. [PubMed] [Google Scholar]
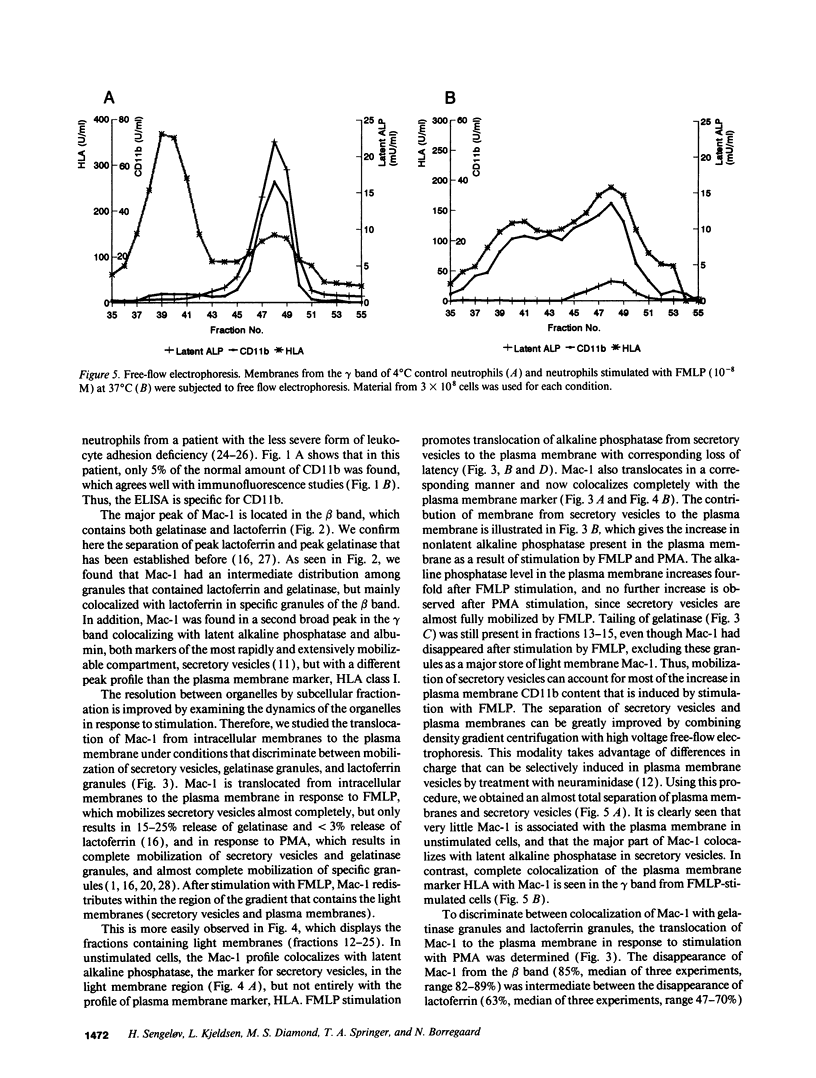
- Sengeløv H., Nielsen M. H., Borregaard N. Separation of human neutrophil plasma membrane from intracellular vesicles containing alkaline phosphatase and NADPH oxidase activity by free flow electrophoresis. J Biol Chem. 1992 Jul 25;267(21):14912–14917. [PubMed] [Google Scholar]
- Singer I. I., Scott S., Kawka D. W., Kazazis D. M. Adhesomes: specific granules containing receptors for laminin, C3bi/fibrinogen, fibronectin, and vitronectin in human polymorphonuclear leukocytes and monocytes. J Cell Biol. 1989 Dec;109(6 Pt 1):3169–3182. doi: 10.1083/jcb.109.6.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A., Thompson W. S., Miller L. J., Schmalstieg F. C., Anderson D. C. Inherited deficiency of the Mac-1, LFA-1, p150,95 glycoprotein family and its molecular basis. J Exp Med. 1984 Dec 1;160(6):1901–1918. doi: 10.1084/jem.160.6.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Arnaout M. A., Rosin R. E., Crowley C. A., Peters W. A., Babior B. M. Subcellular localization of the large subunit of Mo1 (Mo1 alpha; formerly gp 110), a surface glycoprotein associated with neutrophil adhesion. J Clin Invest. 1984 Oct;74(4):1280–1290. doi: 10.1172/JCI111538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wencel-Drake J. D., Plow E. F., Kunicki T. J., Woods V. L., Keller D. M., Ginsberg M. H. Localization of internal pools of membrane glycoproteins involved in platelet adhesive responses. Am J Pathol. 1986 Aug;124(2):324–334. [PMC free article] [PubMed] [Google Scholar]
- Wilchek M., Bayer E. A. Biotin-containing reagents. Methods Enzymol. 1990;184:123–138. doi: 10.1016/0076-6879(90)84267-k. [DOI] [PubMed] [Google Scholar]
- Yoon P. S., Boxer L. A., Mayo L. A., Yang A. Y., Wicha M. S. Human neutrophil laminin receptors: activation-dependent receptor expression. J Immunol. 1987 Jan 1;138(1):259–265. [PubMed] [Google Scholar]
- von Andrian U. H., Chambers J. D., McEvoy L. M., Bargatze R. F., Arfors K. E., Butcher E. C. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]


