Abstract
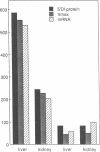
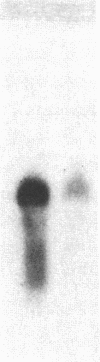
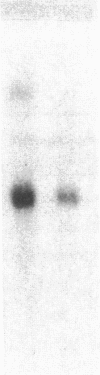
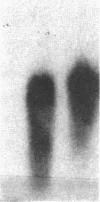
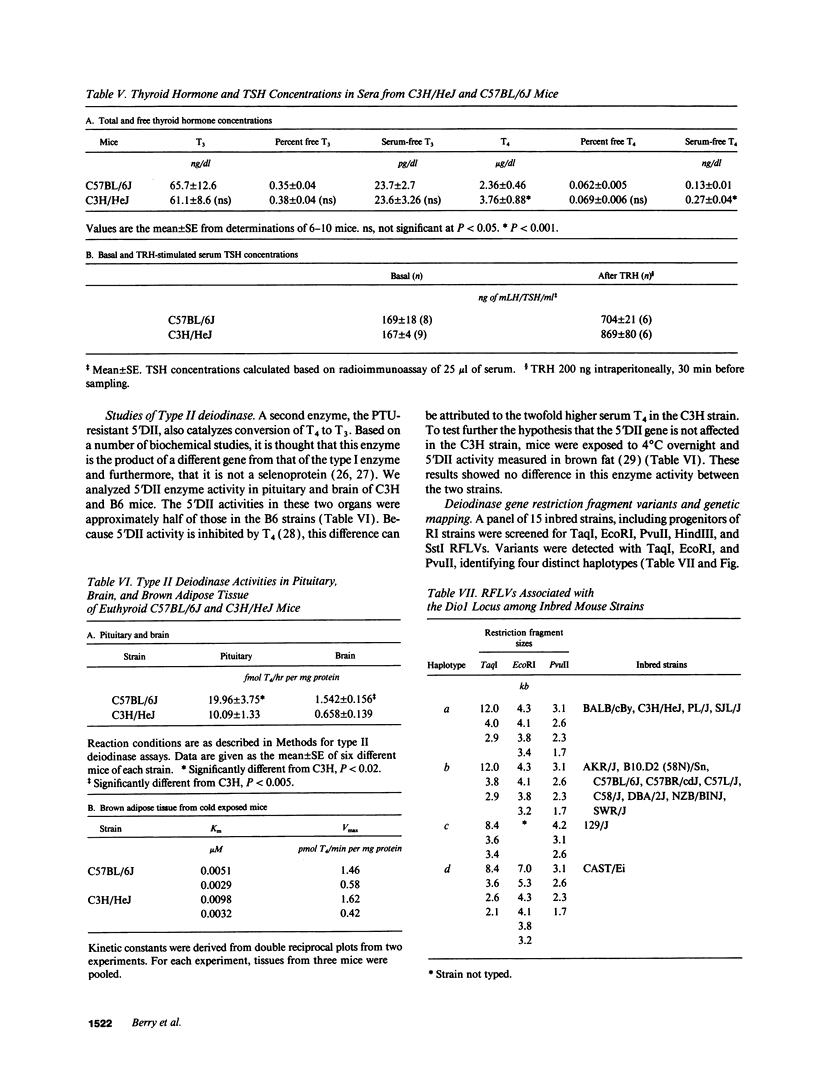
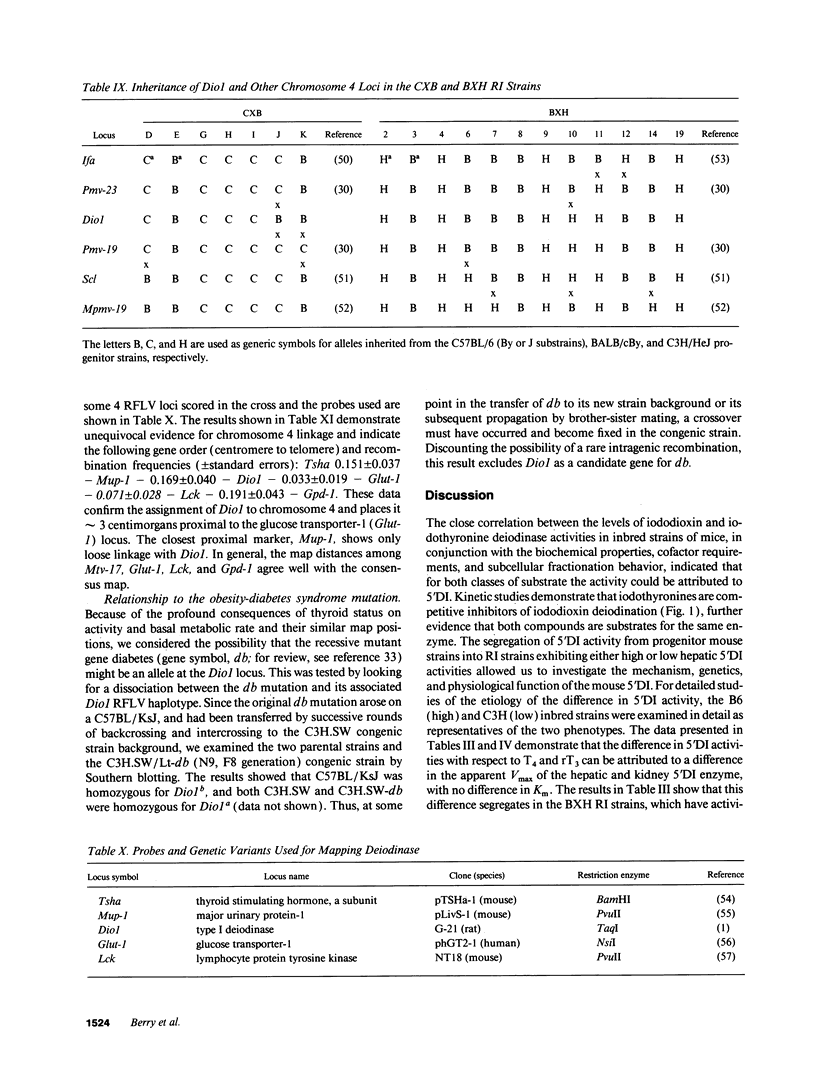
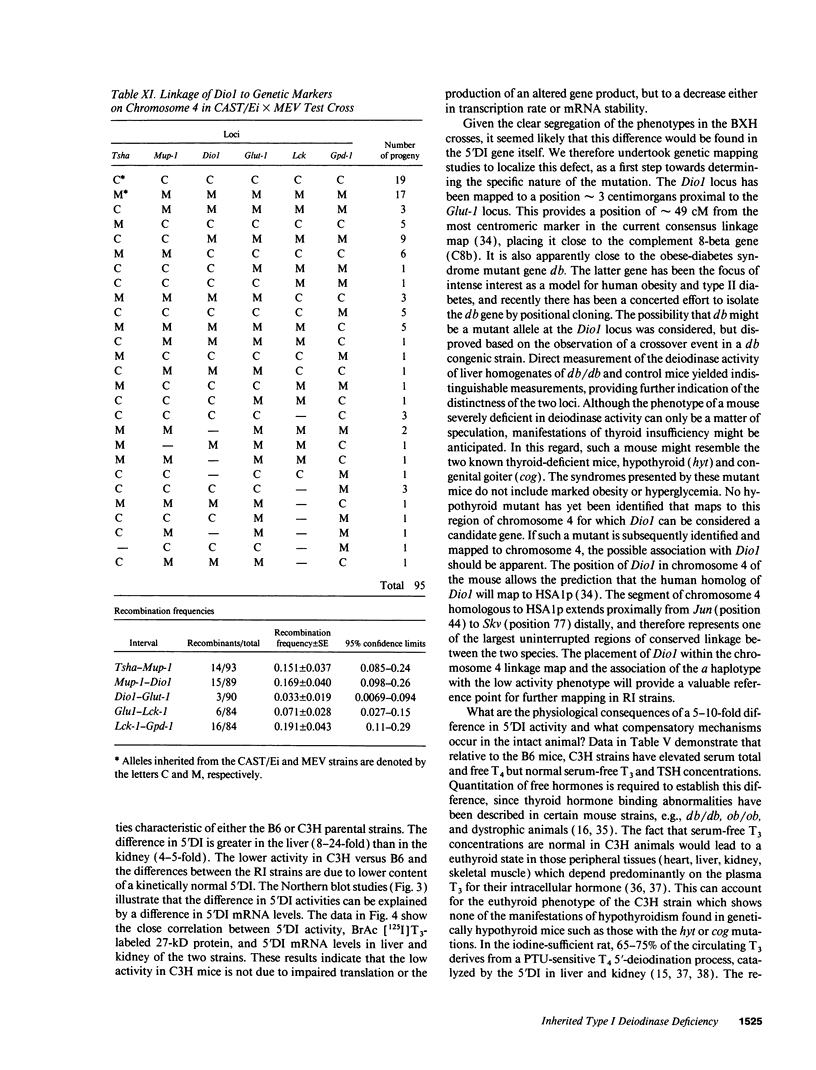
Inbred mouse strains differ in their capacity to deiodinate iododioxin and iodothyronines, with strains segregating into high or low activity groups. Metabolism of iododioxin occurs via the type I iodothyronine 5'deiodinase (5'DI), one of two enzymes that metabolize thyroxine (T4) to 3,5,3'-triiodothyronine (T3). Recombinant inbred strains derived from crosses between high and low activity strains exhibit segregation characteristic of a single allele difference. Hepatic and renal 5'DI mRNA in a high (C57BL/6J) and low (C3H/HeJ) strain paralleled enzyme activity and concentration, in agreement with a recent report. 5'DI-deficient mice had twofold higher serum free T4 but normal free T3 and thyrotropin. Brown adipose tissue 5'DII was invariant between the two strains. Southern analyses using a 5'DI probe identified a restriction fragment length variant that segregated with 5'DI activity in 33 of 35 recombinant inbred strains derived from four different pairs of high and low activity parental strains. Recombination frequencies using previously mapped loci allowed assignment of the 5'DI gene to mouse chromosome 4 and identified its approximate chromosomal position. We propose the symbol Dio1 to denote the mouse 5'DI gene. Conserved linkage between this segment of mouse chromosome 4 and human HSA1p predicts this location for human Dio1.
Full text
PDF

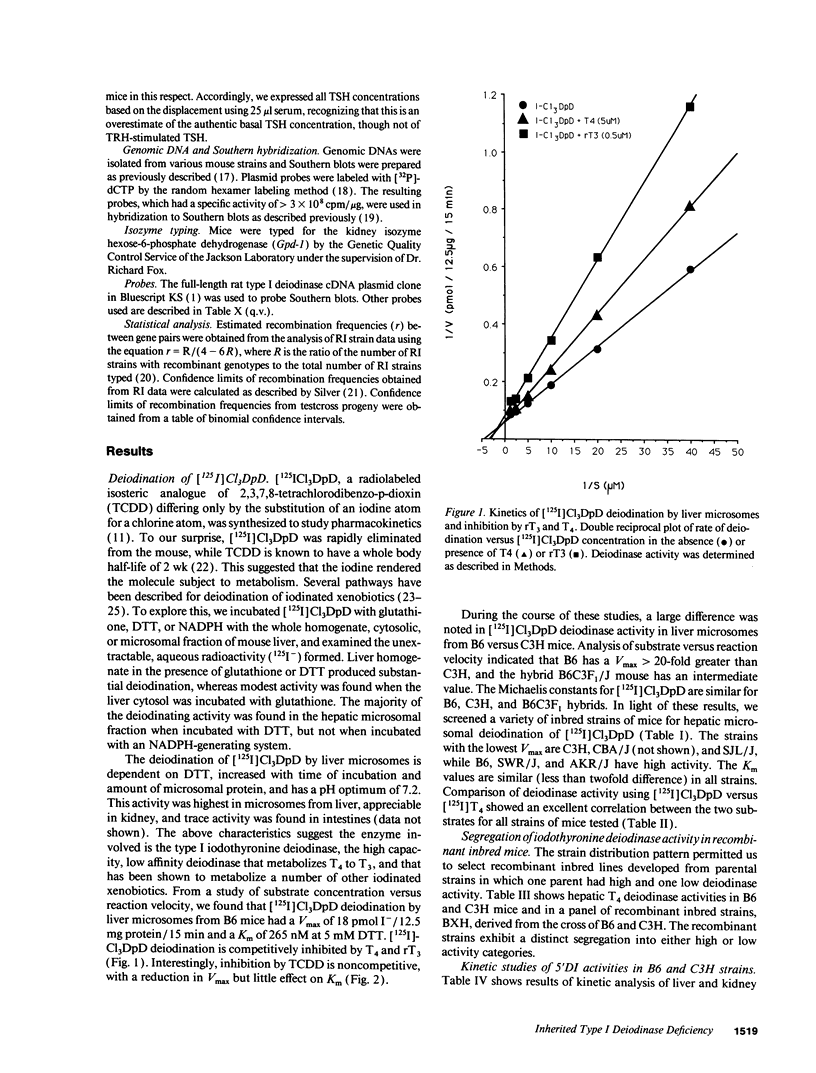




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur J. R., Nicol F., Hutchinson A. R., Beckett G. J. The effects of selenium depletion and repletion on the metabolism of thyroid hormones in the rat. J Inorg Biochem. 1990 Jun;39(2):101–108. doi: 10.1016/0162-0134(90)80018-s. [DOI] [PubMed] [Google Scholar]
- Beckett G. J., MacDougall D. A., Nicol F., Arthur R. Inhibition of type I and type II iodothyronine deiodinase activity in rat liver, kidney and brain produced by selenium deficiency. Biochem J. 1989 May 1;259(3):887–892. doi: 10.1042/bj2590887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley C. G., Visvader J., Green A. R., Aplan P. D., Metcalf D., Kirsch I. R., Gough N. M. Molecular cloning and chromosomal localization of the murine homolog of the human helix-loop-helix gene SCL. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):869–873. doi: 10.1073/pnas.88.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. J., Banu L., Larsen P. R. Type I iodothyronine deiodinase is a selenocysteine-containing enzyme. Nature. 1991 Jan 31;349(6308):438–440. doi: 10.1038/349438a0. [DOI] [PubMed] [Google Scholar]
- Berry M. J., Kates A. L., Larsen P. R. Thyroid hormone regulates type I deiodinase messenger RNA in rat liver. Mol Endocrinol. 1990 May;4(5):743–748. doi: 10.1210/mend-4-5-743. [DOI] [PubMed] [Google Scholar]
- Berry M. J., Kieffer J. D., Harney J. W., Larsen P. R. Selenocysteine confers the biochemical properties characteristic of the type I iodothyronine deiodinase. J Biol Chem. 1991 Aug 5;266(22):14155–14158. [PubMed] [Google Scholar]
- Berry M. J., Kieffer J. D., Larsen P. R. Evidence that cysteine, not selenocysteine, is in the catalytic site of type II iodothyronine deiodinase. Endocrinology. 1991 Jul;129(1):550–552. doi: 10.1210/endo-129-1-550. [DOI] [PubMed] [Google Scholar]
- Berry M. J., Maia A. L., Kieffer J. D., Harney J. W., Larsen P. R. Substitution of cysteine for selenocysteine in type I iodothyronine deiodinase reduces the catalytic efficiency of the protein but enhances its translation. Endocrinology. 1992 Oct;131(4):1848–1852. doi: 10.1210/endo.131.4.1396330. [DOI] [PubMed] [Google Scholar]
- Blank R., Eppig J., Fiedorek F. T., Jr, Frankel W. N., Friedman J. M., Huppi K., Jackson I., Mock B. Mouse chromosome 4. Mamm Genome. 1991;1(Spec No):S51–S78. doi: 10.1007/BF00656486. [DOI] [PubMed] [Google Scholar]
- Buchmann A., Bauer-Hofmann R., Mahr J., Drinkwater N. R., Luz A., Schwarz M. Mutational activation of the c-Ha-ras gene in liver tumors of different rodent strains: correlation with susceptibility to hepatocarcinogenesis. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):911–915. doi: 10.1073/pnas.88.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanoine J. P., Safran M., Farwell A. P., Tranter P., Ekenbarger D. M., Dubord S., Alex S., Arthur J. R., Beckett G. J., Braverman L. E. Selenium deficiency and type II 5'-deiodinase regulation in the euthyroid and hypothyroid rat: evidence of a direct effect of thyroxine. Endocrinology. 1992 Jul;131(1):479–484. doi: 10.1210/endo.131.1.1612029. [DOI] [PubMed] [Google Scholar]
- Chin W. W., Kronenberg H. M., Dee P. C., Maloof F., Habener J. F. Nucleotide sequence of the mRNA encoding the pre-alpha-subunit of mouse thyrotropin. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5329–5333. doi: 10.1073/pnas.78.9.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman D. L. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978 Mar;14(3):141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- Dandoy F., Kelley K. A., DeMaeyer-Guignard J., DeMaeyer E., Pitha P. M. Linkage analysis of the murine interferon-alpha locus on chromosome 4. J Exp Med. 1984 Jul 1;160(1):294–302. doi: 10.1084/jem.160.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Doolittle M. H., LeBoeuf R. C., Warden C. H., Bee L. M., Lusis A. J. A polymorphism affecting apolipoprotein A-II translational efficiency determines high density lipoprotein size and composition. J Biol Chem. 1990 Sep 25;265(27):16380–16388. [PubMed] [Google Scholar]
- Farwell A. P., Lynch R. M., Okulicz W. C., Comi A. M., Leonard J. L. The actin cytoskeleton mediates the hormonally regulated translocation of type II iodothyronine 5'-deiodinase in astrocytes. J Biol Chem. 1990 Oct 25;265(30):18546–18553. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Frankel W. N., Stoye J. P., Taylor B. A., Coffin J. M. A linkage map of endogenous murine leukemia proviruses. Genetics. 1990 Feb;124(2):221–236. doi: 10.1093/genetics/124.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel W. N., Stoye J. P., Taylor B. A., Coffin J. M. Genetic identification of endogenous polytropic proviruses by using recombinant inbred mice. J Virol. 1989 Sep;63(9):3810–3821. doi: 10.1128/jvi.63.9.3810-3821.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz M. J., Vogel S. N. The physical separation of Lps and Ifa loci in BXH recombinant inbred mice. J Immunol. 1989 Nov 1;143(9):3001–3006. [PubMed] [Google Scholar]
- Gasiewicz T. A., Geiger L. E., Rucci G., Neal R. A. Distribution, excretion, and metabolism of 2,3,7,8-tetrachlorodibenzo-p-dioxin in C57BL/6J, DBA/2J, and B6D2F1/J mice. Drug Metab Dispos. 1983 Sep-Oct;11(5):397–403. [PubMed] [Google Scholar]
- Kaplan M. M., Young J. B., Shaw E. A. Abnormal thyroid hormone binding to serum proteins in ob/ob and db/db genetically obese mice. Endocrinology. 1985 Nov;117(5):1858–1863. doi: 10.1210/endo-117-5-1858. [DOI] [PubMed] [Google Scholar]
- Kleinhaus N., Faber J., Kahana L., Schneer J., Scheinfeld M. Euthyroid hyperthyroxinemia due to a generalized 5'-deiodinase defect. J Clin Endocrinol Metab. 1988 Apr;66(4):684–688. doi: 10.1210/jcem-66-4-684. [DOI] [PubMed] [Google Scholar]
- Larsen P. R., Dick T. E., Markovitz B. P., Kaplan M. M., Gard T. G. Inhibition of intrapituitary thyroxine to 3.5.3'-triiodothyronine conversion prevents the acute suppression of thyrotropin release by thyroxine in hypothyroid rats. J Clin Invest. 1979 Jul;64(1):117–128. doi: 10.1172/JCI109430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. R., Frumess R. D. Comparison of the biological effects of thyroxine and triiodothyronine in the rat. Endocrinology. 1977 Apr;100(4):980–988. doi: 10.1210/endo-100-4-980. [DOI] [PubMed] [Google Scholar]
- Larsen P. R., Silva J. E., Kaplan M. M. Relationships between circulating and intracellular thyroid hormones: physiological and clinical implications. Endocr Rev. 1981 Winter;2(1):87–102. doi: 10.1210/edrv-2-1-87. [DOI] [PubMed] [Google Scholar]
- Leonard J. L., Kaplan M. M., Visser T. J., Silva J. E., Larsen P. R. Cerebral cortex responds rapidly to thyroid hormones. Science. 1981 Oct 30;214(4520):571–573. doi: 10.1126/science.7291997. [DOI] [PubMed] [Google Scholar]
- Mandel S. J., Berry M. J., Kieffer J. D., Harney J. W., Warne R. L., Larsen P. R. Cloning and in vitro expression of the human selenoprotein, type I iodothyronine deiodinase. J Clin Endocrinol Metab. 1992 Oct;75(4):1133–1139. doi: 10.1210/jcem.75.4.1400883. [DOI] [PubMed] [Google Scholar]
- Marth J. D., Disteche C., Pravtcheva D., Ruddle F., Krebs E. G., Perlmutter R. M. Localization of a lymphocyte-specific protein tyrosine kinase gene (lck) at a site of frequent chromosomal abnormalities in human lymphomas. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7400–7404. doi: 10.1073/pnas.83.19.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxon H. R., Burman K. D., Premachandra B. N., Chen I. W., Burger A., Levy P., Georges L. P. Familial elevations of total and free thyroxine in healthy, euthyroid subjects without detectable binding protein abnormalities. Acta Endocrinol (Copenh) 1982 Jun;100(2):224–230. doi: 10.1530/acta.0.1000224. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Propylthiouracil inhibits the conversion of L-thyroxine to L-triiodothyronine. An explanation of the antithyroxine effect of propylthiouracil and evidence supporting the concept that triiodothyronine is the active thyroid hormone. J Clin Invest. 1972 Sep;51(9):2493–2497. doi: 10.1172/JCI107063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paigen B., Mitchell D., Holmes P. A., Albee D. Genetic analysis of strains C57BL/6J and BALB/cJ for Ath-1, a gene determining atherosclerosis susceptibility in mice. Biochem Genet. 1987 Dec;25(11-12):881–892. doi: 10.1007/BF00502607. [DOI] [PubMed] [Google Scholar]
- Paigen B., Nesbitt M. N., Mitchell D., Albee D., LeBoeuf R. C. Ath-2, a second gene determining atherosclerosis susceptibility and high density lipoprotein levels in mice. Genetics. 1989 May;122(1):163–168. doi: 10.1093/genetics/122.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland A., Teitelbaum P., Glover E., Kende A. Stimulation of in vivo hepatic uptake and in vitro hepatic binding of [125I]2-lodo-3,7,8-trichlorodibenzo-p-dioxin by the administration of agonist for the Ah receptor. Mol Pharmacol. 1989 Jul;36(1):121–127. [PubMed] [Google Scholar]
- Safran M., Farwell A. P., Leonard J. L. Evidence that type II 5'-deiodinase is not a selenoprotein. J Biol Chem. 1991 Jul 25;266(21):13477–13480. [PubMed] [Google Scholar]
- Schoenmakers C. H., Pigmans I. G., Poland A., Visser T. J. Impairment of the selenoenzyme type I iodothyronine deiodinase in C3H/He mice. Endocrinology. 1993 Jan;132(1):357–361. doi: 10.1210/endo.132.1.8419134. [DOI] [PubMed] [Google Scholar]
- Scott M. T., Sinsheimer J. E. In vitro dehalogenation of para-substituted aromatic halides in rat liver preparations. J Pharm Sci. 1984 Aug;73(8):1101–1104. doi: 10.1002/jps.2600730819. [DOI] [PubMed] [Google Scholar]
- Shows T. B., Eddy R. L., Byers M. G., Fukushima Y., Dehaven C. R., Murray J. C., Bell G. I. Polymorphic human glucose transporter gene (GLUT) is on chromosome 1p31.3----p35. Diabetes. 1987 Apr;36(4):546–549. doi: 10.2337/diab.36.4.546. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Gordon M. B., Crantz F. R., Leonard J. L., Larsen P. R. Qualitative and quantitative differences in the pathways of extrathyroidal triiodothyronine generation between euthyroid and hypothyroid rats. J Clin Invest. 1984 Apr;73(4):898–907. doi: 10.1172/JCI111313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Adrenergic activation of triiodothyronine production in brown adipose tissue. Nature. 1983 Oct 20;305(5936):712–713. doi: 10.1038/305712a0. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Contributions of plasma triiodothyronine and local thyroxine monodeiodination to triiodothyronine to nuclear triiodothyronine receptor saturation in pituitary, liver, and kidney of hypothyroid rats. Further evidence relating saturation of pituitary nuclear triiodothyronine receptors and the acute inhibition of thyroid-stimulating hormone release. J Clin Invest. 1978 May;61(5):1247–1259. doi: 10.1172/JCI109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Pituitary nuclear 3,5,3'-triiodothyronine and thyrotropin secretion: an explanation for the effect of thyroxine. Science. 1977 Nov 11;198(4317):617–620. doi: 10.1126/science.199941. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Leonard J. L. Regulation of rat cerebrocortical and adenohypophyseal type II 5'-deiodinase by thyroxine, triiodothyronine, and reverse triiodothyronine. Endocrinology. 1985 Apr;116(4):1627–1635. doi: 10.1210/endo-116-4-1627. [DOI] [PubMed] [Google Scholar]
- Silver J. Confidence limits for estimates of gene linkage based on analysis of recombinant inbred strains. J Hered. 1985 Nov-Dec;76(6):436–440. doi: 10.1093/oxfordjournals.jhered.a110140. [DOI] [PubMed] [Google Scholar]
- Sinsheimer J. E., Wang T., Röder S., Shum Y. Y. Mechanisms for the biodehalogenation of iodocompounds. Biochem Biophys Res Commun. 1978 Jul 14;83(1):281–286. doi: 10.1016/0006-291x(78)90428-x. [DOI] [PubMed] [Google Scholar]
- St Germain D. L. The effects and interactions of substrates, inhibitors, and the cellular thiol-disulfide balance on the regulation of type II iodothyronine 5'-deiodinase. Endocrinology. 1988 May;122(5):1860–1868. doi: 10.1210/endo-122-5-1860. [DOI] [PubMed] [Google Scholar]
- Taylor B. A., Grieco D. Localization of the gene encoding insulin-like growth factor I on mouse chromosome 10. Cytogenet Cell Genet. 1991;56(1):57–58. doi: 10.1159/000133046. [DOI] [PubMed] [Google Scholar]
- Taylor B. A., Rowe L. A mouse linkage testing stock possessing multiple copies of the endogenous ecotropic murine leukemia virus genome. Genomics. 1989 Aug;5(2):221–232. doi: 10.1016/0888-7543(89)90050-5. [DOI] [PubMed] [Google Scholar]
- Taylor B. A., Rowe L. Genes for serum amyloid A proteins map to Chromosome 7 in the mouse. Mol Gen Genet. 1984;195(3):491–499. doi: 10.1007/BF00341452. [DOI] [PubMed] [Google Scholar]
- Taylor B. A., Rowe L. Localization of the gene encoding the alpha-subunit of the acetylcholine receptor on chromosome 2 of the mouse. Cytogenet Cell Genet. 1989;52(1-2):102–103. doi: 10.1159/000132854. [DOI] [PubMed] [Google Scholar]
- Wataya Y., Santi D. V. Thymidylate synthetase catalyzed dehalogenation of 5-bromo-and 5-iodo-2'-deoxyuridylate. Biochem Biophys Res Commun. 1975 Nov 17;67(2):818–823. doi: 10.1016/0006-291x(75)90886-4. [DOI] [PubMed] [Google Scholar]
- van Doorn J., Roelfsema F., van der Heide D. The effect of propylthiouracil and methimazole on the peripheral conversion of thyroxine to 3,5,3'-triiodothyronine in athyreotic thyroxine-maintained rats. Acta Endocrinol (Copenh) 1983 Aug;103(4):509–520. doi: 10.1530/acta.0.1030509. [DOI] [PubMed] [Google Scholar]