Abstract
In order to promote wound repair and induce tissue regeneration, an engineered hyaluronan (HA) hydrogel – Carbylan GSX, which contains di(thiopropionyl) bishydrazide-modified hyaluronic acid (HA-DTPH), di(thiopropionyl) bishydrazide-modified gelatin (Gtn-DTPH) and polyethylene glycol diacrylate (PEGDA), has been developed for extracellular matrix (ECM) defects of the superficial and middle layers of the lamina propria. The purpose of this study was to evaluate the biocompatibility of Carbylan GSX in a previously established immortalized human vocal fold fibroblast (hVFF) cell line prior to human clinical trials. Immortalized hVFF proliferation, viability, apoptosis and transcript analysis for both ECM constituents and inflammatory markers were measured for two-dimensional and three-dimensional culture conditions. There were no significant differences in morphology, cell marker protein expression, proliferation, viability and apoptosis of hVFF cultured with Carbylan GSX compared to Matrigel, a commercial 3D control, after one week. Gene expression levels for fibromodulin, TGF-β1, and TNF-α were similar between Carbylan GSX and Matrigel. Fibronectin, hyaluronidase 1 and COX2 expression levels were induced by Carbylan GSX; whereas IL6, IL8. COL1 and hyaluronic acid synthase 3 expression levels were decreased by Carbylan GSX. This investigation demonstrates that Carbylan GSX may serve as a natural biomaterial for tissue engineering of human vocal folds.
Keywords: human vocal fold fibroblasts, three-dimensional culture, extracellular matrix, HA hydrogel (Carbylan GSX), cell proliferation, cell viability, gene expression
1. Introduction
Normal vocal fold vibration is crucially dependent upon tissue composition and viscoelasticity. The vocal folds are composed of a stratified squamous epithelial layer, lamina propria and underlying muscle. The lamina propria is multilayered connective tissue, composed of fibroblasts and their secreted extracellular matrix (ECM) containing elastin, collagen, hyaluronic acid, and proteoglycans [1] [2] [3]. It’s well known that these molecules come together to form the structural framework that stabilizes tissues and provides mechanical support for cell attachment. The ECM plays a very important functional role in cell behavior, including cell proliferation, migration, shape, orientation, and most importantly, cell differentiation. The maintenance and induction of cell differentiation is governed by regulation of gene expression, which occurs in a tissue-specific manner and dictates how cells carry out their necessary functions. When the composition of the extracellular matrix (ECM) of the lamina propria is altered, vocal fold vibratory function can be severely disrupted due to alterations in tissue viscoelasticity [4] [5] [6] [7]. However, the mechanisms responsible for regulating fibroblast ECM gene expression and proliferation remain unclear.
Laryngeal cancer, injury and infectious diseases can cause damage or loss of vocal fold lamina propria. Wound healing results in scar formation, including the changes of ECM composition and viscoelastic properties of the tissue, resulting in voice disorders estimated to affect 6.2% of the population [8]. Vocal fold scar is difficult to treat effectively with current surgical paradigms and available biomaterials, including injections of collagen, fat, and implantation of Teflon [9] [10]. Treatment failures have been ascribed to poor understanding of pathologic processes in the ECM, as well as suboptimal materials that may negatively affect vocal fold biomechanical properties. Accordingly, there is a clinical need for improved understanding of the pathophysiology of disrupted ECM and the development of advanced biomaterials relative to the native lamina propria tissue.
As a component of vocal fold lamina propria, hyaluronic acid (HA) regulates cell attachment, proliferation, migration, and differentiation. The primary action of HA on tissue repair appears to be its effect on cell-cell and cell-matrix interactions [11]. In addition, HA is the first macromolecule to appear in the ECM during wound healing [12]. Previous animal work has demonstrated that an injectable HA-based synthetic ECM (sECM) hydrogel - Carbylan GSX, enhances tissue regeneration, restoration of the tissue’s biomechanical properties and overall wound healing [13] [14] [15] [16]. Prior to initiation of human clinical trails with this sECM hydrogel it is fundamental that we evaluate our candidate sECM therapeutics with an in vitro assay of human origin to assess biocompatibility, tolerability and effectiveness. In this investigation we analyzed cell morphology, marked protein distribution, cell proliferation, viability, apoptosis, inflammatory gene and ECM gene expression with human vocal fold fibroblasts (hVFFs) cultured in 3D Carbylan GSX.
2. Materials and Methods
2.1. Preparation of gels
The engineered injectable chemically-modified HA-gelatin hydrogel –Carbylan GSX was developed in conjunction with the Center for Therapeutic Biomaterials at The University of Utah [17] [18] [19]. It was composed of polyethylene glycol diacrylate (PEGDA, Mol. Wt 3400)-crosslinked di(thiopropionyl) bishydrazide-modified hyaluronic acid (HA-DTPH) and di(thiopropionyl) bishydrazide-modified gelatin (Gtn-DTPH). Carbylan-GSX 5% was prepared by mixing a 1.5% solution of Carbylan-SX (HA-DTPH) in phosphate buffered saline (PBS; pH 7.4) and Gtn-DTPH (0.075%) in PBS (pH 7.4) with a 4.0% solution of PEGDA in PBS (pH 7.4) according to a volume ratio of 4 to 1. [17]. The final Carbylan GSX was cast and allowed to gel for 10 minutes in 37°C incubator before adding medium.
As a control to Carbylan GSX, Matrigel (BD Biosciences Discovery Labware, Bedford, MA), a solubilized commercial basement membrane preparation which is commonly used by cell biologists as a substrate for cell culture, was selected. Matrigel (BD Biosciences Discovery Labware, Bedford, MA) gels were made according to the manufacture’s protocol. Briefly, the material was thawed overnight on ice at 4°C. Subsequently, the product was kept on ice and handled with pre-cooled pipettes. After casting, the material was allowed to gel for 10 minutes in 37°C incubator before adding medium.
2.2. Cell culture
2.2.1. Human vocal fold fibroblasts and cell line
The primary hVFF cells were established from a normal human vocal fold specimen, which was harvested from an autopsy of 21-year-old donor, within 4 hours after death. The research protocol was conducted with approval by the Institutional Review Board of the University of Wisconsin-Madison. Primary hVFFs were isolated and cultured as described previous [20]. Fibroblast identification has previously been reported with this culture methodology [21]. The immortalized hVFF line was established by transduction of the primary hVFF with a defective retrovirus expressing hTERT, which was generated from pBABE-hTERT-neo vector [22]. The immortalized hVFFs were grown in DMEM cell culture medium with 10% Fetal Bovine Serum (FBS), 1X non-essential amino acid (NEAA, Sigma Inc. St. Louis, MO). These immortalized cells showed similar morphological features and ECM gene expression until passage 25 as the primary hVFFs. Full immortalization details and characterization of this cell line is described elsewhere [22]. Immortalized hVFF were used in this study because they were a reproducible, characterized source of VFF of human origin, in addition to providing an unlimited supply of cells. Primary fibroblasts from this tissue type is typically very difficult to obtain in adequate numbers to complete the experiments completed. All experiments described in this manuscript were carried out using immortalized hVFF from passages 3–8.
2.2.2. Two-dimensional (2D) and three-dimensional (3D) cell culture
For 2D culture, immortalized hVFFs at 0.5×106/well (6-well plate) were seeded on transwell permeable supports (inserts with 0.4 μm membrane pore size, Millipore Inc. Billerica, MA) with 500 μl gelated hydrogel, Carbylan GSX. For 3D culture, single cell suspension was mixed with Carbylan GSX or Matrigel solutions at a final concentration of 2×106 cells/ml by gently vortex, and then 500μl of this mixture was placed in each well of 6-well plate with the inserts. After gelation (gel thickness was approximately 0.5 mm), cell culture medium (DMEM-10% FBS) was added above and under gel. The media was exchanged every three days.
2.3. Immunocytochemistry
To investigate the immunoreactive response of the fibroblasts to our HA sECM hydrogels, we measured mediators in culture to determine of the whether or not the constructs will have an effect on immune mediated inflammation, which has an intricate cascade with a panoply of players. Active inflammation can be determined in fibroblast cultures by altered expression of biosynthase prolyl 4-hydroxylase (hPH) [23] and intercellular adhesion molecule-1 (ICAM-1), a cell surface ligand known to be critical to the propagation of immune-mediated inflammation [24]. To determine expression of the above mentioned cell marker proteins with confocal microscopy, hVFFs were cultured on or in gel on the 6-well glass bottom culture plates (MatTek, Ashland, MA) for 3 days. Prior to confocal imaging, the gels with cells were fixed in 4% paraformaldehyde for 40 minutes. After washing, the cells were permeabilized with a PBS with 0.1% Triton X-100 for 5 minutes × 3 times. Cells were incubated with 10% BLOCKADE (AbD Serotec, Osaka, Japan) and 5% normal goat serum. Samples were incubated with the primary antibodies directly against hPH (1:200, Millipore, Billerica, MA) and ICAM-1 (also referred to as CD54, 1:100, Santa Cruz Biotechnology, Santa Cruz, CA), respectively, and corresponding fluorescent-conjugated (Alexa 488 and 568) secondary antibodies. Nuclei were labeled by TO-PRO-3 (2 μM, Invitrogen, Carlsbad, CA) for 10 minutes. Control cells were processed identically without application of primary antibodies. Digital images were captured every 2μm in the middle 30–60 μm layer using an Bio-Rad Radiance 2100 MP Rainbow inverted confocal microscope equipped with dual excitation lasers for red, green and blue fluorescence.
2.4. Cell Proliferation Assay
To determine the proliferation of immortalized hVFFs in Carbylan GSX, single cells were mixed into Carbylan GSX or Matrigel at a final concentration of 27 × 103 cells/ml gel and 75 μl of the cell-seeded gel was cast per well of 96-well plates. After gelation, the cells were incubated with DMEM with 10% FBS. For the 2D condition, a volume of 75 μl of Carbylan GSX solution was dispensed into each well of a 96-well plate and allowed to solidify in a 37°C incubator for 10 minutes. 2×103 cells were diluted in 150 μl of media and were rapidly dispensed into each well. Cells growing in untreated wells (polystyrene) served as control. For each condition, cells from the same passage were assessed. On day 0, 1, 3, 5 and 7 of culture, cell numbers were monitored in quadruplicate for each condition using the CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI) as per manufacture’s instructions. Briefly, 100 μl of medium was gently removed from each well and 100 μl of CellTiter-Glo Reagent was added. The plate was rocked for two minutes and incubated an additional eight minutes before reading luminescent output on a Flex Station III plate reader (Molecular Devices, Sunnyvale, CA). The CellTiter-Glo Assay is a homogeneous method for determining the number of viable cells in culture. Detection is based on use of a bioluminescent reaction to measure the amount of ATP in viable cells. The luminescent signal (relative light unit, RLU), reflects ATP levels, and is proportional to the number of viable cells [25] [26]. If cellular health is reduced, ATP is rapidly consumed. RLU levels were plotted against the time course of the assay to yield the growth profile of the cells seeded on/in the sECM.
2.5. Apoptosis/Cytotoxicity Assay
The cytotoxicity of Carbylan GSX on hVFFs was quantitatively analyzed using a Live/Dead viability/cytotoxicity assay (Invitrogen, Carlsbad, CA). This is a two-color fluorescence cell viability assay that is based on the simultaneous identification of live and dead cells with two probes (calcein AM, CaAM and ethidium homodimer-1, EthD-1) that measure recognized parameters of cell viability-intracellular esterase activity and plasma membrane integrity. This assay has been utilized to quantify apoptotic cell death and cell-mediated cytotoxicity [27] [28]. The intracellular esterase activity in live cells was determined by the enzymatic conversion of the virtually nonfluorescent cell-permeate calcein AM to the intensely fluorescent calaein. EthD-1 enters cells with damaged membranes and undergoes a 40-fold enhancement of fluorescence upon binding to nucleic acids, producing a bright red fluorescence in dead cells. Cells were treated with 70% methanol for 40minutes as the maximum dead cell control. To perform the assay, immortalized hVFFs were seeded in 96-well plates at 104 cells/well in 2D and 3D Carbylan GSX or Matrigel with phenol red-free DMEM-10% FBS. After 3 and 7 day incubations, the indicator dyes (1μM CaAM and 2μM EthD-1 in PBS) were directly added on cultured cells for each condition in quadruplicate and the resulting solution was scanned by the fluorescence multiwell plate-reader equipped with a 485/530 nm excitation filter and a 530/645 nm emission filter. After subtracting the background of each culture condition (negative control, medium plus gel without cells), live cell and dead cell percentages were calculated by normalized monolayer culture on polystyrene as a measure of maximum live cells.
2.6. Gene Expression Analysis
2.6.1. RNA extraction and reverse transcription
Total cellular RNA was isolated from hVFFs on/in Carbylan GSX, Matrigel or polystyrene using an RNeasy Mini Kit (Qiagen, Valencia, CA), according to the manufacturer’s instructions. First strand cDNA was synthesized from 1 μg of total RNA by using a QuantiTect Reverse Transcription Kit (Qiagen, CA).
2.6.2 Quantitative real-time PCR
mRNA levels were quantified by real-time PCR -- standard curve method -- using LightCycler System (Roche, IN), with amplification of β-actin as control. mRNA from the cDNA sample was amplified with specific primer pairs for cyclooxygenase II (COX2), interleukin 6 (IL-6), interleukin 8 (IL-8), tumor necrosis factor-alpha (TNF-α), collagen I α-2 (Col I), fibronectin (FN), fibromodulin (FMOD), hyaluronic acid synthase 3 (HAS3), hyaluronidase 1 (HYAL1), transforming growth factor-beta1 (TGF-β1) and β-actin. The primer sequences, gene bank access number and expected PCR product sizes are listed in Table 1. Amplification was carried out for 45 cycles, each of 95°C, 10s, 55°C, 5s, and 72°C 8s in a 20ul reaction mixture containing 2μl cDNA, 0.5 μM of each primer, MgCl2, dNTPs and Tag DNA polymerase from LightCycler FastStart plus DNA Master SYBR Green I (Roche, Indianapolis, IN) by the LightCycler 1.5 System. The exact amplification efficiencies of target and reference genes were assessed by LightCycler software before any calculation of the normalized gene expression was completed. The specificity of every pair of PCR primers was confirmed by melting curves and PCR reactions (Figure 1), which shows the single peak and the predicted sized DNA band of each gene product. Results were shown by the fold of control (polystyrene or Matrigel) of target gene mRNA concentration (ng/μl) normalized by housekeeping gene β-actin mRNA (ng/μl). Each individual sample was tested in triplicate.
Table 1.
Primer Sequences and Products of RT-PCR
| Gene | GenBank # | Forward Primer | Reverse Primer | Size of PCR product |
|---|---|---|---|---|
| COX2 | NM_000963 | 5′-ACAGATGCAATTCCCGGACGTCTA-3′ | 5′-TGGGCATGAAACTGTGGTTTGCTC-3′ | 112bp |
| IL-6 | NM_000600 | 5′-AAGCCAGAGCTGTGCAGATGAGTA-3′ | 5′-GCTGCGCAGAATGAGATGAGTTGT-3′ | 176bp |
| IL-8 | NM_000584 | 5′-AGACATACTCCAAACCTTTCCACCC-3′ | 5′-TCCAGACAGAGCTCTCTTCCATCA-3′ | 122bp |
| TNF-α | NM_000594 | 5′-AATGGTGCTCCTGGTATTGCTGGT-3′ | 5′-ACCAGTGTCTCCTTTGCTGCCA-3′ | 131bp |
| Collagen I α-2 | NM_000089 | 5′-AACAAATAAGCCATCACGCCTGCC-3′ | 5′-TGAAACAGACTGGGCCAATGTCCA-3′ | 101bp |
| Fibronectin | NM_002026 | 5′-ACCTACGGATGACTCGTGCTTTGA-3′ | 5′-CAAAGCCTAAGCACTGGCACAACA-3′ | 116bp |
| Fibromodulin | NM_002023 | 5′-AACCTCAAGTACCTGCCCTTCGTT-3′ | 5′-TATCACTGGTGATCTGGTTGCCGT-3′ | 148bp |
| HAS3 | NM_005329 | 5′-TGTGCAGTGTATTAGTGGGCCCTT-3′ | 5′-TTGGAGCGCGCGGTATACTTAGTT-3′ | 177bp |
| HYAL1 | NM_000088 | 5′-TAACCCTGCCAGTTTCTCCATCCA-3′ | 5′-AGCCAGGGTAGCATCGACATTTGA-3′ | 125bp |
| TGF-β1 | NM_000660 | 5′-TGCTCGCCCTGTACAACAGCA-3′ | 5′-CGTTGTGGGTTTCCACCATTAGCA-3′ | 126bp |
| β-Actin | NM_001101 | 5′-ACGTTGCTATCCAGGCTGTGCTAT-3′ | 5′-CTCGGTGAGGATCTTCATGAGGTAGT-3′ | 188bp |
Figure 1.
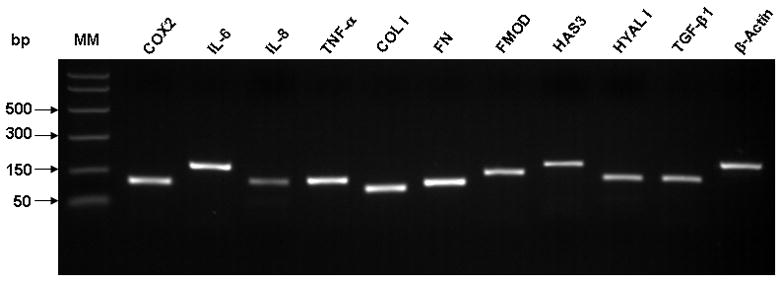
Electrophoresis of PCR products for each pair of real-time PCR primers. PCR products for each gene match a single band of predicted size as in Table 1 confirming the primer specificity for each gene.
2.7. Statistical analysis
The results of cell proliferation and cell viability/cytotoxicity assays are expressed as mean ± standard deviations (SD) of quadruplicated samples per condition at each experimental time interval. For mRNA expression levels, the results are expressed as mean ± SD of mRNA fold change per control. Repeated measure ANOVA was performed to compare the group conditions for cell proliferation, cell viability/cytotoxicity, gene expression for each condition each the experimental time points to determine whether any statistical differences existed among each condition at each time point. If the p value was significant, then pair wise comparisons were examined (Fisher’s protected LSD). All p-values reported are two-sided. A p<0.05 was considered statistically significant difference. Statistical interpretations were made using PROC mixed in SAS version 9.1.3 (SAS Institute, Cary, NC).
3. Results
3.1. 3D culture affected cell morphology
Morphology of fibroblasts cultured in the Carbylan GSX/Matrigel (3D), or on thin layer (2D) of Carbylan GSX, or a tissue culture plate was observed on Day 7 after seeding. Two major morphological patterns were observed: stretched, spindle-like shaped fibroblasts and single rounded fibroblasts (Figure 2). Cells grown on polystyrene (Figure 2A) and on a thin layer of Carbylan GSX (Figure 2B), showed typical spindle-shaped appearance, with several cells penetrating into the surface of Carbylan GSX (data not shown). In contrast, the cells in 3D Carbylan GSX (Figure 2C) showed rounded, isolated morphology and evenly distributed in Carbylan gel without significant migration, which was similar to those grown in 3D Matrigel (Figure 3A). This experiment was repeated more three times with consistent results, demonstrating that the observed the difference in cellular morphology was clearly attributable to cell the 2D versus 3D microenvironments.
Figure 2.
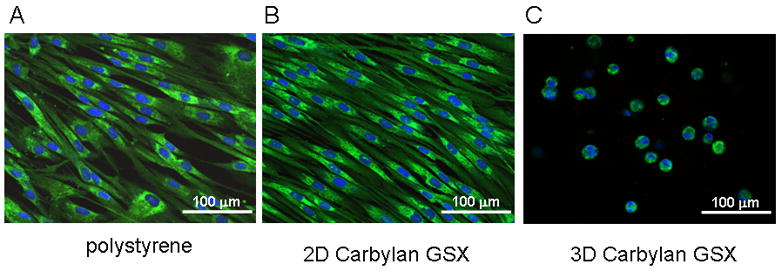
Morphologic appearance and fibroblast marker (hPH) distribution of immortalized hVFFs in various culture environments. After 72h of in vitro culture, fluorescent images of hVFF on polystyrene (A), on a thin layer of Carbylan GSX (2D condition) or in Carbylan GSX (3D condition) were captured by an inverted confocal microscope equipped with dual excitation lasers for green and blue fluorescence.
Figure 3.
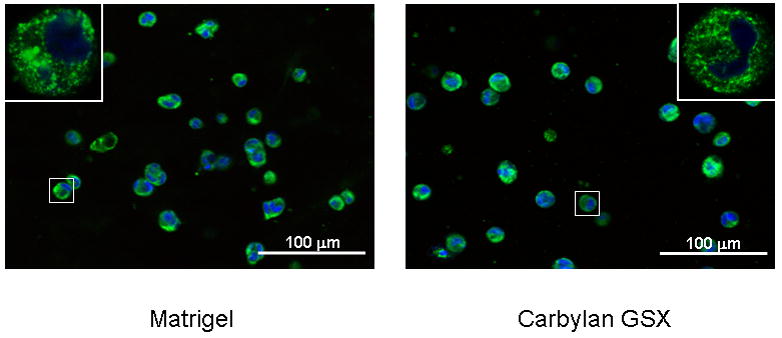
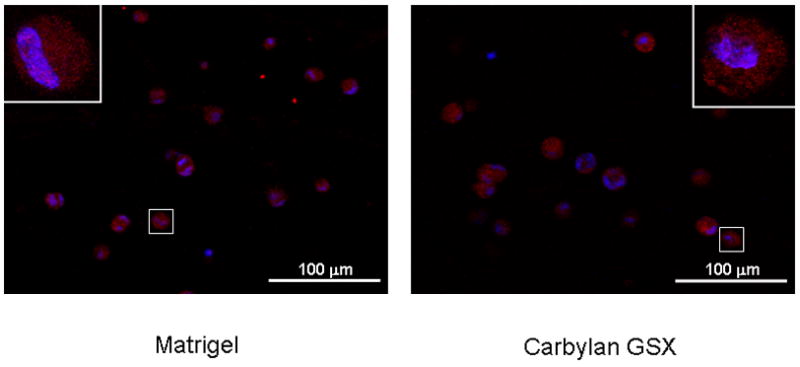
The distribution of hPH (A) and ICAM-1 (B) in hVFF cultured in 3D Matrigel and Carbylan GSX environments. After 72h of in vitro culture, the fluorescent images of hVFF in Matrigel and Carbylan GSX (3D condition) were captured by an inverted confocal microscope equipped with dual excitation lasers for red, green and blue fluorescence.
3.2. Presence of fibroblast markers on hVFFs in 3D Carbylan GSX
In order to evaluate the immunogenic and inflammatory response of the Carbylan GSX environment for hVFFs, the distribution of collagen biosynthase prolyl 4-hydroxylase (hPH) were observed for the different culture conditions. On 2D Carbylan GSX culture (Figure 2B), hPH staining was punctuate in the hVFF cytoplasm around nucleoli; this distributing style was similar to the polystyrene condition (Figure 2A). Under 3D culture conditions (Figure 2C), Carbylan GSX did not affect the hPH staining compared to Matrigel, staining was similar to those in 2D. For ICAM-1, there was similar distributional phenomenon observed in both 3D Carbylan GSX and Matrigel (Figure 3B).
3.3. Quantification of cell proliferation in 3D culture
In order to assess hVFF proliferation rates for culturing conditions, cells were seeded on a thin layer of Carbylan GSX or in Carbylan GSX. The cell number for each condition was quantified by luminescent assay every other day over a 7 day period. The cells on Carbylan GSX (2D) displayed a significant higher proliferation rate comparing those in Carbylan GSX (3D) at each experiment time points (p<0.0001, Figure. 4A), and both proliferation rates were significantly lower than control (polystyrene, p<0.0001). For 3D culture (Figure 4B), there was less cell proliferation over the 7 day period yet similar proliferation rates were measured for Carbylan GSX and Matrigel (p=0.0598).
Figure 4.
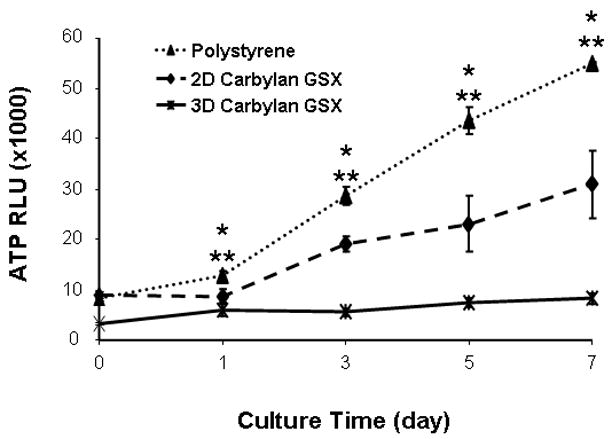
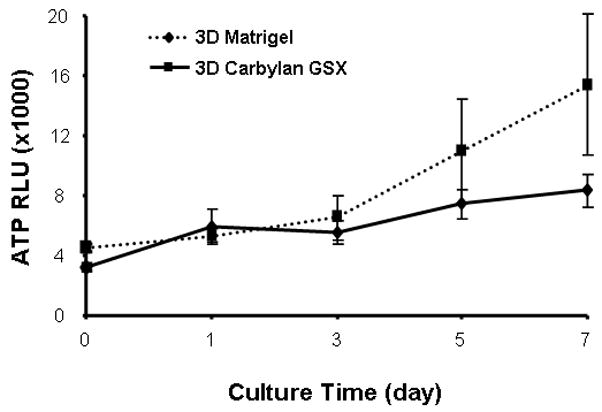
Proliferation rates of immortalized hVFF cells in various culture conditions. The cells were initially seeded at 2×103/well of 96-well plate and cell number was quadruplicated determined by CellTiter-Glo Luminescence Cell Viability assay over the period of 7 days. The ATP RLU is proportional to the number of viability cells. (A) Cell proliferation rates on polystyrene, 2D Carbylan GSX and in 3D Carbylan GSX; (B) Cell proliferation rates in 3D Matrigel and Carbylan GSX: similar proliferation rates were shown for Carbylan GSX and Matrigel. * cell proliferation rate in 2D Carbylan shown significantly lower comparing to polystyrene (p<0.01); ** cell proliferation rate in 3D Carbylan GSX was significanty lower comparing to polystyrene (p<0.01).
3.4. Carbylan GSX affected cell viability/apoptosis in 3D culture
hVFF viability/apoptosis was determined by a two-color fluorescence cell viability assay, which simultaneously determines live and dead cells using two probes for measuring intercellular esterase activity and membrane integrity. At Day 3 and 7, live cells in 3D Carbylan GSX were estimated to be 63.7 and 67% respectively for the total cell population based on the fluorescence microplate reader (Figure 5A), which were significantly different from cells grown on Carbylan GSX (p=0.0074 and 0.0004), but not significantly different from those grown on polystyrene control (p=0.2373 and 0.5626). For 3D culture conditions (Figure 5B), the live cell percentage in Carbylan GSX was significantly lower than Matrigel (p=0.0.0018), however, at Day 7 this difference was ameliorated (p=0.1062).
Figure 5.
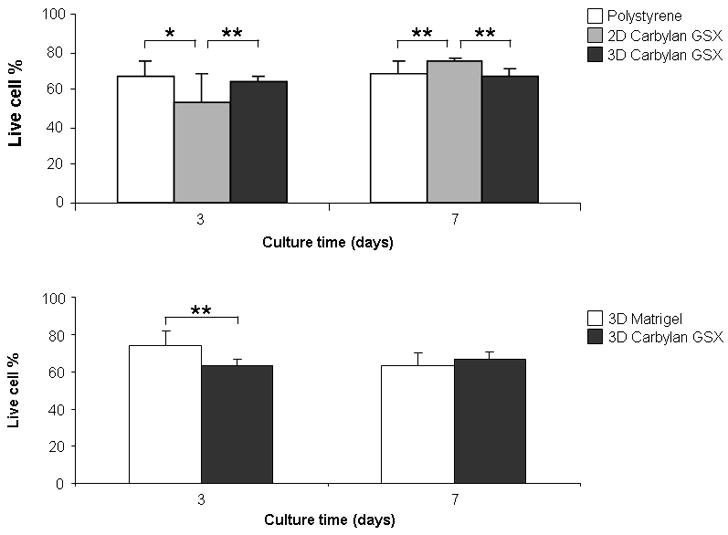
Live/Dead viability/apoptosis assay of immortalized hVFFs seeded in various culture conditions at Day 3 and Day 7. Y-axis indicates the live cell % in total cell population. (A) Total live cell percentage on polystyrene and 2D Carbylan GSX, and in 3D Carbylan GSX; (B) Total live cell percentage in 3D Matrigel and Carbylan GSX. * p<0.05, ** p<0.01
3.5. Carbylan GSX affected the cellular inflammation response in 3D culture
In order to investigate the hVFFs inflammatory response to Carbylan GSX before progression to human clinical trails for prophylactic use, the inflammatory marker genes COX2, IL-6, IL-8 and TNF-α were measured for cells cultured in/on Carbylan GSX and Matrigel (Figure 6). At Day 7, Carbylan GSX (2D) up-regulated IL-8 and TNF-α mRNA expression compared to control polystyrene (both p<0.0001), but did not induce COX2 and IL-6 mRNA expression (Figure 6A). In 3D Carbylan GSX, IL-8 and TNF-a expression were elevated compared to polystyrene (p≤0.0001), but COX2 and IL-6 expression levels were significantly lower than polystyrene (p<0.0089). When comparing the cells cultured in 3D Matrigel (Figure 6B), Carbylan GSX (3D) induced COX2 expression significantly (p=0.0053), but down-regulated the IL-6, IL-8 and TNF-α expression (p=0.0008, 0.0007 and 0.2558 respectively).
Figure 6.
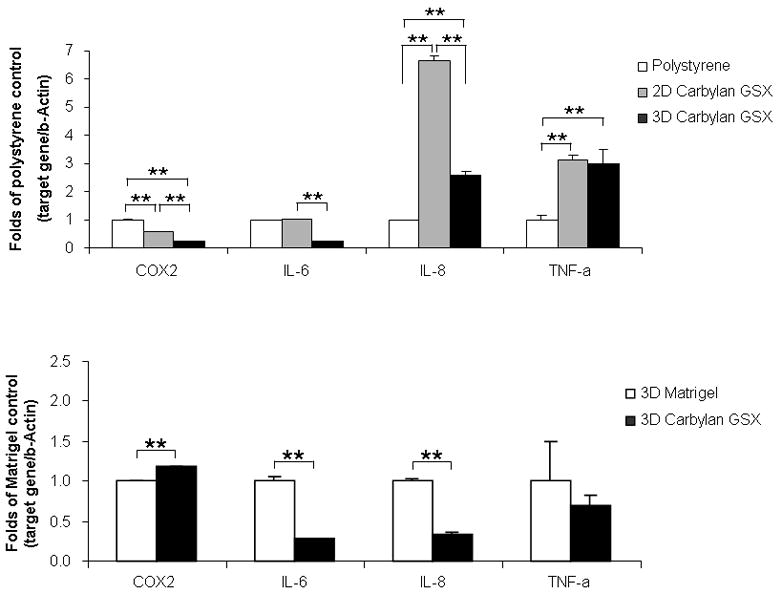
Gene expression levels (mRNA) of COX2, IL-6, IL-8 and TNF-α for immortalized hVFFs cultured in/on Carbylan GSX or Matrigel at Day 7. Y-axis demonstrates fold of control (polystyrene or Matrigel) of each target gene (mRNA concentration ng/μl), normalized by housekeeping gene, β-actin mRNA (ng/μl). (A) Polystyrene was used as control and mRNA level on polystyrene after seven days of culture was defined as 1.0. Y-axis showed the fold change of mRNA levels in 2D and 3D Carbylan GSX; (B) Matrigel was used as control and mRNA level in Matrigel after seven days of culture was defined as 1.0. The y-axis showed the fold change of mRNA levels in 3D Carbylan GSX. ** p<0.01
3.6. Carbylan GSX affected ECM gene expression in 3-D culture
In order to evaluate changes in fibroblast gene expression when cultured with Carbylan GSX, ECM genes - Col I (collagen I α-2), FN (fibronectin), FMOD (fibromodulin), HAS3 (hyaluronic acid synthase 3), Hyal1 (hyaluronidase1) and TGF-β1, were measured (Figure 7). At Day 7 (Figure 7A), Col I expression levels for those hVFF cultured in 3D Carbylan GSX were significantly lower than those grown on Carbylan GSX and control polystyrene (both p<0.001). Carbylan GSX (3D) significantly up-regulated FN expression compared to polystyrene (p<0.0001). There were no significant differences found for FMOD, HAS3 and HYAL1 between the 3D Carbylan GSX and polystyrene control groups; for the 2D Carbylan GSX culture condition, the HAS3 gene was significantly up-regulated when compared to polystyrene and 3-D Carbylan GSX (p=0.002 and p<0.001, respectively). Lastly, Carbylan GSX (both 2D and 3D) significantly up-regulated the TGF-β1 expression compared to control polystyrene (p<0.001).
Figure 7.
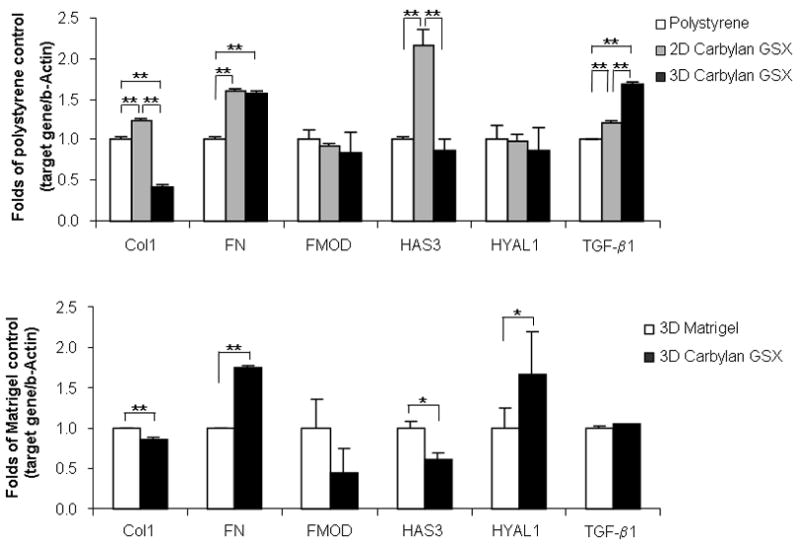
ECM related gene expression levels (mRNA) of collagen type I α-2 (Col I), fibronectin (FN), fibromodulin (FMOD), hyaluronic acid synthase 3 (HAS3), hyaluronidase 1 (HYAL1) and TGF-β1 in immortalized hVFFs responding to Carbylan GSX and Matrigel at Day 7. Y-axis shows the fold of control (polystyrene or Matrigel) of each target gene (mRNA concentration ng/μl), normalized by housekeeping gene, β-actin mRNA (ng/μl). (A) Tissue culture polystyrene was used as control and mRNA level on polystyrene after seven days of culture was defined as 1.0. Y-axis demonstrates fold change of mRNA levels in 2D and 3D Carbylan GSX: Carbylan GSX; (B) Matrigel was used as control and mRNA level in Matrigel after seven days of culture was defined as 1.0. Y-axis demonstrates fold change of mRNA levels in 3D Carbylan GSX. * p<0.05, ** p<0.01.
For 3D Carbylan GSX and Matrigel comparisons (Figure 7B), Carbylan GSX significantly up-regulated FN and HYAL1 gene expression (p=0.0003 and p= 0.0197, respectively), whereas significantly down-regulated the levels of Col I (p=0.0069) and HAS3 (p=0.0188) mRNA were found for cells cultured in Carbylan GSX. There were no significant differences for TGF-β1 and FMOD gene expression between 3D Carbylan GSX and Matrigel.
4. Discussion
In vitro research with primary or immortalized cells is undoubtedly a convenient, relatively cheap, and reliable way to acquire preliminary information about various biological functions. However, in vitro cultures are inferior to in vivo studies in many ways. One concern is that growing cells on 2D substrata creates an artificial lower and upper surface polarity, an artifact reflected in many physiological properties. However, in vivo studies are expensive, slow and often present difficulty in isolating single studied processes/mechanisms. Therefore, combining the convenience of a controlled, relatively cheap, and rapid experimental environment, 2D cell culture has been developed over the past several years and has proven to be a bridge between 2D cultures and in vivo studies. Its experimental results are the closed to physiologic situations [29] [30] [31]. Because both the cell and matrix contents are easily controlled, 3D culture has been widely applied in the tissue engineering field. In this study, we have used 3D culture to gain fundamental insights into cell behavior including cell morphology, marker-distribution, proliferation, viability and gene expression for human vocal fold fibroblasts cultured in Carbylan GSX. In an animal model Carbylan GSX has been shown to improve wound healing and provide prophylaxis against vocal fold scarring.
Commonly in in vitro investigations, cells are plated on tissue cultured certified polystyrene surfaces, and cell proliferation is assayed for a predetermined period of time. Our experimental results show clear differences in cell morphology and proliferation rates that appear to be dependent on the surface materials of culturing and microenvironment. HVFF easily attached and spread out in the typical spindle shape on polystyrene and Carbylan GSX, but they were rounded in 3D conditions for both Carbylan GSX and Matrigel. This is a similar morphological phenomenon to what has been reported for embryonic carcinoma cells cultured in 3D Matrigel [32]. For these cell types it has been reported that after 4 weeks, cells are able to leave their cell cluster and show spindle-shaped cell morphology. The attachment and spreading of cells in the 3D environment is very dependent upon the elasticity modulation, adhesion and proteolytic susceptibility of the artificial ECM. The presence of ECM molecules can stimulate cell differentiation selectively, activating transcriptional regulatory factors, and such regulation occurs in co-ordination with ECM-promoted changes in the cell shape [33] [34] [35]. In both of our 3D conditions -- Carbylan GSX and Matrigel -- hVFFs had the same morphological shape -- isolated and round with similar proliferation rates over the seven day culture period. After 7 days cells in Carbylan GSX and Matrigel started to expand and gradually become spindle in shape (data not shown). This was more evident for those cells grown in Matrigel. Matrigel, a solubilized basement membrane matrix extracted from the Engelbreth-Holm-Swarm mouse sarcoma, was selected as control as it is effective for the attachment and differentiation for both normal and transformed anchorage dependent cell lines. It contains several structural proteins (laminin and collagen IV) and growth factors (i.e. TGF-β, epidermal growth factor, insulin-like growth factor, fibroblast growth factor and tissue plasminogen activator), which promote cell differentiation, proliferation and gene expression. Matrigel has broad applicability as a cell culture substrate/supporter for both in vitro and in vivo studies [36] [37] [38] [39]. Carbylan GSX mainly contains modified hyaluronic acid, gelatin and a PEGDA cross-linker. Cells grown in PEGDA are known to be encapsulated and take a rounded morphology. Hahn et al report similar morphology for porcine vocal fold fibroblasts without changes in vocal fold responses as compared to collagen gels [40]. The differences in the chemical composition between these two gels may explain the greater number of cells having a typical fibroblast shape after day 7 for Matrigel versus Carbylan GSX. Further investigation is necessary to determine the time period in which the spindle shaped fibroblast morphology is prevalent in 3D Carbylan GSX.
Regarding proliferation, hVFF proliferation rate on polystyrene was noted to be faster than cells on/in Carbylan GSX. This may have been secondary to the cell growth environment. The stiffness of the gel is higher than media. The increased stiffness of the environment has been hypothesized to cause limitations for cell elongation and migration [40]. In vivo, cells including hVFFs are surrounded by tissue (lamina propria-connective tissue) that exert particular stiffness. Therefore, in spite of the low cell growth rate, the 3D culturing condition may more closely represent the in vivo condition.
Cytotoxicity/cell viability assays are very useful tools in providing valuable information regarding the safety of various drugs and materials. Several different assays are commonly used to evaluate survival of cells inside 3D carriers. The most common technique is the image analysis of 3D scaffold sections, which could be stained by lactate dehydrogenase (LDH), calcein AM/ethidium homodimer-1 (CaAM/EthD-1) and trypan blue [41] [42] [43] [44] [45]. This method can provide qualitative and semi-quantitative results, however it is very time consuming as it requires multiple steps (fixing, sectioning and staining). Recently, fluorescence-activated cell sorting (FACS) analysis has been used to determine cells that have survived culture in a 3D carrier [32] [46] [47]. The advantage of FACS is its quantitative nature, however one of the main limitations is the need to isolate sufficient single cells from 3D hydrogels. It is difficult to isolate a sufficient number single cells from 3D cultured in hydrogels, as the cells can not be isolated without hydrogel coating each cell, limiting uptake by the flow cytometer. Consequently, we utilized the Invitrogen Live/Dead Viability/Cytotoxicity fluorescence microplate assay as it provided the most consistently reliable quantitative results for 3D culture. At Day 7 no significant differences for hVFF viability were found between Carbylan GSX and Matrigel conditions, indicating that Carbylan GSX provided an adequate environment for living cells. For 3D cultures, proliferation was not as robust compared to 2D cultures. This phenomenon has been reported in the literature for other cell types. [45] [48] [46] Cell function of the hVFF cultured in 3D with lower proliferation, as implied by measured transcription levels for common proteins found in the extracellular matrix of the vocal folds, appears to allow for the potential regeneration of tissue.
Cytokines are important mediators that allow different cell types to communicate with each other. They play a role in the control and orientation of different physiological and pathophysiological processes. In particular, pro-inflammatory cytokines are involved in the early events of wound healing, inflammatory reactions, and foreign body reactions. The acute and sub-acute phases of inflammation can cause abnormal wound healing, and damage host tissue. Therefore, for any new biomaterials, it is very important to investigate their cellular inflammatory responses as measured by inflammatory cytokine production. We selected COX2, IL-6, IL-8 and TNF-α as the inflammatory markers because COX2 (responsible for the prostanoid biosynthesis involved in inflammation and mitogenesis), IL-6 (a pro-inflammatory and anti-inflammatory cytokine), IL-8 (an important mediator of the immune reaction in the innate immune system response) and TNF-α (involve in systemic inflammation) are known to be important signaling molecules involved in conducting the inflammatory response to foreign materials. In a previous rabbit vocal fold in vivo study [15], Carbylan GSX did not cause a substantial inflammatory reaction. The inflammatory cytokines were temporary up-regulated however after 14 days, their levels were returned to baseline. In our study our inflammatory responses was minimal at 7 days with up-regulation IL-8 and TNF-α mRNA only at Day 7 after exposure to Carbylan GSX or Matrigel. No differences were evident in the immunohistochemistry for expression of hPH and ICAM-1, two markers of active inflammation. By in large, Carbylan GSX appears to induce a minimal inflammatory response in human cells. Lastly, compared to Matrigel upregulated FN and HYAL1 gene expression with significantly down-regulated the levels of Col I for cells in 3D Carbylan GSX appears to correspond to in vivo animal experiments that have shown improved wound healing and decreased scaring with Carbylan GSX [13,14].
5. Conclusions
Cumulatively, the present results suggest that Carbylan GSX does not induce significant toxicity or inflammation as measured by cell viability, proliferation, inflammatory response compared to Matrigel over a seven-day period. Additionally the hVFFs were able to produce mRNA for ECM constituents found in lamina propria tissue. This unique synthesized ECM may be an ideal biomaterial for the clinical management of wound healing in human vocal fold.
Acknowledgments
The authors would like to acknowledge the National Institute of Deafness and Other Communication Disorders—R21 DC008428 for supporting this research project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Xia Chen, Email: chenx@surgery.wisc.edu, Division of Otolaryngology – Head and Neck Surgery, University of Wisconsin – Madison, 5136 WIMR, 1111 Highland Ave, Madison, Wisconsin, 53705-2275, Phone 6082634635.
Susan L. Thibeault, Email: thibeault@surgery.wisc.edu, Division of Otolaryngology – Head and Neck Surgery, University of Wisconsin -- Madison, 5107 WIMR 1111 Highland Ave, Madison, Wisconsin 53705-2275, Phone 6082636751
References
- 1.Gray SD, Pignatari SS, Harding P. Morphologic ultrastructure of anchoring fibers in normal vocal fold basement membrane zone. J Voice. 1994;8(1):48–52. doi: 10.1016/s0892-1997(05)80318-2. [DOI] [PubMed] [Google Scholar]
- 2.Pawlak AS, Hammond T, Hammond E, Gray SD. Immunocytochemical study of proteoglycans in vocal folds. Ann Otol Rhinol Laryngol. 1996;105(1):6–11. doi: 10.1177/000348949610500102. [DOI] [PubMed] [Google Scholar]
- 3.Hammond TH, Zhou R, Hammond EH, Pawlak A, Gray SD. The intermediate layer: a morphologic study of the elastin and hyaluronic acid constituents of normal human vocal folds. J Voice. 1997;11(1):59–66. doi: 10.1016/s0892-1997(97)80024-0. [DOI] [PubMed] [Google Scholar]
- 4.Gray SD, Titze IR, Alipour F, Hammond TH. Biomechanical and histologic observations of vocal fold fibrous proteins. Ann Otol Rhinol Laryngol. 2000;109(1):77–85. doi: 10.1177/000348940010900115. [DOI] [PubMed] [Google Scholar]
- 5.Chan RW, Titze IR. Viscoelastic shear properties of human vocal fold mucosa: theoretical characterization based on constitutive modeling. J Acoust Soc Am. 2000;107(1):565–580. doi: 10.1121/1.428354. [DOI] [PubMed] [Google Scholar]
- 6.Titze IR. The physics of small-amplitude oscillation of the vocal folds. J Acoust Soc Am. 1988;83(4):1536–1552. doi: 10.1121/1.395910. [DOI] [PubMed] [Google Scholar]
- 7.Titze IR. Phonation threshold pressure: a missing link in glottal aerodynamics. J Acoust Soc Am. 1992;91(5):2926–2935. doi: 10.1121/1.402928. [DOI] [PubMed] [Google Scholar]
- 8.Roy N, Merrill RM, Thibeault S, Gray SD, Smith EM. Voice disorders in teachers and the general population: effects on work performance, attendance, and future career choices. J Speech Lang Hear Res. 2004;47(3):542–551. doi: 10.1044/1092-4388(2004/042). [DOI] [PubMed] [Google Scholar]
- 9.Dedo HH, Urrea RD, Lawson L. Intracordal injection of Teflon in the treatment of 135 patients with dysphonia. Ann Otol Rhinol Laryngol. 1973;82(5):661–667. doi: 10.1177/000348947308200509. [DOI] [PubMed] [Google Scholar]
- 10.Ford CN, Bless DM. Selected problems treated by vocal fold injection of collagen. Am J Otolaryngol. 1993;14(4):257–261. doi: 10.1016/0196-0709(93)90071-e. [DOI] [PubMed] [Google Scholar]
- 11.Chen WY, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999;7(2):79–89. doi: 10.1046/j.1524-475x.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- 12.Agren UM, Tammi M, Ryynanen M, Tammi R. Developmentally programmed expression of hyaluronan in human skin and its appendages. J Invest Dermatol. 1997;109(2):219–224. doi: 10.1111/1523-1747.ep12319412. [DOI] [PubMed] [Google Scholar]
- 13.Duflo S, Thibeault SL, Li W, Shu XZ, Prestwich G. Effect of a synthetic extracellular matrix on vocal fold lamina propria gene expression in early wound healing. Tissue Eng. 2006;12(11):3201–3207. doi: 10.1089/ten.2006.12.3201. [DOI] [PubMed] [Google Scholar]
- 14.Duflo S, Thibeault SL, Li W, Shu XZ, Prestwich GD. Vocal fold tissue repair in vivo using a synthetic extracellular matrix. Tissue Eng. 2006;12(8):2171–2180. doi: 10.1089/ten.2006.12.2171. [DOI] [PubMed] [Google Scholar]
- 15.Thibeault SL, Duflo S. Inflammatory cytokine responses to synthetic extracellular matrix injection to the vocal fold lamina propria. Ann Otol Rhinol Laryngol. 2008;117(3):221–226. doi: 10.1177/000348940811700310. [DOI] [PubMed] [Google Scholar]
- 16.Prestwich GD, Shu XZ, Liu Y, Cai S, Walsh JF, Hughes CW, et al. Injectable synthetic extracellular matrices for tissue engineering and repair. Adv Exp Med Biol. 2006;585:125–133. doi: 10.1007/978-0-387-34133-0_9. [DOI] [PubMed] [Google Scholar]
- 17.Shu XZ, Liu Y, Luo Y, Roberts MC, Prestwich GD. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules. 2002;3(6):1304–1311. doi: 10.1021/bm025603c. [DOI] [PubMed] [Google Scholar]
- 18.Shu XZ, Liu Y, Palumbo F, Prestwich GD. Disulfide-crosslinked hyaluronan-gelatin hydrogel films: a covalent mimic of the extracellular matrix for in vitro cell growth. Biomaterials. 2003;24(21):3825–3834. doi: 10.1016/s0142-9612(03)00267-9. [DOI] [PubMed] [Google Scholar]
- 19.Xiao Zheng Shu YL, Palumbo Fabio S, Luo Yi, Prestwich Glenn D. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials. 2004;25:1339–1348. doi: 10.1016/j.biomaterials.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Thibeault SL. Characteristics of age-related changes in cultured human vocal fold fibroblasts. Laryngoscope. 2008;118(9):1700–1704. doi: 10.1097/MLG.0b013e31817aec6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thibeault SL, Li W, Bartley S. A method for identification of vocal fold lamina propria fibroblasts in culture. Otolaryngol Head Neck Surg. 2008;139(6):816–822. doi: 10.1016/j.otohns.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X, Thibeault SL. Novel isolation and biochemical characterization of immortalized fibroblasts for tissue engineering vocal fold lamina propria. Tissue Eng Part C Methods. 2009;15(2):201–212. doi: 10.1089/ten.tec.2008.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saika S, Ooshima A, Yamanaka O, Tonoe O, Okada Y, Ohnishi Y, et al. Immunolocalization of prolyl 4-hydroxylase in fibroblasts cultured from Tenon’s capsule of humans. Graefes Arch Clin Exp Ophthalmol. 1996;234(4):251–257. doi: 10.1007/BF00430418. [DOI] [PubMed] [Google Scholar]
- 24.Gao J, Morgan G, Tieu D, Schwalb TA, Luo JY, Wheeler LA, et al. ICAM-1 expression predisposes ocular tissues to immune-based inflammation in dry eye patients and Sjogrens syndrome-like MRL/lpr mice. Exp Eye Res. 2004;78(4):823–835. doi: 10.1016/j.exer.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 25.Crouch SP, Kozlowski R, Slater KJ, Fletcher J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J Immunol Methods. 1993;160(1):81–88. doi: 10.1016/0022-1759(93)90011-u. [DOI] [PubMed] [Google Scholar]
- 26.Kangas L, Gronroos M, Nieminen AL. Bioluminescence of cellular ATP: a new method for evaluating cytotoxic agents in vitro. Med Biol. 1984;62(6):338–343. [PubMed] [Google Scholar]
- 27.Lichtenfels R, Biddison WE, Schulz H, Vogt AB, Martin R. CARE-LASS (calcein-release-assay), an improved fluorescence-based test system to measure cytotoxic T lymphocyte activity. J Immunol Methods. 1994;172(2):227–239. doi: 10.1016/0022-1759(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 28.Xin Ming Wang PIT, George W, Rankin, Chia David, Zhong Hui Ping, Hardy Steven. A New Microcellular Cytotoxicity Test Based on Calcein AM Release. Human Immunology. 1993;37:264–270. doi: 10.1016/0198-8859(93)90510-8. [DOI] [PubMed] [Google Scholar]
- 29.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294(5547):1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 30.Abbott A. Cell culture: biology’s new dimension. Nature. 2003;424(6951):870–872. doi: 10.1038/424870a. [DOI] [PubMed] [Google Scholar]
- 31.Prestwich GD. Simplifying the extracellular matrix for 3-D cell culture and tissue engineering: a pragmatic approach. J Cell Biochem. 2007;101(6):1370–1383. doi: 10.1002/jcb.21386. [DOI] [PubMed] [Google Scholar]
- 32.Kraehenbuehl TP, Zammaretti P, Van der Vlies AJ, Schoenmakers RG, Lutolf MP, Jaconi ME, et al. Three-dimensional extracellular matrix-directed cardioprogenitor differentiation: systematic modulation of a synthetic cell-responsive PEG-hydrogel. Biomaterials. 2008;29(18):2757–2766. doi: 10.1016/j.biomaterials.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Ben-Ze’ev A, Robinson GS, Bucher NL, Farmer SR. Cell-cell and cell-matrix interactions differentially regulate the expression of hepatic and cytoskeletal genes in primary cultures of rat hepatocytes. Proc Natl Acad Sci U S A. 1988;85(7):2161–2165. doi: 10.1073/pnas.85.7.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DiPersio CM, Jackson DA, Zaret KS. The extracellular matrix coordinately modulates liver transcription factors and hepatocyte morphology. Mol Cell Biol. 1991;11(9):4405–4414. doi: 10.1128/mcb.11.9.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mooney D, Hansen L, Vacanti J, Langer R, Farmer S, Ingber D. Switching from differentiation to growth in hepatocytes: control by extracellular matrix. J Cell Physiol. 1992;151(3):497–505. doi: 10.1002/jcp.1041510308. [DOI] [PubMed] [Google Scholar]
- 36.Kubota Y, Kleinman HK, Martin GR, Lawley TJ. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988;107(4):1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maeshima Y, Manfredi M, Reimer C, Holthaus KA, Hopfer H, Chandamuri BR, et al. Identification of the anti-angiogenic site within vascular basement membrane-derived tumstatin. J Biol Chem. 2001;276(18):15240–15248. doi: 10.1074/jbc.M007764200. [DOI] [PubMed] [Google Scholar]
- 38.Passaniti A, Taylor RM, Pili R, Guo Y, Long PV, Haney JA, et al. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest. 1992;67(4):519–528. [PubMed] [Google Scholar]
- 39.Isaji M, Miyata H, Ajisawa Y, Takehana Y, Yoshimura N. Tranilast inhibits the proliferation, chemotaxis and tube formation of human microvascular endothelial cells in vitro and angiogenesis in vivo. Br J Pharmacol. 1997;122(6):1061–1066. doi: 10.1038/sj.bjp.0701493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munoz-Pinto DJ, Jimenez-Vergara AC, Gelves LM, McMahon RE, Guiza-Arguello V, Hahn MS. Probing vocal fold fibroblast response to hyaluronan in 3D contexts. Biotechnol Bioeng. 2009;104(4):821–831. doi: 10.1002/bit.22436. [DOI] [PubMed] [Google Scholar]
- 41.Thevenot P, Nair A, Dey J, Yang J, Tang L. Method to analyze three-dimensional cell distribution and infiltration in degradable scaffolds. Tissue Eng Part C Methods. 2008;14(4):319–331. doi: 10.1089/ten.tec.2008.0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gantenbein-Ritter B, Potier E, Zeiter S, van der Werf M, Sprecher CM, Ito K. Accuracy of three techniques to determine cell viability in 3D tissues or scaffolds. Tissue Eng Part C Methods. 2008;14(4):353–358. doi: 10.1089/ten.tec.2008.0313. [DOI] [PubMed] [Google Scholar]
- 43.Hudalla GA, Eng TS, Murphy WL. An approach to modulate degradation and mesenchymal stem cell behavior in poly(ethylene glycol) networks. Biomacromolecules. 2008;9(3):842–849. doi: 10.1021/bm701179s. [DOI] [PubMed] [Google Scholar]
- 44.Jongpaiboonkit L, King WJ, Lyons GE, Paguirigan AL, Warrick JW, Beebe DJ, et al. An adaptable hydrogel array format for 3-dimensional cell culture and analysis. Biomaterials. 2008;29(23):3346–3356. doi: 10.1016/j.biomaterials.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serban MA, Liu Y, Prestwich GD. Effects of extracellular matrix analogues on primary human fibroblast behavior. Acta Biomater. 2008;4(1):67–75. doi: 10.1016/j.actbio.2007.09.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nuttelman CR, Tripodi MC, Anseth KS. Synthetic hydrogel niches that promote hMSC viability. Matrix Biol. 2005;24(3):208–218. doi: 10.1016/j.matbio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Kraehenbuehl TP, Ferreira LS, Zammaretti P, Hubbell JA, Langer R. Cell-responsive hydrogel for encapsulation of vascular cells. Biomaterials. 2009;30(26):4318–4324. doi: 10.1016/j.biomaterials.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fischbach C, Kong HJ, Hsiong SX, Evangelista MB, Yuen W, Mooney DJ. Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement. Proc Natl Acad Sci U S A. 2009;106(2):399–404. doi: 10.1073/pnas.0808932106. [DOI] [PMC free article] [PubMed] [Google Scholar]


