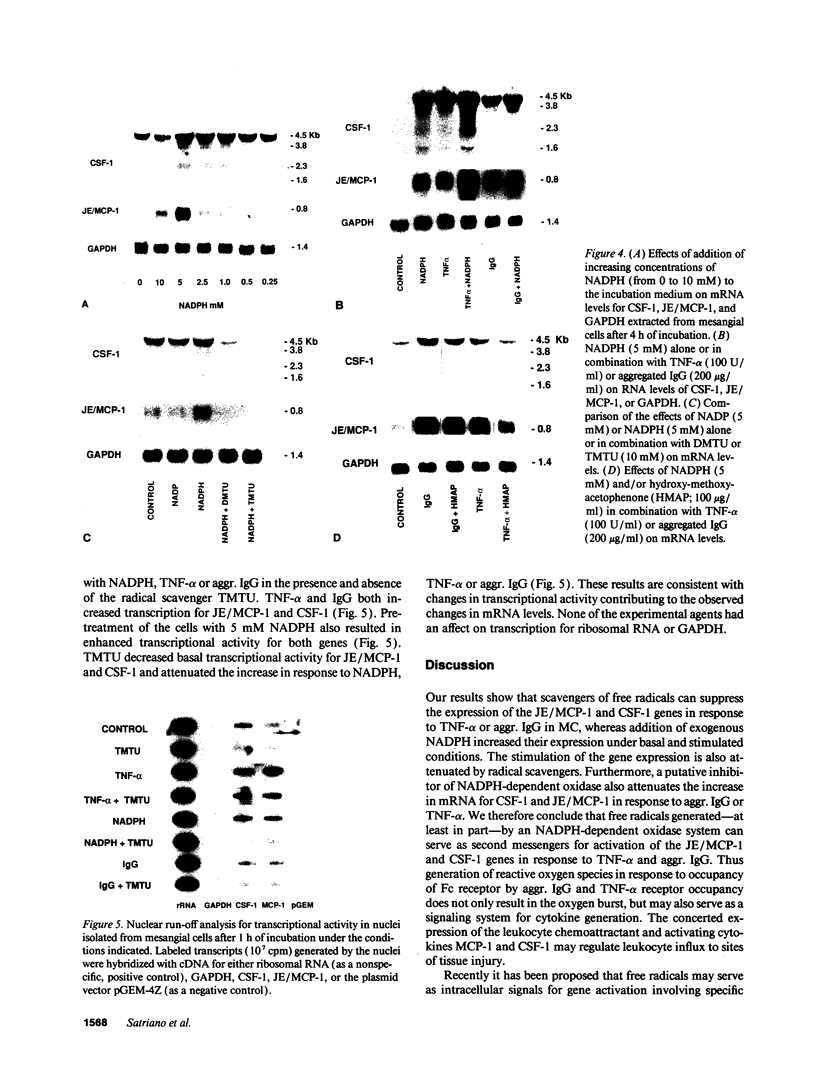
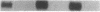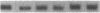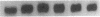Abstract
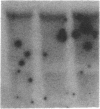
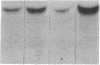
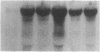
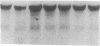
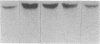
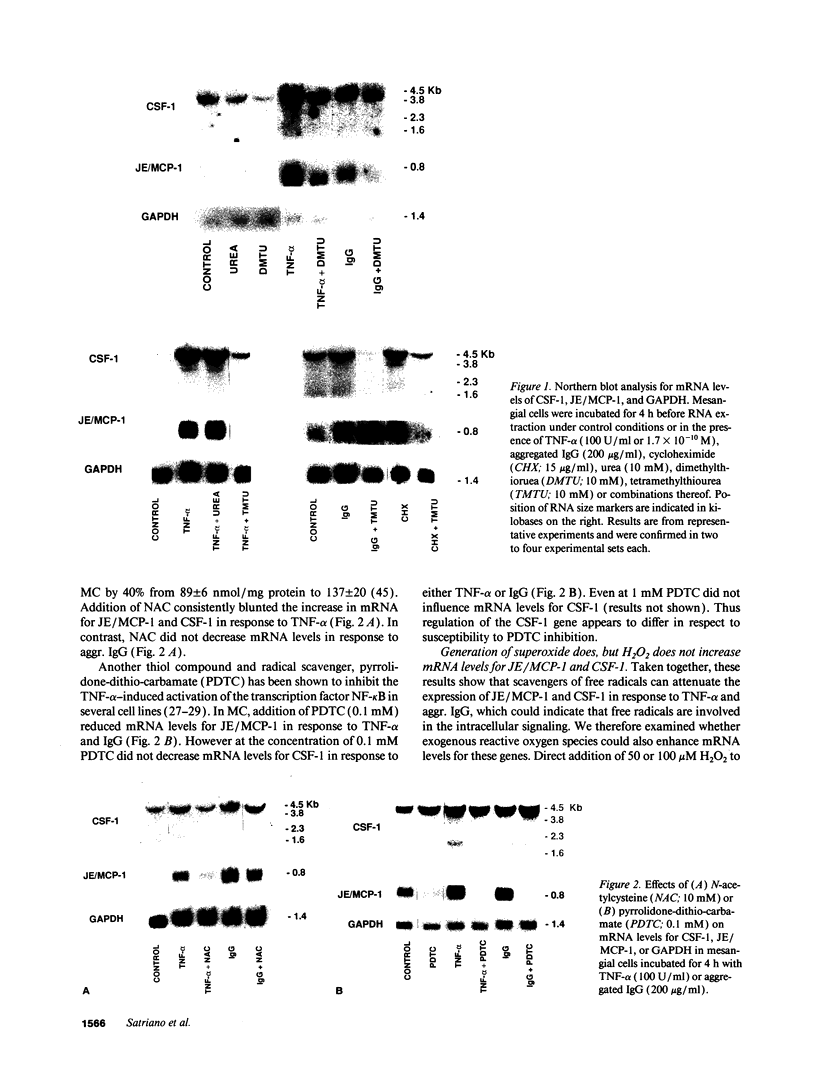
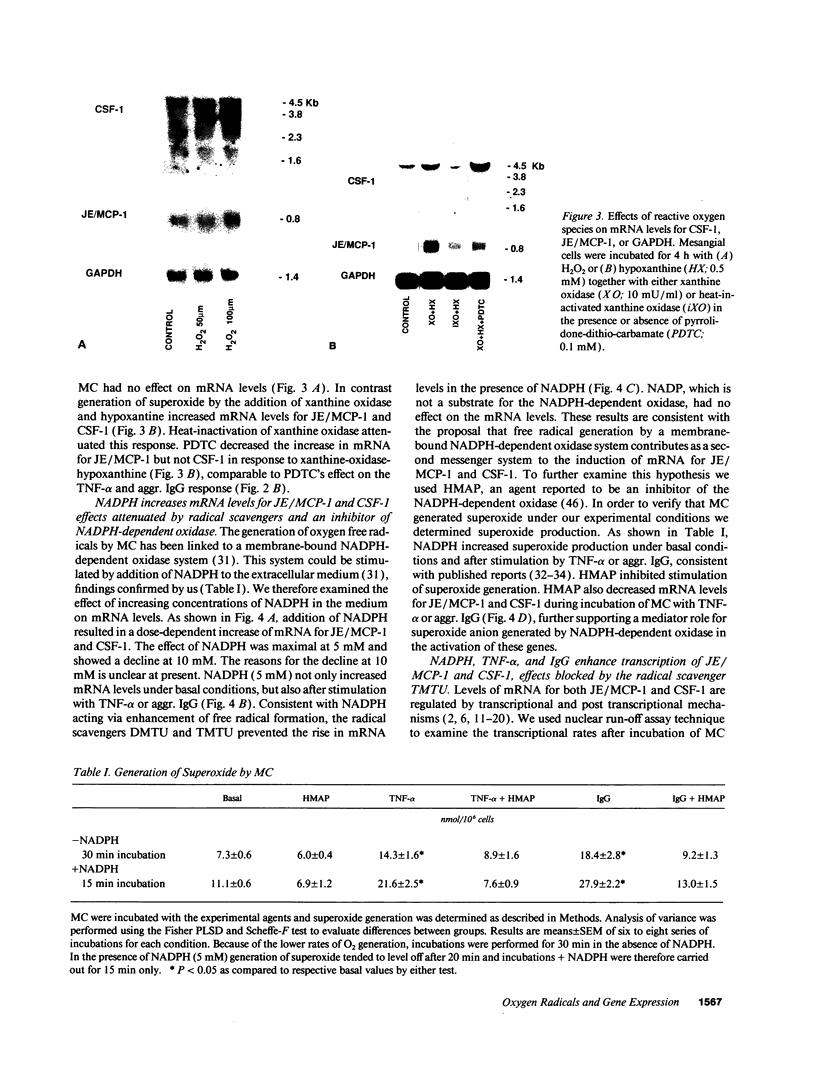
The potential involvement of reactive oxygen species in the expression of genes involved in immune response was examined in mesangial cells. Tumor necrosis factor (TNF-alpha) and aggregated (aggr.) IgG increased mRNA levels for the monocyte chemoattractant protein, JE/MCP-1, and the colony-stimulating factor, CSF-1. Scavengers for free radicals such as di- and tetra-methylthiourea (DMTU and TMTU) attenuated the increase in mRNA levels in response to TNF-alpha and aggr. IgG. Generation of superoxide anion by xanthine oxidase and hypoxanthine increased mRNA levels of these genes, but exogenous H2O2 did not. Addition of NADPH to activate a membrane-bound NADPH-oxidase generated superoxide and caused a dose-dependent increase in mRNA levels and further enhanced the stimulation by TNF-alpha or aggr. IgG. An inhibitor of NADPH-dependent oxidase 4'-hydroxy-3'-methoxy-acetophenone attenuated the rise in mRNA levels in response to TNF-alpha and aggr. IgG. By nuclear run-on experiments TNF-alpha, aggr. IgG and NADPH increased the transcription rates for JE/MCP-1 and CSF-1, effects inhibited by TMTU. We conclude that generation of reactive oxygen species, possibly by NADPH-dependent oxidase, are involved in the induction of the JE/MCP-1 and CSF-1 genes by TNF-alpha and IgG complexes. The concerted expression of leukocyte-directed cytokines represents a general response to tissue injury.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Patel L., Rauscher F. J., 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990 Sep 7;249(4973):1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- Adler S., Baker P. J., Johnson R. J., Ochi R. F., Pritzl P., Couser W. G. Complement membrane attack complex stimulates production of reactive oxygen metabolites by cultured rat mesangial cells. J Clin Invest. 1986 Mar;77(3):762–767. doi: 10.1172/JCI112372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeuerle P. A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991 Apr 16;1072(1):63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Baud L., Hagege J., Sraer J., Rondeau E., Perez J., Ardaillou R. Reactive oxygen production by cultured rat glomerular mesangial cells during phagocytosis is associated with stimulation of lipoxygenase activity. J Exp Med. 1983 Dec 1;158(6):1836–1852. doi: 10.1084/jem.158.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D., Zurawski S. M., Mosmann T. R., Zurawski G. A family of small inducible proteins secreted by leukocytes are members of a new superfamily that includes leukocyte and fibroblast-derived inflammatory agents, growth factors, and indicators of various activation processes. J Immunol. 1989 Jan 15;142(2):679–687. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Ernst T. J., Ritchie A. R., Demetri G. D., Griffin J. D. Regulation of granulocyte- and monocyte-colony stimulating factor mRNA levels in human blood monocytes is mediated primarily at a post-transcriptional level. J Biol Chem. 1989 Apr 5;264(10):5700–5703. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Harrington M. A., Edenberg H. J., Saxman S., Pedigo L. M., Daub R., Broxmeyer H. E. Cloning and characterization of the murine promoter for the colony-stimulating factor-1-encoding gene. Gene. 1991 Jun 30;102(2):165–170. doi: 10.1016/0378-1119(91)90074-l. [DOI] [PubMed] [Google Scholar]
- Hora K., Satriano J. A., Santiago A., Mori T., Stanley E. R., Shan Z., Schlondorff D. Receptors for IgG complexes activate synthesis of monocyte chemoattractant peptide 1 and colony-stimulating factor 1. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1745–1749. doi: 10.1073/pnas.89.5.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi J., Sariban E., Kufe D. Transcriptional and posttranscriptional regulation of CSF-1 gene expression in human monocytes. Mol Cell Biol. 1988 Sep;8(9):3951–3954. doi: 10.1128/mcb.8.9.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Introna M., Bast R. C., Jr, Tannenbaum C. S., Hamilton T. A., Adams D. O. The effect of LPS on expression of the early "competence" genes JE and KC in murine peritoneal macrophages. J Immunol. 1987 Jun 1;138(11):3891–3896. [PubMed] [Google Scholar]
- Kawahara R. S., Deng Z. W., Deuel T. F. Glucocorticoids inhibit the transcriptional induction of JE, a platelet-derived growth factor-inducible gene. J Biol Chem. 1991 Jul 15;266(20):13261–13266. [PubMed] [Google Scholar]
- Knauss T. C., Mené P., Ricanati S. A., Kester M., Dubyak G. R., Emancipator S. N., Sedor J. R. Immune complex activation of rat glomerular mesangial cells: dependence on the Fc region of antibody. Am J Physiol. 1989 Sep;257(3 Pt 2):F478–F485. doi: 10.1152/ajprenal.1989.257.3.F478. [DOI] [PubMed] [Google Scholar]
- Ladner M. B., Martin G. A., Noble J. A., Wittman V. P., Warren M. K., McGrogan M., Stanley E. R. cDNA cloning and expression of murine macrophage colony-stimulating factor from L929 cells. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6706–6710. doi: 10.1073/pnas.85.18.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard E. J., Yoshimura T. Human monocyte chemoattractant protein-1 (MCP-1). Immunol Today. 1990 Mar;11(3):97–101. doi: 10.1016/0167-5699(90)90035-8. [DOI] [PubMed] [Google Scholar]
- Meichle A., Schütze S., Hensel G., Brunsing D., Krönke M. Protein kinase C-independent activation of nuclear factor kappa B by tumor necrosis factor. J Biol Chem. 1990 May 15;265(14):8339–8343. [PubMed] [Google Scholar]
- Meier B., Cross A. R., Hancock J. T., Kaup F. J., Jones O. T. Identification of a superoxide-generating NADPH oxidase system in human fibroblasts. Biochem J. 1991 Apr 1;275(Pt 1):241–245. doi: 10.1042/bj2750241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberle G. P., Niemeyer J., Thaiss F., Schoeppe W., Stahl R. A. Increased oxygen radical and eicosanoid formation in immune-mediated mesangial cell injury. Kidney Int. 1992 Jul;42(1):69–74. doi: 10.1038/ki.1992.262. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Pick E., Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46(2):211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- Radeke H. H., Cross A. R., Hancock J. T., Jones O. T., Nakamura M., Kaever V., Resch K. Functional expression of NADPH oxidase components (alpha- and beta-subunits of cytochrome b558 and 45-kDa flavoprotein) by intrinsic human glomerular mesangial cells. J Biol Chem. 1991 Nov 5;266(31):21025–21029. [PubMed] [Google Scholar]
- Radeke H. H., Meier B., Topley N., Flöge J., Habermehl G. G., Resch K. Interleukin 1-alpha and tumor necrosis factor-alpha induce oxygen radical production in mesangial cells. Kidney Int. 1990 Feb;37(2):767–775. doi: 10.1038/ki.1990.44. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Morrison E. D., Stiles C. D. Cloning and expression of JE, a gene inducible by platelet-derived growth factor and whose product has cytokine-like properties. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3738–3742. doi: 10.1073/pnas.85.11.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J., Morton C. C., Ledbetter D. H., Eddy R. L., Jr, Shows T. B. Assignment of the human small inducible cytokine A2 gene, SCYA2 (encoding JE or MCP-1), to 17q11.2-12: evolutionary relatedness of cytokines clustered at the same locus. Genomics. 1991 Jun;10(2):489–492. doi: 10.1016/0888-7543(91)90338-f. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Stier P., Ernst T., Wong G. G. The human homolog of the JE gene encodes a monocyte secretory protein. Mol Cell Biol. 1989 Nov;9(11):4687–4695. doi: 10.1128/mcb.9.11.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J., Yoshimura T., Leonard E. J., Pober J. S. Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. Am J Pathol. 1990 Jun;136(6):1229–1233. [PMC free article] [PubMed] [Google Scholar]
- Rovin B. H., Yoshiumura T., Tan L. Cytokine-induced production of monocyte chemoattractant protein-1 by cultured human mesangial cells. J Immunol. 1992 Apr 1;148(7):2148–2153. [PubMed] [Google Scholar]
- Safirstein R., Megyesi J., Saggi S. J., Price P. M., Poon M., Rollins B. J., Taubman M. B. Expression of cytokine-like genes JE and KC is increased during renal ischemia. Am J Physiol. 1991 Dec;261(6 Pt 2):F1095–F1101. doi: 10.1152/ajprenal.1991.261.6.F1095. [DOI] [PubMed] [Google Scholar]
- Satriano J. A., Hora K., Shan Z., Stanley E. R., Mori T., Schlondorff D. Regulation of monocyte chemoattractant protein-1 and macrophage colony-stimulating factor-1 by IFN-gamma, tumor necrosis factor-alpha, IgG aggregates, and cAMP in mouse mesangial cells. J Immunol. 1993 Mar 1;150(5):1971–1978. [PubMed] [Google Scholar]
- Schlondorff D., Mori T. Contributions of mesangial cells to glomerular immune functions. Klin Wochenschr. 1990 Nov 16;68(22):1138–1144. doi: 10.1007/BF01798065. [DOI] [PubMed] [Google Scholar]
- Schlondorff D. The glomerular mesangial cell: an expanding role for a specialized pericyte. FASEB J. 1987 Oct;1(4):272–281. doi: 10.1096/fasebj.1.4.3308611. [DOI] [PubMed] [Google Scholar]
- Schreck R., Baeuerle P. A. A role for oxygen radicals as second messengers. Trends Cell Biol. 1991 Aug;1(2-3):39–42. doi: 10.1016/0962-8924(91)90072-h. [DOI] [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedor J. R., Carey S. W., Emancipator S. N. Immune complexes bind to cultured rat glomerular mesangial cells to stimulate superoxide release. Evidence for an Fc receptor. J Immunol. 1987 Jun 1;138(11):3751–3757. [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Sherman M. L., Weber B. L., Datta R., Kufe D. W. Transcriptional and posttranscriptional regulation of macrophage-specific colony stimulating factor gene expression by tumor necrosis factor. Involvement of arachidonic acid metabolites. J Clin Invest. 1990 Feb;85(2):442–447. doi: 10.1172/JCI114457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyy Y. J., Li Y. S., Kolattukudy P. E. Structure of human monocyte chemotactic protein gene and its regulation by TPA. Biochem Biophys Res Commun. 1990 Jun 15;169(2):346–351. doi: 10.1016/0006-291x(90)90338-n. [DOI] [PubMed] [Google Scholar]
- Staal F. J., Roederer M., Herzenberg L. A., Herzenberg L. A. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standiford T. J., Kunkel S. L., Phan S. H., Rollins B. J., Strieter R. M. Alveolar macrophage-derived cytokines induce monocyte chemoattractant protein-1 expression from human pulmonary type II-like epithelial cells. J Biol Chem. 1991 May 25;266(15):9912–9918. [PubMed] [Google Scholar]
- Stanley E. R., Guilbert L. J., Tushinski R. J., Bartelmez S. H. CSF-1--a mononuclear phagocyte lineage-specific hemopoietic growth factor. J Cell Biochem. 1983;21(2):151–159. doi: 10.1002/jcb.240210206. [DOI] [PubMed] [Google Scholar]
- Stanley E. R. The macrophage colony-stimulating factor, CSF-1. Methods Enzymol. 1985;116:564–587. doi: 10.1016/s0076-6879(85)16044-1. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Wiggins R., Phan S. H., Wharram B. L., Showell H. J., Remick D. G., Chensue S. W., Kunkel S. L. Monocyte chemotactic protein gene expression by cytokine-treated human fibroblasts and endothelial cells. Biochem Biophys Res Commun. 1989 Jul 31;162(2):694–700. doi: 10.1016/0006-291x(89)92366-8. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Wang W., Vu T. H., Raffin T. A. Effect of NADPH oxidase inhibition on endothelial cell ELAM-1 mRNA expression. Biochem Biophys Res Commun. 1992 May 15;184(3):1339–1343. doi: 10.1016/s0006-291x(05)80029-4. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledano M. B., Leonard W. J. Modulation of transcription factor NF-kappa B binding activity by oxidation-reduction in vitro. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4328–4332. doi: 10.1073/pnas.88.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasil M., Halliwell B., Grootveld M., Moorhouse C. P., Hutchison D. C., Baum H. The specificity of thiourea, dimethylthiourea and dimethyl sulphoxide as scavengers of hydroxyl radicals. Their protection of alpha 1-antiproteinase against inactivation by hypochlorous acid. Biochem J. 1987 May 1;243(3):867–870. doi: 10.1042/bj2430867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf G., Haberstroh U., Neilson E. G. Angiotensin II stimulates the proliferation and biosynthesis of type I collagen in cultured murine mesangial cells. Am J Pathol. 1992 Jan;140(1):95–107. [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T., Robinson E. A., Tanaka S., Appella E., Leonard E. J. Purification and amino acid analysis of two human monocyte chemoattractants produced by phytohemagglutinin-stimulated human blood mononuclear leukocytes. J Immunol. 1989 Mar 15;142(6):1956–1962. [PubMed] [Google Scholar]
- Yoshimura T., Yuhki N., Moore S. K., Appella E., Lerman M. I., Leonard E. J. Human monocyte chemoattractant protein-1 (MCP-1). Full-length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 1989 Feb 27;244(2):487–493. doi: 10.1016/0014-5793(89)80590-3. [DOI] [PubMed] [Google Scholar]
- Zoja C., Wang J. M., Bettoni S., Sironi M., Renzi D., Chiaffarino F., Abboud H. E., Van Damme J., Mantovani A., Remuzzi G. Interleukin-1 beta and tumor necrosis factor-alpha induce gene expression and production of leukocyte chemotactic factors, colony-stimulating factors, and interleukin-6 in human mesangial cells. Am J Pathol. 1991 Apr;138(4):991–1003. [PMC free article] [PubMed] [Google Scholar]