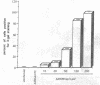
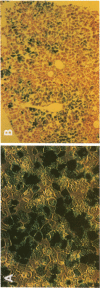
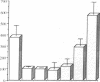
Abstract
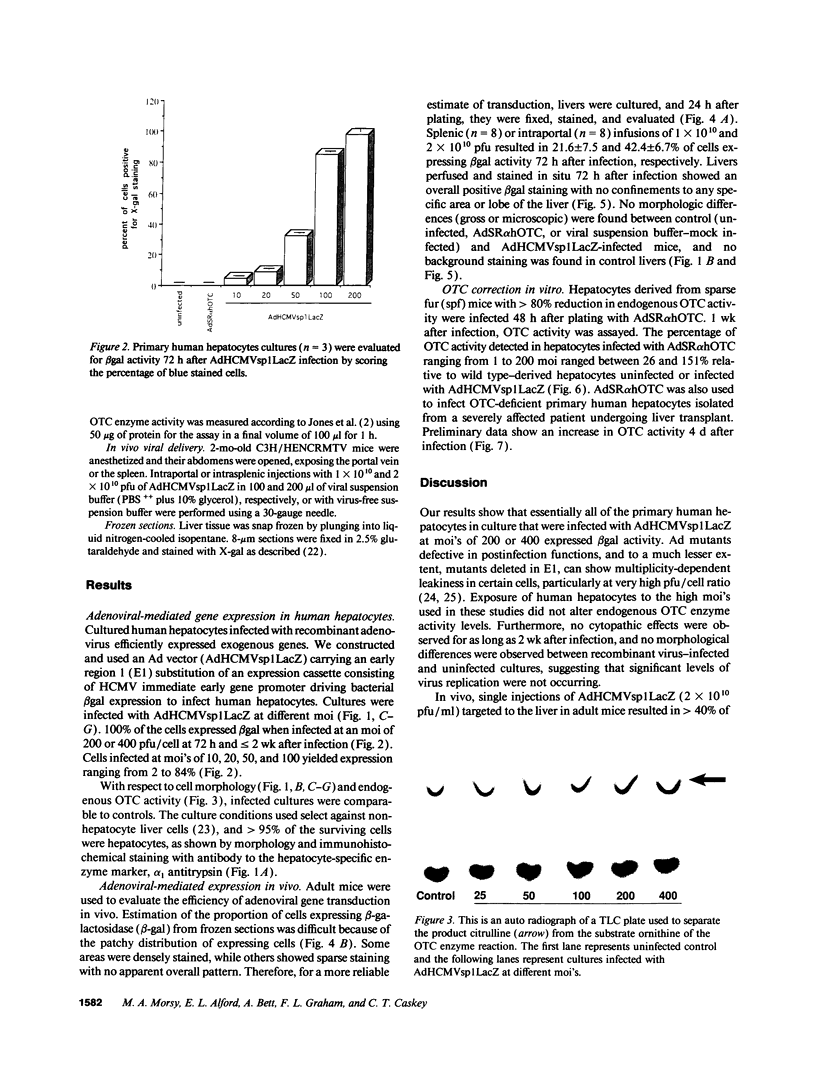
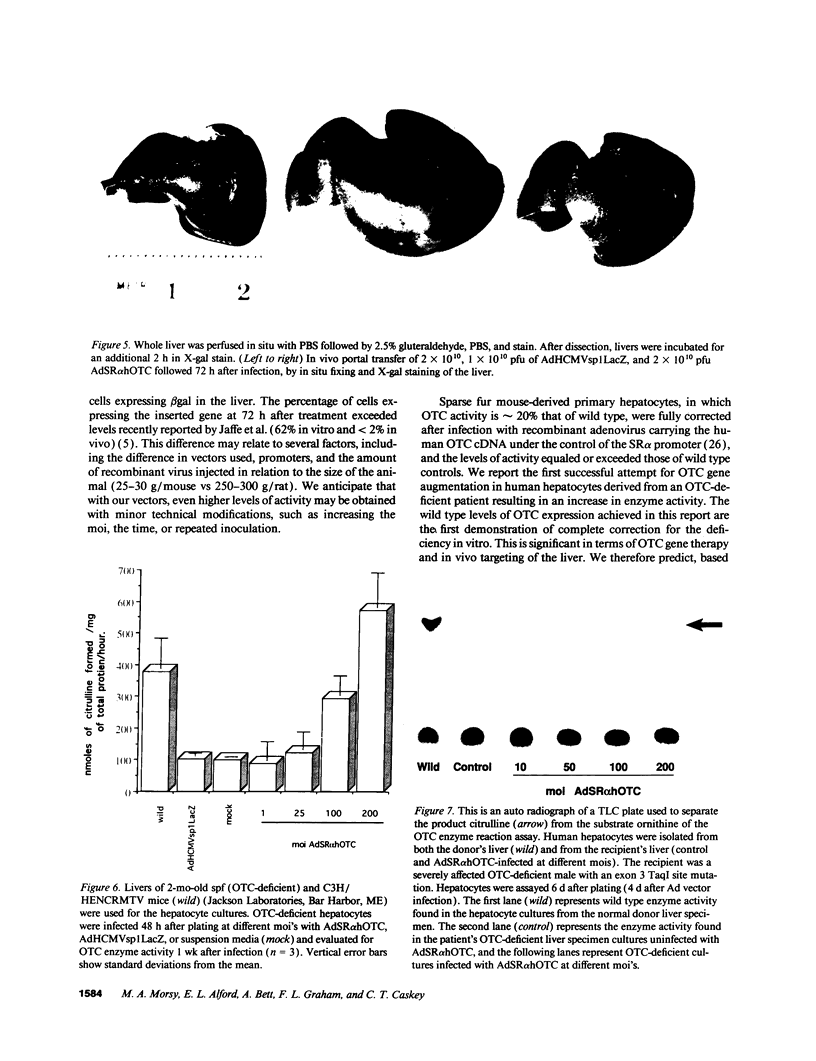
100% of primary human hepatocytes infected with an adenoviral vector carrying beta-galactosidase expressed the exogenous gene. Expression was also achieved in > 40% of adult mouse hepatocytes in vivo. Normal levels of activity were achieved in mouse ornithine transcarbamylase (OTC)-deficient primary hepatocytes using another adenoviral vector carrying human OTC cDNA. Study of OTC-deficient primary human hepatocytes from a single patient confirmed the utility of adenoviral delivery of OTC. We describe adenoviral-mediated exogenous gene expression in human and mouse hepatocytes in vitro and in mouse liver in vivo. Data suggest that adenoviral vectors may be useful for correcting OTC deficiency.
Full text
PDF
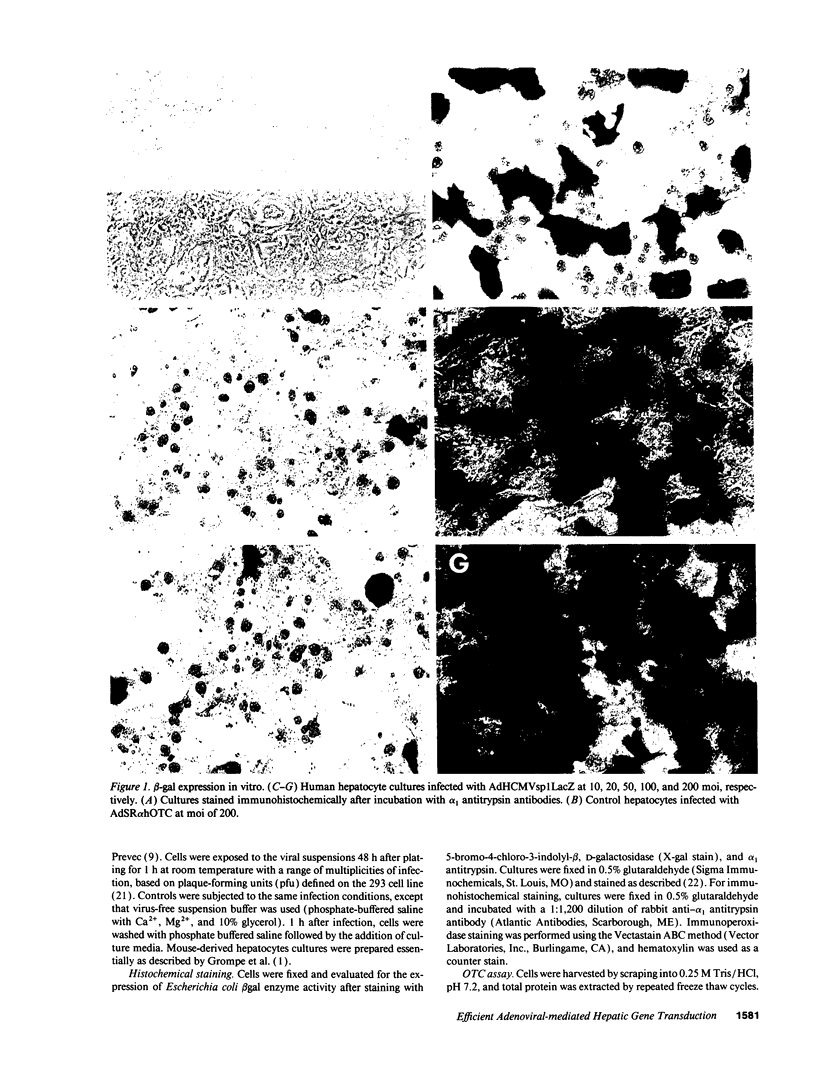



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaloner-Larsson G., Contreras G., Furesz J., Boucher D. W., Krepps D., Humphreys G. R., Mohanna S. M. Immunization of Canadian Armed Forces personnel with live types 4 and 7 adenovirus vaccines. Can J Public Health. 1986 Sep-Oct;77(5):367–370. [PubMed] [Google Scholar]
- Enat R., Jefferson D. M., Ruiz-Opazo N., Gatmaitan Z., Leinwand L. A., Reid L. M. Hepatocyte proliferation in vitro: its dependence on the use of serum-free hormonally defined medium and substrata of extracellular matrix. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1411–1415. doi: 10.1073/pnas.81.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. P., Brandt C. D., Wassermann F. E., Hall C. E., Spigland I., Kogon A., Elveback L. R. The virus watch program: a continuing surveillance of viral infections in metropolitan New York families. VI. Observations of adenovirus infections: virus excretion patterns, antibody response, efficiency of surveillance, patterns of infections, and relation to illness. Am J Epidemiol. 1969 Jan;89(1):25–50. doi: 10.1093/oxfordjournals.aje.a120913. [DOI] [PubMed] [Google Scholar]
- GIRARDI A. J., HILLEMAN M. R., ZWICKEY R. E. TESTS IN HAMSTERS FOR ONCOGENIC QUALITY OF ORDINARY VIRUSES INCLUDING ADENOVIRUS TYPE 7. Proc Soc Exp Biol Med. 1964 Apr;115:1141–1150. doi: 10.3181/00379727-115-29138. [DOI] [PubMed] [Google Scholar]
- Gaynor R. B., Berk A. J. Cis-acting induction of adenovirus transcription. Cell. 1983 Jul;33(3):683–693. doi: 10.1016/0092-8674(83)90011-9. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Griffiths P. D., Ellis D. S., Zuckerman A. J. Other common types of viral hepatitis and exotic infections. Br Med Bull. 1990 Apr;46(2):512–532. doi: 10.1093/oxfordjournals.bmb.a072413. [DOI] [PubMed] [Google Scholar]
- Grompe M., Jones S. N., Loulseged H., Caskey C. T. Retroviral-mediated gene transfer of human ornithine transcarbamylase into primary hepatocytes of spf and spf-ash mice. Hum Gene Ther. 1992 Feb;3(1):35–44. doi: 10.1089/hum.1992.3.1-35. [DOI] [PubMed] [Google Scholar]
- Jaffe H. A., Danel C., Longenecker G., Metzger M., Setoguchi Y., Rosenfeld M. A., Gant T. W., Thorgeirsson S. S., Stratford-Perricaudet L. D., Perricaudet M. Adenovirus-mediated in vivo gene transfer and expression in normal rat liver. Nat Genet. 1992 Aug;1(5):372–378. doi: 10.1038/ng0892-372. [DOI] [PubMed] [Google Scholar]
- Jones S. N., Grompe M., Munir M. I., Veres G., Craigen W. J., Caskey C. T. Ectopic correction of ornithine transcarbamylase deficiency in sparse fur mice. J Biol Chem. 1990 Aug 25;265(24):14684–14690. [PubMed] [Google Scholar]
- Kalser S. C., Hoofnagle J. H. Reports from the NIH DDDN. Human liver tissue for research. Gastroenterology. 1990 Apr;98(4):1086–1087. doi: 10.1016/0016-5085(90)90038-3. [DOI] [PubMed] [Google Scholar]
- Ledley F. D., Woo S. L., Ferry G. D., Whisennand H. H., Brandt M. L., Darlington G. J., Demmler G. J., Finegold M. J., Pokorny W. J., Rosenblatt H. Hepatocellular transplantation in acute hepatic failure and targeting genetic markers to hepatic cells. Hum Gene Ther. 1991 Winter;2(4):331–358. doi: 10.1089/hum.1991.2.4-331. [DOI] [PubMed] [Google Scholar]
- MacGregor G. R., Mogg A. E., Burke J. F., Caskey C. T. Histochemical staining of clonal mammalian cell lines expressing E. coli beta galactosidase indicates heterogeneous expression of the bacterial gene. Somat Cell Mol Genet. 1987 May;13(3):253–265. doi: 10.1007/BF01535207. [DOI] [PubMed] [Google Scholar]
- McGrory W. J., Bautista D. S., Graham F. L. A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology. 1988 Apr;163(2):614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- McKinnon R. D., Bacchetti S., Graham F. L. Tn5 mutagenesis of the transforming genes of human adenovirus type 5. Gene. 1982 Jul-Aug;19(1):33–42. doi: 10.1016/0378-1119(82)90186-x. [DOI] [PubMed] [Google Scholar]
- Qureshi I. A., Letarte J., Ouellet R. Ornithine transcarbamylase deficiency in mutant mice I. Studies on the characterization of enzyme defect and suitability as animal model of human disease. Pediatr Res. 1979 Jul;13(7):807–811. doi: 10.1203/00006450-197907000-00003. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. A., Siegfried W., Yoshimura K., Yoneyama K., Fukayama M., Stier L. E., Päkkö P. K., Gilardi P., Stratford-Perricaudet L. D., Perricaudet M. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science. 1991 Apr 19;252(5004):431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. A., Yoshimura K., Trapnell B. C., Yoneyama K., Rosenthal E. R., Dalemans W., Fukayama M., Bargon J., Stier L. E., Stratford-Perricaudet L. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992 Jan 10;68(1):143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- Shenk T., Jones N., Colby W., Fowlkes D. Functional analysis of adenovirus-5 host-range deletion mutants defective for transformation of rat embryo cells. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):367–375. doi: 10.1101/sqb.1980.044.01.041. [DOI] [PubMed] [Google Scholar]
- Southard J. H., van Gulik T. M., Ametani M. S., Vreugdenhil P. K., Lindell S. L., Pienaar B. L., Belzer F. O. Important components of the UW solution. Transplantation. 1990 Feb;49(2):251–257. doi: 10.1097/00007890-199002000-00004. [DOI] [PubMed] [Google Scholar]
- Spessot R., Inchley K., Hupel T. M., Bacchetti S. Cloning of the herpes simplex virus ICP4 gene in an adenovirus vector: effects on adenovirus gene expression and replication. Virology. 1989 Feb;168(2):378–387. doi: 10.1016/0042-6822(89)90279-1. [DOI] [PubMed] [Google Scholar]
- Stratford-Perricaudet L. D., Levrero M., Chasse J. F., Perricaudet M., Briand P. Evaluation of the transfer and expression in mice of an enzyme-encoding gene using a human adenovirus vector. Hum Gene Ther. 1990 Fall;1(3):241–256. doi: 10.1089/hum.1990.1.3-241. [DOI] [PubMed] [Google Scholar]
- Stratford-Perricaudet L. D., Makeh I., Perricaudet M., Briand P. Widespread long-term gene transfer to mouse skeletal muscles and heart. J Clin Invest. 1992 Aug;90(2):626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevethia M. J., Spector D. J., Leisure K. M., Stinski M. F. Participation of two human cytomegalovirus immediate early gene regions in transcriptional activation of adenovirus promoters. Virology. 1987 Dec;161(2):276–285. doi: 10.1016/0042-6822(87)90119-x. [DOI] [PubMed] [Google Scholar]
- Top F. H., Jr Control of adenovirus acute respiratory disease in U.S. Army trainees. Yale J Biol Med. 1975 Jul;48(3):185–195. [PMC free article] [PubMed] [Google Scholar]
- Tremblay M. L., Yee S. P., Persson R. H., Bacchetti S., Smiley J. R., Branton P. E. Activation and inhibition of expression of the 72,000-Da early protein of adenovirus type 5 in mouse cells constitutively expressing an immediate early protein of herpes simplex virus type 1. Virology. 1985 Jul 15;144(1):35–45. doi: 10.1016/0042-6822(85)90302-2. [DOI] [PubMed] [Google Scholar]
- Wong K. M., Levine A. J. Identification and mapping of Epstein-Barr virus early antigens and demonstration of a viral gene activator that functions in trans. J Virol. 1986 Oct;60(1):149–156. doi: 10.1128/jvi.60.1.149-156.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]