Abstract
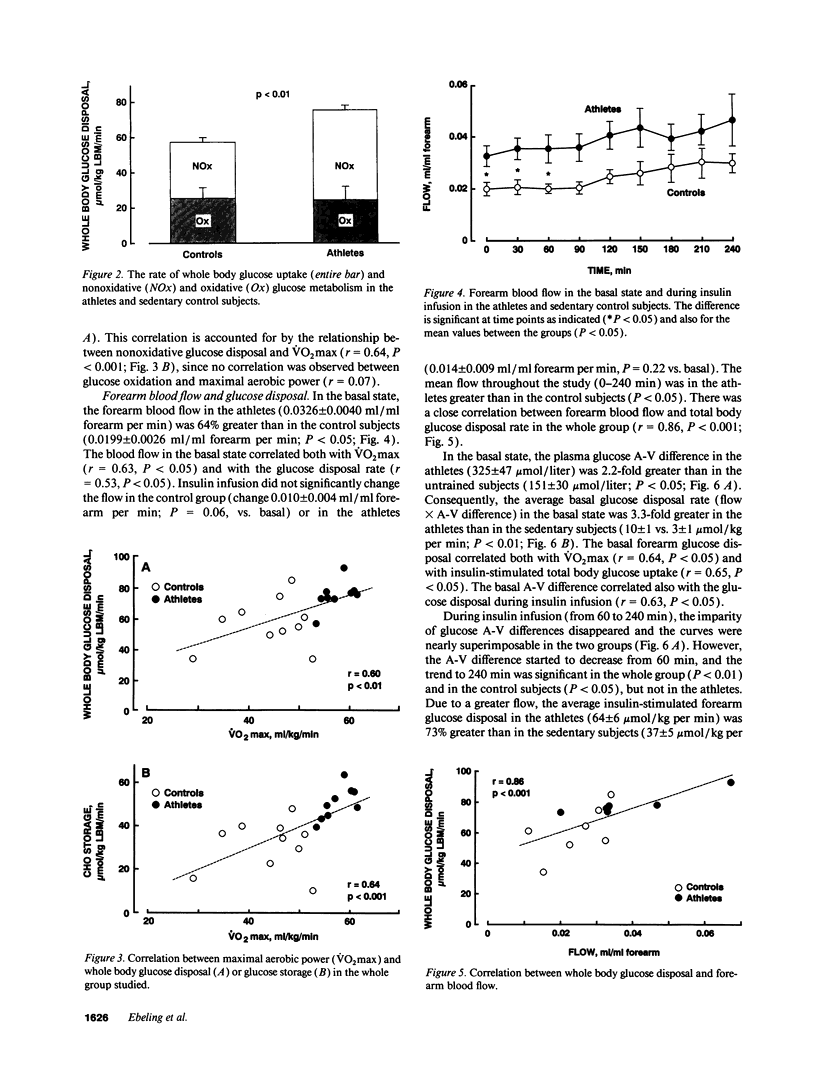
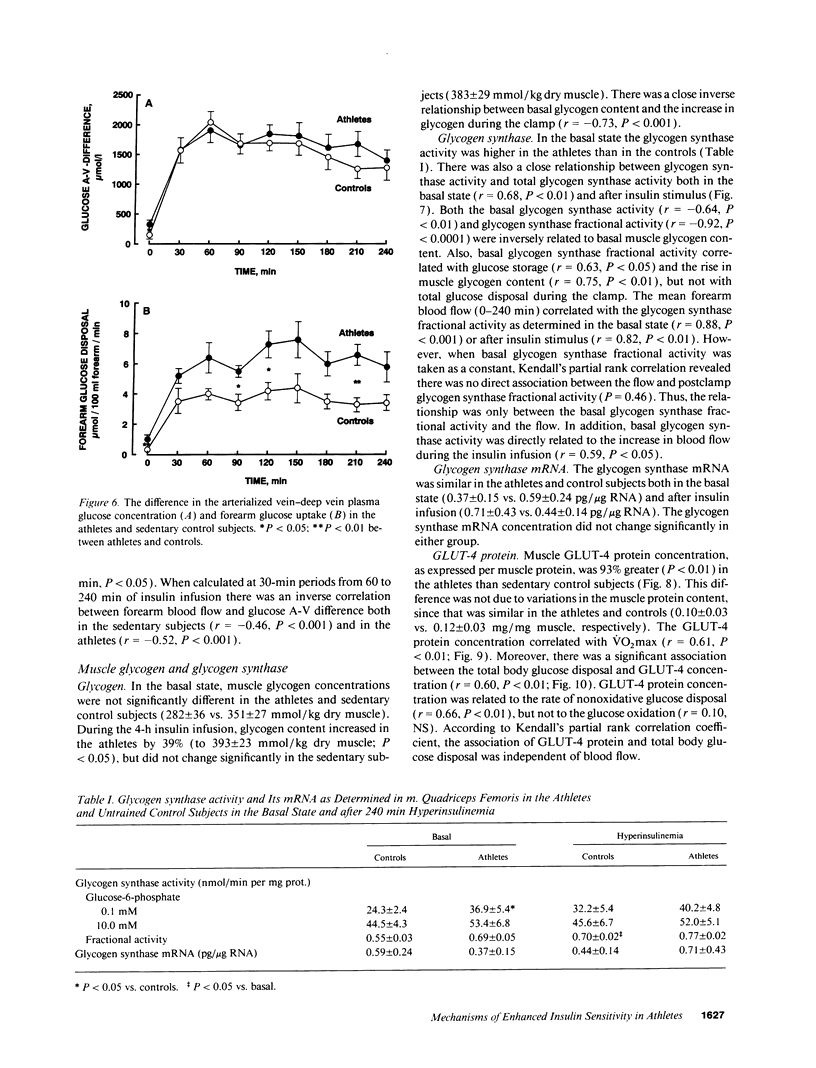
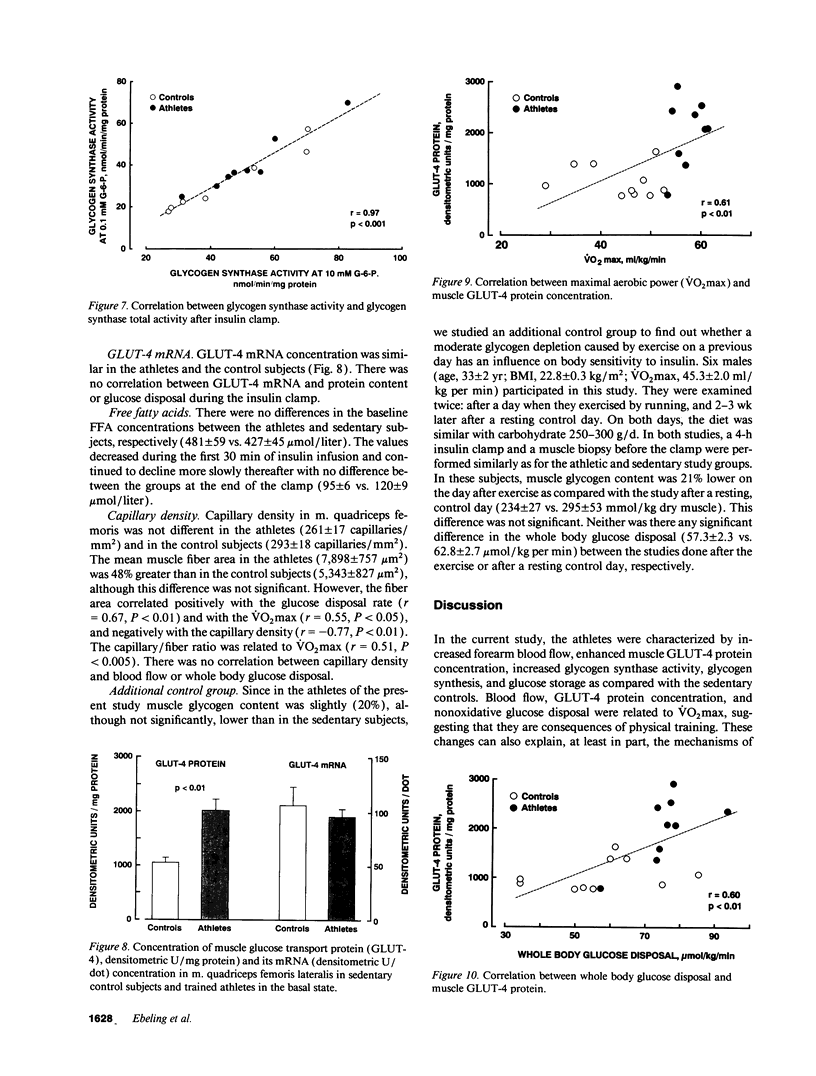
We examined the mechanisms of enhanced insulin sensitivity in 9 male healthy athletes (age, 25 +/- 1 yr; maximal aerobic power [VO2max], 57.6 +/- 1.0 ml/kg per min) as compared with 10 sedentary control subjects (age, 28 +/- 2 yr; VO2max, 44.1 +/- 2.3 ml/kg per min). In the athletes, whole body glucose disposal (240-min insulin clamp) was 32% (P < 0.01) and nonoxidative glucose disposal (indirect calorimetry) was 62% higher (P < 0.01) than in the controls. Muscle glycogen content increased by 39% in the athletes (P < 0.05) but did not change in the controls during insulin clamp. VO2max correlated with whole body (r = 0.60, P < 0.01) and nonoxidative glucose disposal (r = 0.64, P < 0.001). In the athletes forearm blood flow was 64% greater (P < 0.05) than in the controls, whereas their muscle capillary density was normal. Basal blood flow was related to VO2max (r = 0.63, P < 0.05) and glucose disposal during insulin infusion (r = 0.65, P < 0.05). The forearm glucose uptake in the athletes was increased by 3.3-fold (P < 0.01) in the basal state and by 73% (P < 0.05) during insulin infusion. Muscle glucose transport protein (GLUT-4) concentration was 93% greater in the athletes than controls (P < 0.01) and it was related to VO2max (r = 0.61, P < 0.01) and to whole body glucose disposal (r = 0.60, P < 0.01). Muscle glycogen synthase activity was 33% greater in the athletes than in the controls (P < 0.05), and the basal glycogen synthase fractional activity was closely related to blood flow (r = 0.88, P < 0.001). In conclusion: (a) athletes are characterized by enhanced muscle blood flow and glucose uptake. (b) The cellular mechanisms of glucose uptake are increased GLUT-4 protein content, glycogen synthase activity, and glucose storage as glycogen. (c) A close correlation between glycogen synthase fractional activity and blood flow suggests that they are causally related in promoting glucose disposal.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P. Capillary density in skeletal muscle of man. Acta Physiol Scand. 1975 Oct;95(2):203–205. doi: 10.1111/j.1748-1716.1975.tb10043.x. [DOI] [PubMed] [Google Scholar]
- Andersen P., Henriksson J. Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol. 1977 Sep;270(3):677–690. doi: 10.1113/jphysiol.1977.sp011975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Henriksson J. Training induced changes in the subgroups of human type II skeletal muscle fibres. Acta Physiol Scand. 1977 Jan;99(1):123–125. doi: 10.1111/j.1748-1716.1977.tb10361.x. [DOI] [PubMed] [Google Scholar]
- Baron A. D., Laakso M., Brechtel G., Edelman S. V. Reduced capacity and affinity of skeletal muscle for insulin-mediated glucose uptake in noninsulin-dependent diabetic subjects. Effects of insulin therapy. J Clin Invest. 1991 Apr;87(4):1186–1194. doi: 10.1172/JCI115117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström J., Hultman E. Muscle glycogen synthesis after exercise: an enhancing factor localized to the muscle cells in man. Nature. 1966 Apr 16;210(5033):309–310. doi: 10.1038/210309a0. [DOI] [PubMed] [Google Scholar]
- Browner M. F., Nakano K., Bang A. G., Fletterick R. J. Human muscle glycogen synthase cDNA sequence: a negatively charged protein with an asymmetric charge distribution. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1443–1447. doi: 10.1073/pnas.86.5.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLES D. R., COOPER K. E., MOTTRAM R. F., OCCLESHAW J. V. The source of blood samples withdrawn from deep forearm veins via catheters passed upstream from the median cubital vein. J Physiol. 1958 Jul 14;142(2):323–328. doi: 10.1113/jphysiol.1958.sp006019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Daub W. D., Green H. J., Houston M. E., Thomson J. A., Fraser I. G., Ranney D. A. Cross-adaptive responses to different forms of leg training: skeletal muscle biochemistry and histochemistry. Can J Physiol Pharmacol. 1982 May;60(5):628–633. doi: 10.1139/y82-085. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J. P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981 Dec;30(12):1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- Edelman S. V., Laakso M., Wallace P., Brechtel G., Olefsky J. M., Baron A. D. Kinetics of insulin-mediated and non-insulin-mediated glucose uptake in humans. Diabetes. 1990 Aug;39(8):955–964. doi: 10.2337/diab.39.8.955. [DOI] [PubMed] [Google Scholar]
- Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism. 1988 Mar;37(3):287–301. doi: 10.1016/0026-0495(88)90110-2. [DOI] [PubMed] [Google Scholar]
- Friedman J. E., Sherman W. M., Reed M. J., Elton C. W., Dohm G. L. Exercise training increases glucose transporter protein GLUT-4 in skeletal muscle of obese Zucker (fa/fa) rats. FEBS Lett. 1990 Jul 30;268(1):13–16. doi: 10.1016/0014-5793(90)80960-q. [DOI] [PubMed] [Google Scholar]
- Fushiki T., Kano T., Inoue K., Sugimoto E. Decrease in muscle glucose transporter number in chronic physical inactivity in rats. Am J Physiol. 1991 Mar;260(3 Pt 1):E403–E410. doi: 10.1152/ajpendo.1991.260.3.E403. [DOI] [PubMed] [Google Scholar]
- Goodyear L. J., Hirshman M. F., Smith R. J., Horton E. S. Glucose transporter number, activity, and isoform content in plasma membranes of red and white skeletal muscle. Am J Physiol. 1991 Nov;261(5 Pt 1):E556–E561. doi: 10.1152/ajpendo.1991.261.5.E556. [DOI] [PubMed] [Google Scholar]
- Goodyear L. J., Hirshman M. F., Valyou P. M., Horton E. S. Glucose transporter number, function, and subcellular distribution in rat skeletal muscle after exercise training. Diabetes. 1992 Sep;41(9):1091–1099. doi: 10.2337/diab.41.9.1091. [DOI] [PubMed] [Google Scholar]
- Henriksen E. J., Rodnick K. J., Mondon C. E., James D. E., Holloszy J. O. Effect of denervation or unweighting on GLUT-4 protein in rat soleus muscle. J Appl Physiol (1985) 1991 May;70(5):2322–2327. doi: 10.1152/jappl.1991.70.5.2322. [DOI] [PubMed] [Google Scholar]
- Hirshman M. F., Wardzala L. J., Goodyear L. J., Fuller S. P., Horton E. D., Horton E. S. Exercise training increases the number of glucose transporters in rat adipose cells. Am J Physiol. 1989 Oct;257(4 Pt 1):E520–E530. doi: 10.1152/ajpendo.1989.257.4.E520. [DOI] [PubMed] [Google Scholar]
- Hornbrook K. R., Burch H. B., Lowry O. H. The effects of adrenalectomy and hydrocortisone on rat liver metabolites and glycogen synthetase activity. Mol Pharmacol. 1966 Mar;2(2):106–116. [PubMed] [Google Scholar]
- Horton E. S. Exercise and physical training: effects on insulin sensitivity and glucose metabolism. Diabetes Metab Rev. 1986;2(1-2):1–17. doi: 10.1002/dmr.5610020101. [DOI] [PubMed] [Google Scholar]
- Houmard J. A., Egan P. C., Neufer P. D., Friedman J. E., Wheeler W. S., Israel R. G., Dohm G. L. Elevated skeletal muscle glucose transporter levels in exercise-trained middle-aged men. Am J Physiol. 1991 Oct;261(4 Pt 1):E437–E443. doi: 10.1152/ajpendo.1991.261.4.E437. [DOI] [PubMed] [Google Scholar]
- Kahn B. B., Charron M. J., Lodish H. F., Cushman S. W., Flier J. S. Differential regulation of two glucose transporters in adipose cells from diabetic and insulin-treated diabetic rats. J Clin Invest. 1989 Aug;84(2):404–411. doi: 10.1172/JCI114180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King D. S., Dalsky G. P., Staten M. A., Clutter W. E., Van Houten D. R., Holloszy J. O. Insulin action and secretion in endurance-trained and untrained humans. J Appl Physiol (1985) 1987 Dec;63(6):2247–2252. doi: 10.1152/jappl.1987.63.6.2247. [DOI] [PubMed] [Google Scholar]
- Koivisto V. A., Bourey R. E., Vuorinen-Markkola H., Koranyi L. Exercise reduces muscle glucose transport protein (GLUT-4) mRNA in type 1 diabetic patients. J Appl Physiol (1985) 1993 Apr;74(4):1755–1760. doi: 10.1152/jappl.1993.74.4.1755. [DOI] [PubMed] [Google Scholar]
- Koivisto V. A., Yki-Järvinen H., DeFronzo R. A. Physical training and insulin sensitivity. Diabetes Metab Rev. 1986;1(4):445–481. doi: 10.1002/dmr.5610010407. [DOI] [PubMed] [Google Scholar]
- Koivisto V. A., Yki-Järvinen H., Puhakainen I., Virkamäki A., Kolaczynski J., DeFronzo R. No evidence for isotope discrimination of tritiated glucose tracers in measurements of glucose turnover rates in man. Diabetologia. 1990 Mar;33(3):168–173. doi: 10.1007/BF00404045. [DOI] [PubMed] [Google Scholar]
- Koranyi L. I., Bourey R. E., Vuorinen-Markkola H., Koivisto V. A., Mueckler M., Permutt M. A., Yki-Järvinen H. Level of skeletal muscle glucose transporter protein correlates with insulin-stimulated whole body glucose disposal in man. Diabetologia. 1991 Oct;34(10):763–765. doi: 10.1007/BF00401526. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laakso M., Edelman S. V., Brechtel G., Baron A. D. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest. 1990 Jun;85(6):1844–1852. doi: 10.1172/JCI114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso M., Edelman S. V., Olefsky J. M., Brechtel G., Wallace P., Baron A. D. Kinetics of in vivo muscle insulin-mediated glucose uptake in human obesity. Diabetes. 1990 Aug;39(8):965–974. doi: 10.2337/diab.39.8.965. [DOI] [PubMed] [Google Scholar]
- Lohmann D., Liebold F., Heilmann W., Senger H., Pohl A. Diminished insulin response in highly trained athletes. Metabolism. 1978 May;27(5):521–524. doi: 10.1016/0026-0495(78)90017-3. [DOI] [PubMed] [Google Scholar]
- Mikines K. J., Sonne B., Farrell P. A., Tronier B., Galbo H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol. 1988 Mar;254(3 Pt 1):E248–E259. doi: 10.1152/ajpendo.1988.254.3.E248. [DOI] [PubMed] [Google Scholar]
- Mikines K. J., Sonne B., Farrell P. A., Tronier B., Galbo H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol. 1988 Mar;254(3 Pt 1):E248–E259. doi: 10.1152/ajpendo.1988.254.3.E248. [DOI] [PubMed] [Google Scholar]
- Mikines K. J., Sonne B., Tronier B., Galbo H. Effects of acute exercise and detraining on insulin action in trained men. J Appl Physiol (1985) 1989 Feb;66(2):704–711. doi: 10.1152/jappl.1989.66.2.704. [DOI] [PubMed] [Google Scholar]
- Miles J., Glasscock R., Aikens J., Gerich J., Haymond M. A microfluorometric method for the determination of free fatty acids in plasma. J Lipid Res. 1983 Jan;24(1):96–99. [PubMed] [Google Scholar]
- Nuutila P., Koivisto V. A., Knuuti J., Ruotsalainen U., Teräs M., Haaparanta M., Bergman J., Solin O., Voipio-Pulkki L. M., Wegelius U. Glucose-free fatty acid cycle operates in human heart and skeletal muscle in vivo. J Clin Invest. 1992 Jun;89(6):1767–1774. doi: 10.1172/JCI115780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploug T., Stallknecht B. M., Pedersen O., Kahn B. B., Ohkuwa T., Vinten J., Galbo H. Effect of endurance training on glucose transport capacity and glucose transporter expression in rat skeletal muscle. Am J Physiol. 1990 Dec;259(6 Pt 1):E778–E786. doi: 10.1152/ajpendo.1990.259.6.E778. [DOI] [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- ROHTER F. D., ROCHELLE R. H., HYMAN C. Exercise blood flow changes in the human forearm during physical t raining. J Appl Physiol. 1963 Jul;18:789–793. doi: 10.1152/jappl.1963.18.4.789. [DOI] [PubMed] [Google Scholar]
- Rodnick K. J., Holloszy J. O., Mondon C. E., James D. E. Effects of exercise training on insulin-regulatable glucose-transporter protein levels in rat skeletal muscle. Diabetes. 1990 Nov;39(11):1425–1429. doi: 10.2337/diab.39.11.1425. [DOI] [PubMed] [Google Scholar]
- Schalin-Jäntti C., Härkonen M., Groop L. C. Impaired activation of glycogen synthase in people at increased risk for developing NIDDM. Diabetes. 1992 May;41(5):598–604. doi: 10.2337/diab.41.5.598. [DOI] [PubMed] [Google Scholar]
- Schantz P., Henriksson J., Jansson E. Adaptation of human skeletal muscle to endurance training of long duration. Clin Physiol. 1983 Apr;3(2):141–151. doi: 10.1111/j.1475-097x.1983.tb00685.x. [DOI] [PubMed] [Google Scholar]
- Segal K. R., Van Loan M., Fitzgerald P. I., Hodgdon J. A., Van Itallie T. B. Lean body mass estimation by bioelectrical impedance analysis: a four-site cross-validation study. Am J Clin Nutr. 1988 Jan;47(1):7–14. doi: 10.1093/ajcn/47.1.7. [DOI] [PubMed] [Google Scholar]
- Snell P. G., Martin W. H., Buckey J. C., Blomqvist C. G. Maximal vascular leg conductance in trained and untrained men. J Appl Physiol (1985) 1987 Feb;62(2):606–610. doi: 10.1152/jappl.1987.62.2.606. [DOI] [PubMed] [Google Scholar]
- Soman V. R., Koivisto V. A., Deibert D., Felig P., DeFronzo R. A. Increased insulin sensitivity and insulin binding to monocytes after physical training. N Engl J Med. 1979 Nov 29;301(22):1200–1204. doi: 10.1056/NEJM197911293012203. [DOI] [PubMed] [Google Scholar]
- Thorens B., Charron M. J., Lodish H. F. Molecular physiology of glucose transporters. Diabetes Care. 1990 Mar;13(3):209–218. doi: 10.2337/diacare.13.3.209. [DOI] [PubMed] [Google Scholar]
- Vuorinen-Markkola H., Koivisto V. A., Yki-Jarvinen H. Mechanisms of hyperglycemia-induced insulin resistance in whole body and skeletal muscle of type I diabetic patients. Diabetes. 1992 May;41(5):571–580. doi: 10.2337/diab.41.5.571. [DOI] [PubMed] [Google Scholar]
- Wallberg-Henriksson H., Constable S. H., Young D. A., Holloszy J. O. Glucose transport into rat skeletal muscle: interaction between exercise and insulin. J Appl Physiol (1985) 1988 Aug;65(2):909–913. doi: 10.1152/jappl.1988.65.2.909. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H., Koivisto V. A. Effects of body composition on insulin sensitivity. Diabetes. 1983 Oct;32(10):965–969. doi: 10.2337/diab.32.10.965. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H., Sahlin K., Ren J. M., Koivisto V. A. Localization of rate-limiting defect for glucose disposal in skeletal muscle of insulin-resistant type I diabetic patients. Diabetes. 1990 Feb;39(2):157–167. doi: 10.2337/diab.39.2.157. [DOI] [PubMed] [Google Scholar]
- Young D. A., Wallberg-Henriksson H., Sleeper M. D., Holloszy J. O. Reversal of the exercise-induced increase in muscle permeability to glucose. Am J Physiol. 1987 Oct;253(4 Pt 1):E331–E335. doi: 10.1152/ajpendo.1987.253.4.E331. [DOI] [PubMed] [Google Scholar]




