Abstract
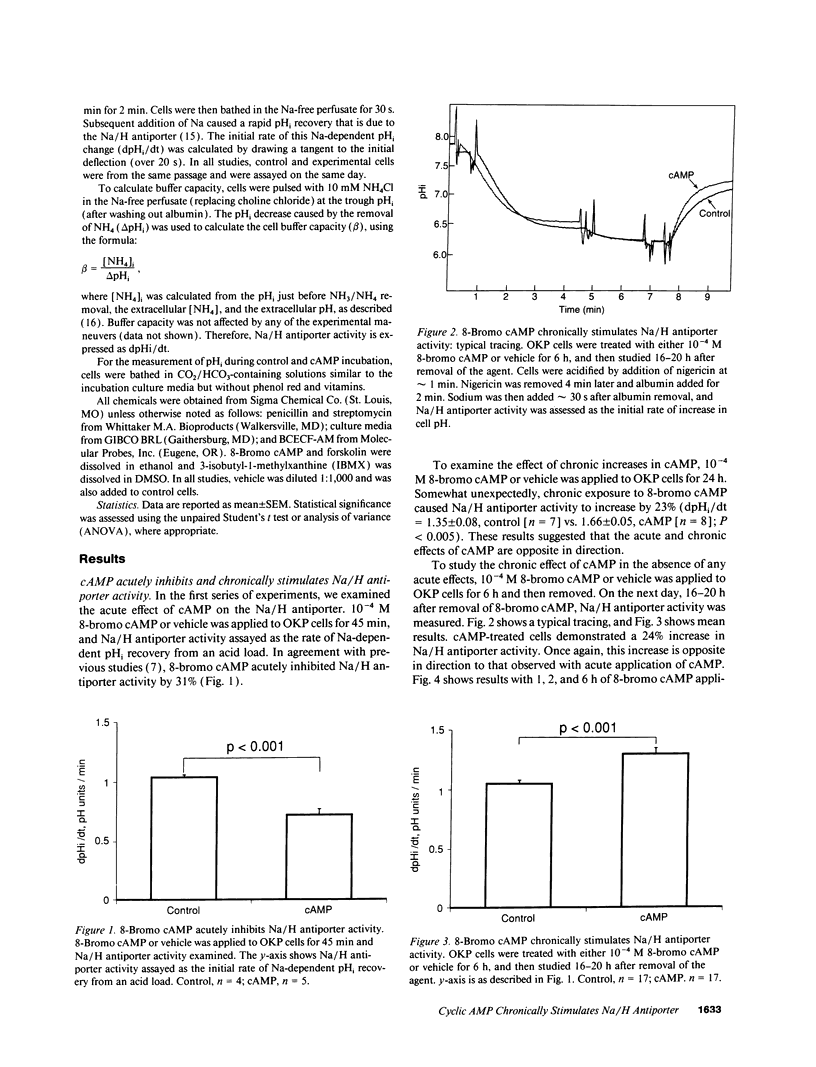
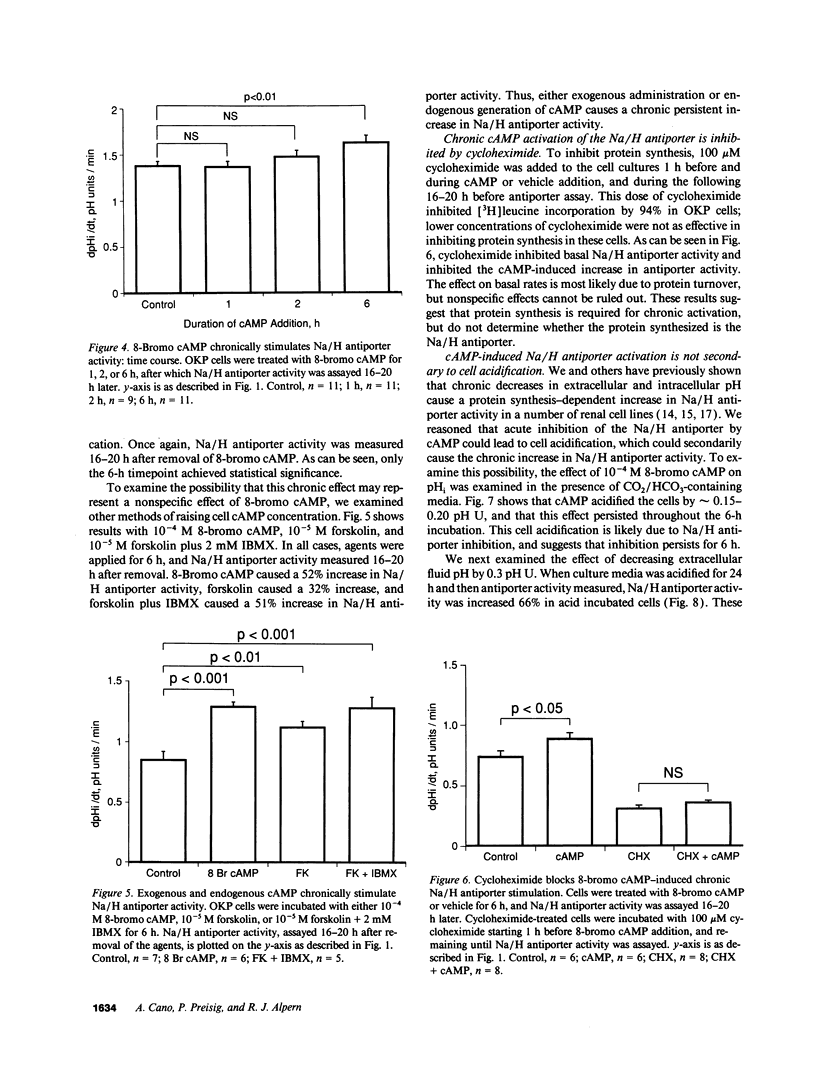
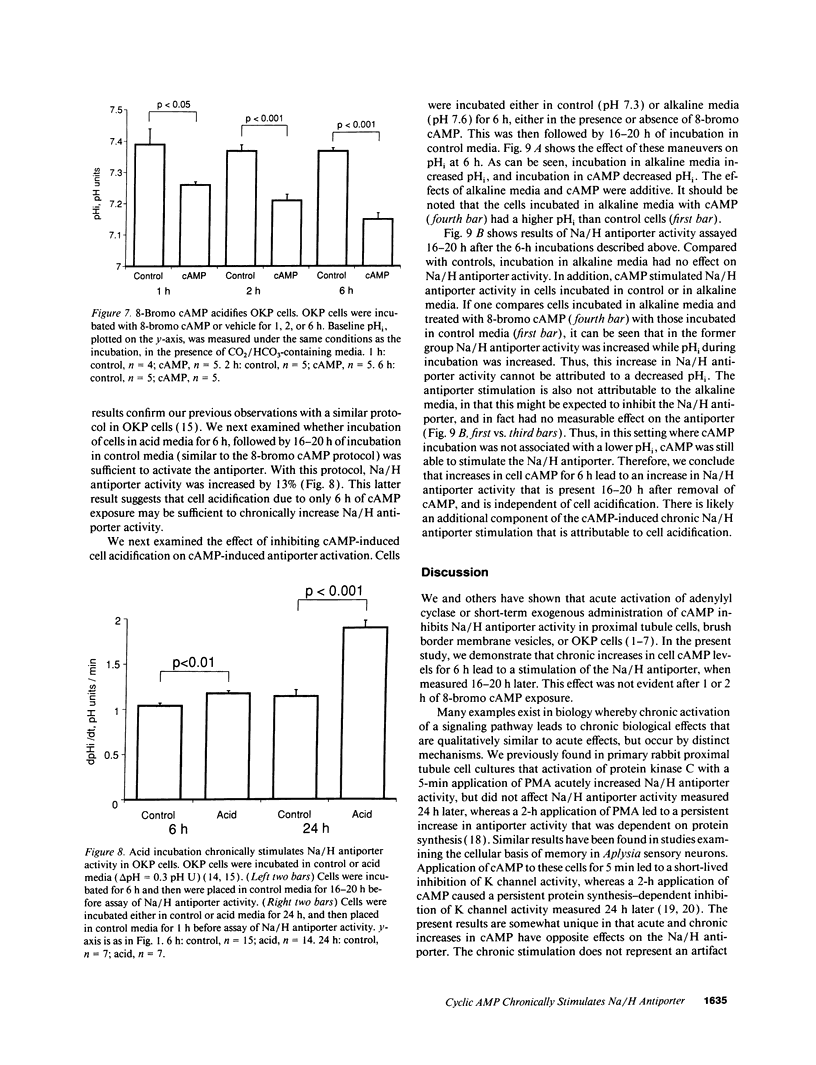
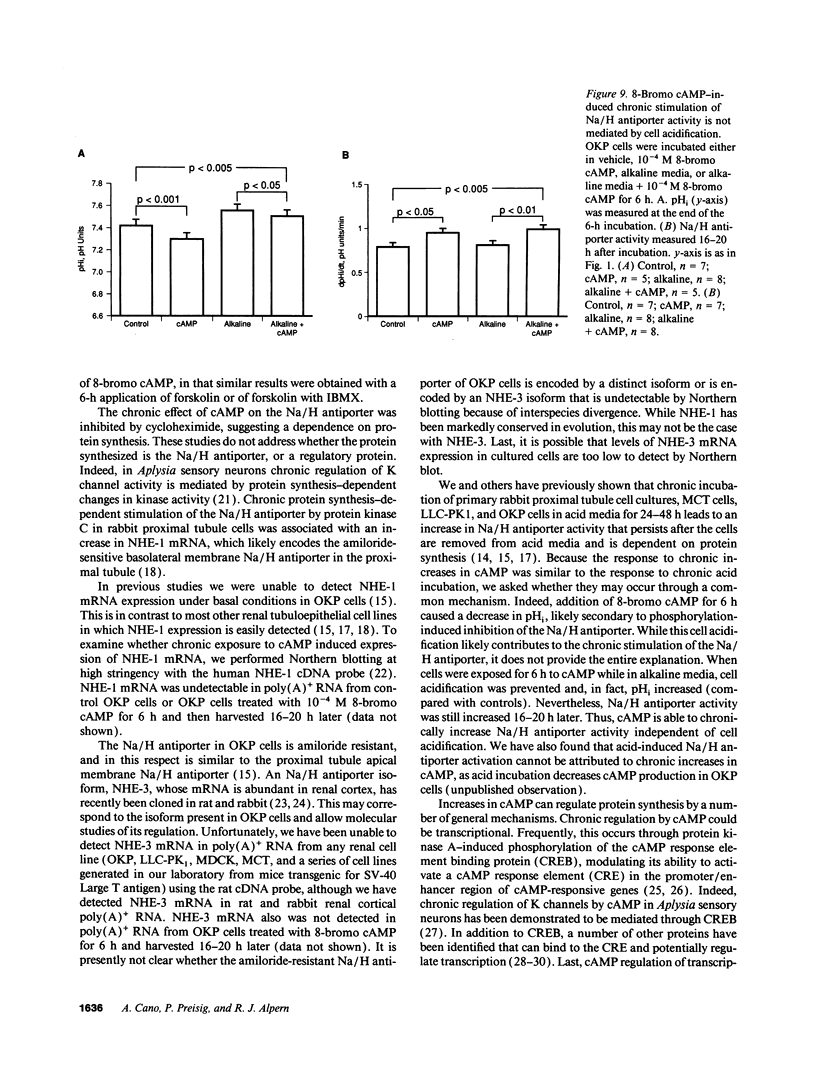
Parathyroid hormone, dopamine, alpha-adrenergic catecholamines, and angiotensin II regulate renal Na excretion, at least in part through modulation of acute cyclic (c)AMP-induced proximal tubule Na/H antiporter inhibition. The present studies examined the effect of chronic increases in cell cAMP on Na/H antiporter activity in OKP cells. Whereas 8-bromo cAMP acutely inhibited Na/H antiporter activity, chronic application for 6 h led to a 24% increase in Na/H antiporter activity measured 16-20 h after cAMP removal. This chronic persistent activation of the Na/H antiporter required > 2 h exposure. This effect was not a nonspecific effect of 8-bromo cAMP, in that addition of forskolin or forskolin + 3-isobutyl-1-methylxanthine for 6 h also led to a chronic persistent increase in Na/H antiporter activity. Inhibition of protein synthesis with cycloheximide prevented 8-bromo cAMP-induced Na/H antiporter stimulation. Although 8-bromo cAMP addition decreased cell pH by 0.15-0.20 pH U, Na/H antiporter stimulation could be dissociated from cell acidification. In summary, while cAMP acutely inhibits Na/H antiporter activity, it chronically increases antiporter activity. This chronic activation occurs with exogenous addition or endogenous generation of cAMP. These results imply that for hormones that modulate renal Na excretion and proximal tubule Na/H antiporter activity via cAMP and protein kinase A, acute effects may not predict chronic effects.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpern R. J. Mechanism of basolateral membrane H+/OH-/HCO-3 transport in the rat proximal convoluted tubule. A sodium-coupled electrogenic process. J Gen Physiol. 1985 Nov;86(5):613–636. doi: 10.1085/jgp.86.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank N., Aynediian H. S. A micropuncture study of the effect of parathyroid hormone on renal bicarbonate reabsorption. J Clin Invest. 1976 Aug;58(2):336–344. doi: 10.1172/JCI108477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. A., Forte L. R., Krause W. J., Thorne P. K. Clonal sublines that are morphologically and functionally distinct from parental OK cells. Am J Physiol. 1989 Apr;256(4 Pt 2):F672–F679. doi: 10.1152/ajprenal.1989.256.4.F672. [DOI] [PubMed] [Google Scholar]
- Dash P. K., Hochner B., Kandel E. R. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990 Jun 21;345(6277):718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- Deutsch P. J., Hoeffler J. P., Jameson J. L., Habener J. F. Cyclic AMP and phorbol ester-stimulated transcription mediated by similar DNA elements that bind distinct proteins. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7922–7926. doi: 10.1073/pnas.85.21.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas J. G. Angiotensin receptor subtypes of the kidney cortex. Am J Physiol. 1987 Jul;253(1 Pt 2):F1–F7. doi: 10.1152/ajprenal.1987.253.1.F1. [DOI] [PubMed] [Google Scholar]
- Felder C. C., Campbell T., Albrecht F., Jose P. A. Dopamine inhibits Na(+)-H+ exchanger activity in renal BBMV by stimulation of adenylate cyclase. Am J Physiol. 1990 Aug;259(2 Pt 2):F297–F303. doi: 10.1152/ajprenal.1990.259.2.F297. [DOI] [PubMed] [Google Scholar]
- Foulkes N. S., Borrelli E., Sassone-Corsi P. CREM gene: use of alternative DNA-binding domains generates multiple antagonists of cAMP-induced transcription. Cell. 1991 Feb 22;64(4):739–749. doi: 10.1016/0092-8674(91)90503-q. [DOI] [PubMed] [Google Scholar]
- Goelet P., Castellucci V. F., Schacher S., Kandel E. R. The long and the short of long-term memory--a molecular framework. 1986 Jul 31-Aug 6Nature. 322(6078):419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez G. A., Montminy M. R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989 Nov 17;59(4):675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Hoeffler J. P., Deutsch P. J., Lin J., Habener J. F. Distinct adenosine 3',5'-monophosphate and phorbol ester-responsive signal transduction pathways converge at the level of transcriptional activation by the interactions of DNA-binding proteins. Mol Endocrinol. 1989 May;3(5):868–880. doi: 10.1210/mend-3-5-868. [DOI] [PubMed] [Google Scholar]
- Horie S., Moe O., Miller R. T., Alpern R. J. Long-term activation of protein kinase c causes chronic Na/H antiporter stimulation in cultured proximal tubule cells. J Clin Invest. 1992 Feb;89(2):365–372. doi: 10.1172/JCI115594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie S., Moe O., Tejedor A., Alpern R. J. Preincubation in acid medium increases Na/H antiporter activity in cultured renal proximal tubule cells. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4742–4745. doi: 10.1073/pnas.87.12.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulter H. N., Peterson J. C. Acid-base homeostasis during chronic PTH excess in humans. Kidney Int. 1985 Aug;28(2):187–192. doi: 10.1038/ki.1985.139. [DOI] [PubMed] [Google Scholar]
- Hulter H. N., Toto R. D., Ilnicki L. P., Halloran B., Sebastian A. Metabolic alkalosis in models of primary and secondary hyperparathyroid states. Am J Physiol. 1983 Oct;245(4):F450–F461. doi: 10.1152/ajprenal.1983.245.4.F450. [DOI] [PubMed] [Google Scholar]
- Igarashi P., Freed M. I., Ganz M. B., Reilly R. F. Effects of chronic metabolic acidosis on Na(+)-H+ exchangers in LLC-PK1 renal epithelial cells. Am J Physiol. 1992 Jul;263(1 Pt 2):F83–F88. doi: 10.1152/ajprenal.1992.263.1.F83. [DOI] [PubMed] [Google Scholar]
- Iino Y., Burg M. B. Effect of parathyroid hormone on bicarbonate absorption by proximal tubules in vitro. Am J Physiol. 1979 Apr;236(4):F387–F391. doi: 10.1152/ajprenal.1979.236.4.F387. [DOI] [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Ivashkiv L. B., Liou H. C., Kara C. J., Lamph W. W., Verma I. M., Glimcher L. H. mXBP/CRE-BP2 and c-Jun form a complex which binds to the cyclic AMP, but not to the 12-O-tetradecanoylphorbol-13-acetate, response element. Mol Cell Biol. 1990 Apr;10(4):1609–1621. doi: 10.1128/mcb.10.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn A. M., Dolson G. M., Hise M. K., Bennett S. C., Weinman E. J. Parathyroid hormone and dibutyryl cAMP inhibit Na+/H+ exchange in renal brush border vesicles. Am J Physiol. 1985 Feb;248(2 Pt 2):F212–F218. doi: 10.1152/ajprenal.1985.248.2.F212. [DOI] [PubMed] [Google Scholar]
- Liu F. Y., Cogan M. G. Angiotensin II stimulates early proximal bicarbonate absorption in the rat by decreasing cyclic adenosine monophosphate. J Clin Invest. 1989 Jul;84(1):83–91. doi: 10.1172/JCI114174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa T., Sakura H., Kanei-Ishii C., Sudo T., Yoshimura T., Fujisawa J., Yoshida M., Ishii S. Leucine zipper structure of the protein CRE-BP1 binding to the cyclic AMP response element in brain. EMBO J. 1989 Jul;8(7):2023–2028. doi: 10.1002/j.1460-2075.1989.tb03610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney T. D., Myers P. Bicarbonate transport by proximal tubules: effect of parathyroid hormone and dibutyryl cyclic AMP. Am J Physiol. 1980 Mar;238(3):F166–F174. doi: 10.1152/ajprenal.1980.238.3.F166. [DOI] [PubMed] [Google Scholar]
- Mitnick P., Greenberg A., Coffman T., Kelepouris E., Wolf C. J., Goldfarb S. Effects of two models of hypercalcemia on renal acid base metabolism. Kidney Int. 1982 Apr;21(4):613–620. doi: 10.1038/ki.1982.68. [DOI] [PubMed] [Google Scholar]
- Moe O. W., Miller R. T., Horie S., Cano A., Preisig P. A., Alpern R. J. Differential regulation of Na/H antiporter by acid in renal epithelial cells and fibroblasts. J Clin Invest. 1991 Nov;88(5):1703–1708. doi: 10.1172/JCI115487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord E. P., Howard M. J., Hafezi A., Moradeshagi P., Vaystub S., Insel P. A. Alpha 2 adrenergic agonists stimulate Na+-H+ antiport activity in the rabbit renal proximal tubule. J Clin Invest. 1987 Dec;80(6):1755–1762. doi: 10.1172/JCI113268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski J., Kandasamy R. A., Shull G. E. Molecular cloning of putative members of the Na/H exchanger gene family. cDNA cloning, deduced amino acid sequence, and mRNA tissue expression of the rat Na/H exchanger NHE-1 and two structurally related proteins. J Biol Chem. 1992 May 5;267(13):9331–9339. [PubMed] [Google Scholar]
- Pollock A. S., Warnock D. G., Strewler G. J. Parathyroid hormone inhibition of Na+-H+ antiporter activity in a cultured renal cell line. Am J Physiol. 1986 Feb;250(2 Pt 2):F217–F225. doi: 10.1152/ajprenal.1986.250.2.F217. [DOI] [PubMed] [Google Scholar]
- Puschett J. B., Zurbach P., Sylk D. Acute effects of parathyroid hormone on proximal bicarbonate transport in the dog. Kidney Int. 1976 Jun;9(6):501–510. doi: 10.1038/ki.1976.64. [DOI] [PubMed] [Google Scholar]
- Roesler W. J., Vandenbark G. R., Hanson R. W. Cyclic AMP and the induction of eukaryotic gene transcription. J Biol Chem. 1988 Jul 5;263(19):9063–9066. [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Sardet C., Franchi A., Pouysségur J. Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell. 1989 Jan 27;56(2):271–280. doi: 10.1016/0092-8674(89)90901-x. [DOI] [PubMed] [Google Scholar]
- Schacher S., Castellucci V. F., Kandel E. R. cAMP evokes long-term facilitation in Aplysia sensory neurons that requires new protein synthesis. Science. 1988 Jun 17;240(4859):1667–1669. doi: 10.1126/science.2454509. [DOI] [PubMed] [Google Scholar]
- Sweatt J. D., Kandel E. R. Persistent and transcriptionally-dependent increase in protein phosphorylation in long-term facilitation of Aplysia sensory neurons. Nature. 1989 May 4;339(6219):51–54. doi: 10.1038/339051a0. [DOI] [PubMed] [Google Scholar]
- Tse C. M., Brant S. R., Walker M. S., Pouyssegur J., Donowitz M. Cloning and sequencing of a rabbit cDNA encoding an intestinal and kidney-specific Na+/H+ exchanger isoform (NHE-3). J Biol Chem. 1992 May 5;267(13):9340–9346. [PubMed] [Google Scholar]
- Weinman E. J., Dubinsky W. P., Shenolikar S. Reconstitution of cAMP-dependent protein kinase regulated renal Na+-H+ exchanger. J Membr Biol. 1988;101(1):11–18. doi: 10.1007/BF01872815. [DOI] [PubMed] [Google Scholar]
- Weinman E. J., Shenolikar S., Kahn A. M. cAMP-associated inhibition of Na+-H+ exchanger in rabbit kidney brush-border membranes. Am J Physiol. 1987 Jan;252(1 Pt 2):F19–F25. doi: 10.1152/ajprenal.1987.252.1.F19. [DOI] [PubMed] [Google Scholar]


