Abstract
CD38 is a multifunctional enzyme that uses nicotinamide adenine dinucleotide (NAD) as a substrate to generate second messengers. Recently, CD38 was also identified as one of the main cellular NADases in mammalian tissues and appears to regulate cellular levels of NAD in multiple tissues and cells. Due to the emerging role of NAD as a key molecule in multiple signaling pathways, and metabolic conditions it is imperative to determine the cellular mechanisms that regulate the synthesis and degradation of this nucleotide. In fact, recently it has been shown that NAD participates in multiple physiological processes such as insulin secretion, control of energy metabolism, neuronal and cardiac cell survival, airway constriction, asthma, aging and longevity. The discovery of CD38 as the main cellular NADase in mammalian tissues, and the characterization of its role on the control of cellular NAD levels indicate that CD38 may serve as a pharmacological target for multiple conditions.
Keywords: CD38, NAD, SIRT1, aging, obesity, metabolic syndrome
1. INTRODUCTION
Nicotinamide adenine dinucleotide (NAD) is a key cellular metabolite that is involved in cellular energetics. In addition, NAD has recently emerged as a crucial regulator of signaling pathways implicated in multiple physiological conditions [1–12]. The two main signaling roles of NAD include its importance as a substrate for the generation of second messengers such as cyclic-ADP-ribose (cADPR) [1–9] and its role as a substrate and regulator of the NAD dependent deacetylases sirtuins [10–12]. Both these signaling pathways have been shown to be very important in many physiological conditions from egg fertilization all the way to the cellular mechanisms of aging, longevity, and death [1–12]. In these regards, a great new interest in NAD functions and metabolism has emerged. In fact, we have seen almost a second discovery of this molecule in recent years [13]. Due to the key role of NAD in cells, it is crucial to characterize the mechanisms that control NAD metabolism. In recent years, we have learned much about the cellular echanisms of NAD synthesis [12–14]. Intense research in this field culminated with the discovery of the role of the protein nicotinamide phosphoribosyltransferase (Nampt) (also known as visfatin or PBEF) as a key enzyme involved in de novo synthesis of NAD [15]. In fact, Nampt has been recently shown to modulate NAD levels and some of its cellular functions [15,16]. On the other hand, until recently, very little was known about the mechanisms that regulate NAD degradation in mammalian cells. Our recent studies clearly show that the multifunctional enzyme CD38 is a key enzyme involved in the degradation of NAD and appears to control cellular NAD levels [17–19]. In this review, I will focus on the role of CD38 as one of the main cellular NADases, and will discuss its potential role as a pharmacological target for the control of conditions regulated by ellular NAD.
2. CD38 IS A SECOND MESSENGER ENZYME: SYNTHESIS AND DEGRADATION OF CADPR
2.1. Biochemistry and Metabolism of cADPR
Cyclic-ADP-ribose is a second messenger that induces calcium release from intracellular stores [2–9]. Cyclic-ADPribose is synthesized from β-NAD+ by an enzymatic activity named ADP-ribosyl cyclase and converted to ADPR in a reaction call cADPR hydrolase (Fig. 1). It is interesting that cADPR metabolism resembles the cyclic AMP system; where a cyclic nucleotide compound with active biological activity (cADPR and cAMP) is hydrolyzed into an inactive non-cyclic compound (ADPR and 5’-AMP). It has to be noted, however, that in many cell types, studied so far, the precise nature of the enzyme(s) responsible for physiological ADP-ribosyl cyclase and cADPR hydrolase activities has not been well established. Nevertheless, ADP-ribosyl cyclase activity has been found across different species spanning from unicellular organisms, invertebrates (sea urchin eggs, Aplysia) to mammalian cells, plants, and parasites suggesting that cADPR metabolism has been preserved in evolution as an ubiquitous second messenger [20–39]. ADP-ribosyl cyclases have been found both in soluble and membrane-bound forms. The first characterization of ADP-ribosyl cyclase was performed in Aplysia californica ovotestis [5, 38, 39]; this soluble 30-KDa enzyme was purified and found to have pure cyclase but no hydrolase activity [39]. It can use β-NAD+ as a substrate, but no α-NAD+ or NADH. It can also metabolize analogs of NAD+, such as nicotinamide guanine dinucleotide (NGD+) and nicotinamide hypoxanthine dinucleotide (NHD+), generating cyclic compounds (cGDPR and cIDPR, respectively) with fluorescent properties, but lacking calcium-releasing activity [40]. These fluorescent compounds are very useful as biochemical tools for studies of ADP-ribosyl cyclase activity [40]. The amino acid sequence of ADP-ribosyl cyclase from Aplysia has considerable homology with the human lymphocyte surface antigen CD38, which led to the discovery that CD38 has also ADP-ribosyl cyclase activity [41]. However, in contrast to Aplysia cyclase, CD38 is a transmembrane protein and has both ADP-ribosyl cyclase and cADPR hydrolase activities. Surprisingly, CD38 bound to the plasma membrane has its catalytic site located on the extracellular domain of the cell, which poses theoretical difficulties for understanding how CD38 generates cADPR in the cytoplasm, where it should be available to interact with calcium channels. Several mechanisms for generation of intracellular cADPR have been proposed, including the translocation of cADPR promoted by CD38 and internalization of CD38 molecules [42], but a clear molecular model remains to be established by further experimental evidence. In cells from vertebrates, the majority of data gathered about cADPR metabolism comes from the studies of cyclase and hydrolase activities of CD38. However, other enzymes though not as extensively characterized as CD38. Another lymphocyte surface antigen named BST-1 (CD157) also has ADP-ribosyl cyclase activity [43]. BST-1 appears to be the product of a gene duplication of CD38 [43].
Fig. (1).
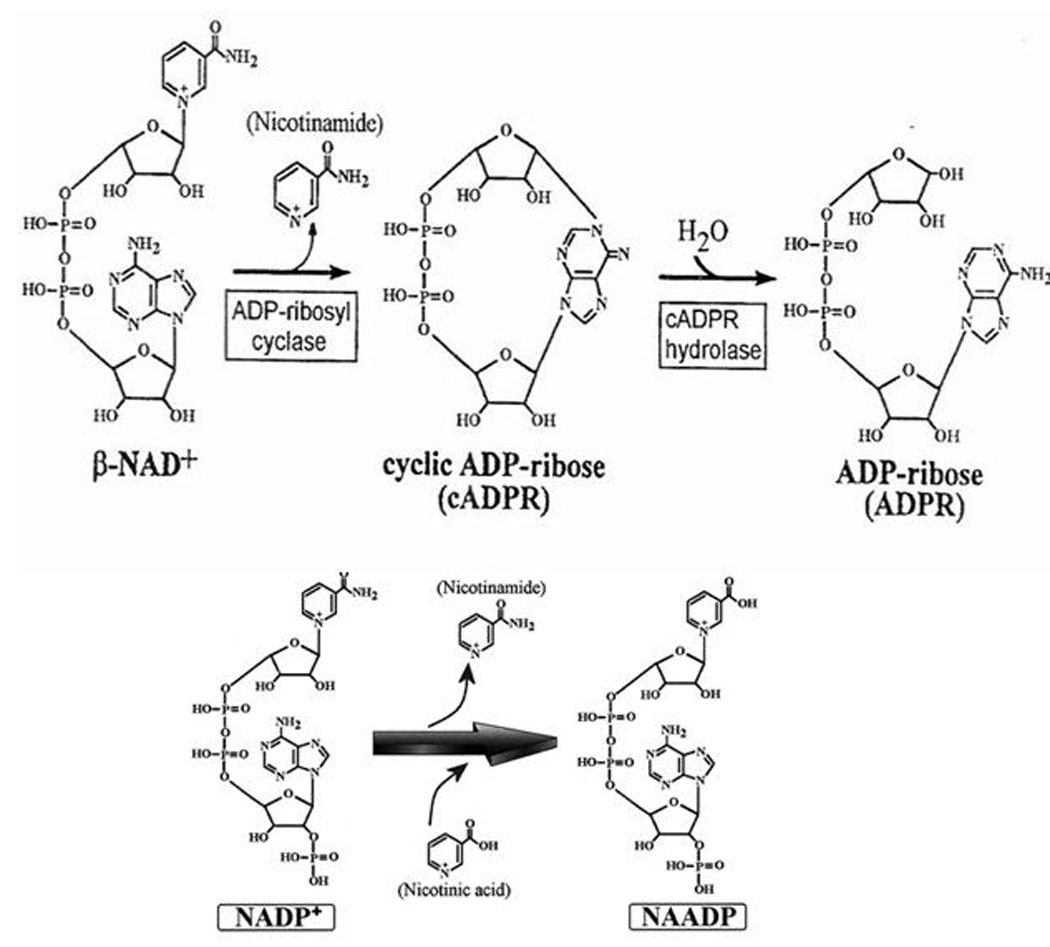
NADase activity: Synthesis and degradation of cyclic ADP-ribose is shown. (A). Synthesis of nicotinic acid adenine dinucleotide phosphate [NAADP+] from nicotinamide adenine dinucleotide phosphate [NADP+] by base-exchange reaction is shown.
Although it appears that CD38 clearly generates and degrades the second messenger cADPR and plays key roles in the regulation of intracellular calcium transients, CD38 may have other functions. For example, CD38 has been implicated as the enzyme responsible for the generation of other second messengers including NAADP via the base-exchange reaction [44–50]. However, the role of CD38 in NAADP generation is still controversial [50, 51]. In fact, we and others have recently described that CD38 is not necessary for the intracellular generation of NAADP in some mammalian cells [50–52]. These indicate that yet another cellular enzyme maybe responsible for some of the functions previously attributed to CD38. It is quite interesting that CD38 appears to be a very inefficient second messenger enzyme, as it will hydrolyze almost a hundred molecules of NAD to generate one molecule of the second messenger cADPR [53, 54]. In this regard, we have recently focused on the possible role of CD38 not only as a second messenger enzyme, but also as a NADase that can control cellular levels of NAD and its physiological functions [17–19].
3. CD38 IS A CELLULAR NADase: ROLE ON THE DEGRADATION OF NAD AND CONTROL OF NAD LEVELS
CD38 is a multifunctional enzyme, ubiquitously distributed in mammalian tissues [26–36]. As discussed above its major enzymatic activity is the hydrolysis of NAD [21, 24, 26, 35, 53, 54]. In fact, as discussed above, CD38 will generate one molecule of cADPR for almost every 100 molecules of NAD hydrolyzed [21]. Until recently, the role of CD38 as a modulator of NAD levels had not been explored. Recently, we postulated that CD38 is the major NADase in mammalian cells and that it regulates intracellular NAD levels (Fig. 2). In fact, we examined the NADase activities and NAD levels in a variety of tissues from both wild-type and CD38 deficient mice [17]. In accordance with our hypothesis, we found that tissue levels of NAD in CD38 deficient mice were 10 to 20 fold higher than in wild-type animals [17], a result confirmed by others [55]. In addition, NADase activity was essentially absent in most of the tissues, from CD38 deficient mice [17–19]. These data support the novel concept that CD38 is a major regulator of cellular NAD levels. Since CD38 is distributed in nearly every mammalian tissue and cellular compartment, I have postulated that CD38 regulates cellular NAD levels. In particular, CD38 may have a role not only in the regulation of intracellular but also extracellular NAD, and may modulate the availability on extracellular applied NAD in some cellular systems. In addition, the presence of CD38 in different intracellular compartments may have a crucial role on the regulation of NAD functions in specific organelles. The role of nuclear CD38 has been recently explored. CD38 is located at the nuclear membrane and regulates the generation of the second messenger cADPR and nuclear stores calcium release [56]. In addition, we have also found that nuclear CD38 regulates the activity of the nuclear enzymes sirtuins [18]. In particular, we observed that CD38 degrades NAD and decreases the accessibility of NAD to the NAD-dependent acetylase SIRT1 [18, 19]. It is also possible that generation of nicotinamide by CD38 may regulate SIRT1 activity (Fig. 2) [18]; this is possible by the fact that nicotinamide is an endogenous inhibitor of the SIRT1 enzyme. The potential role of CD38 as a regulator of NAD levels and SIRT1 activity opens the possibility that CD38 may be a regulator of many of the SIRT1 functions including energy homeostasis, obesity, aging, and longevity. Next, I will briefly describe some key aspects of the SIRT1 pathway.
Fig. (2).
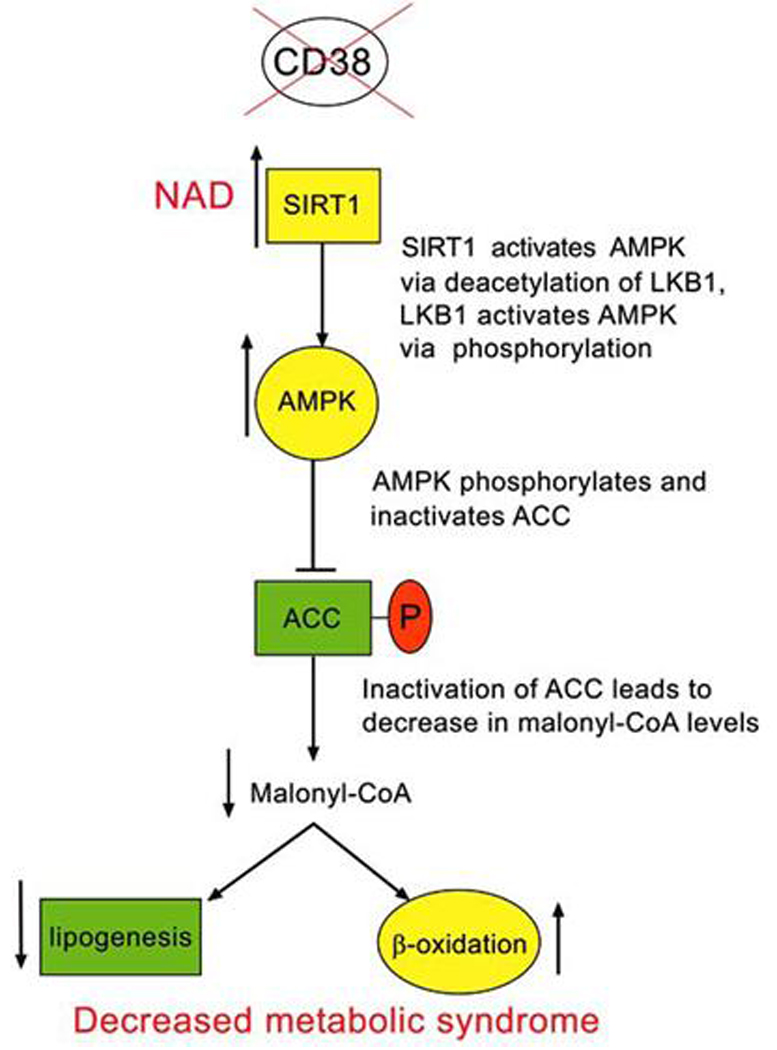
Possible mechanism of regulation of SIRT1 and AMPK pathway by CD38 inhibition.
4. SIRT1 AND THE RENEWED INTEREST IN NAD
4.1. SIRT1, a New Key Regulator Energy Metabolism, Aging and Longevity
One of the main recent advances on the understanding of energy metabolism, and the subsequent development of metabolic syndrome, has been the discovery and characterization of the metabolic roles of the NAD dependent deacetylases sirtuins. In particular, activation of the sirtuin enzyme SIRT1 has been shown to regulate glucose and fat metabolism and protect animals from high fat diet (HFD)-induced metabolic syndrome, liver steatosis, and obesity. The protective effects of SIRT1 activation maybe mediated by both a systemic melioration of the metabolic syndrome and specific effect on tissue glucose and fat metabolism [57–60].
4.2. SIRT1 is a Mediator of Caloric Restriction
Several studies have clearly demonstrated that moderate caloric restriction (CR) slows aging, extending life span up to 30–50% [61], furthermore CR can protect animals from the development of metabolic syndrome [61]. Recently it has been shown that the effects of CR are mediated, at least in part, by SIRT1 [62]. SIRT1 uses NAD as a substrate to promote deacetylation of several target proteins. Increased activity of sirtuins leads to life extension in yeast, fruit flies, and C. elegans [62–64]. Sirtuins also have an important role in the regulation of body weight, and recently it has been clearly shown that activation of SIRT1 can lead to protection against the development of obesity and liver steatosis [10–12].
4.3. SIRT1, a Regulator of Systemic and Hepatic Glucose and Fat Metabolism
SIRT1 regulates energy metabolism, glucose and fat homeostasis both at the systemic and cellular level. In addition to its systemic effects, SIRT1 has liver specific affects on both glucose and fat metabolism [57–60]. SIRT1 induced mitochondrial biogenesis, and gene expression in several cell types [10, 11]. Furthermore, hepatic SIRT1 activation leads to a protection against high fat diet (HFD)-induced steatosis, decreases SREBP1 expression, and the development of inflammation [10, 11, 59]. In addition, SIRT1 regulates the expression of the antioxidant genes SOD2 (manganese superoxide dismutase), NRF1, and UCP3. In cultured hepatocytes, SIRT1 inhibits glucose induced cellular fat accumulation via an AMPK dependent mechanism [65, 66]. It appears that all these effects may play a role on the protective effect of SIRT1 activation upon the development of metabolic syndrome, liver steatosis and obesity. Thus, activation of SIRT1 with small molecules, such as resveratrol, may represent a promising strategy for the treatment of metabolic syndrome, and obesity [10, 11, 19].
4.4. NAD, Sirtuins (SIRT1) and Obesity
NAD and nicotinamide play key roles in many cellular functions [1–11, 19]. In addition to its well known importance in energy metabolism, NAD and nicotinamide also play a role in signal transduction. New evidence suggests that NAD and nicotinamide are regulators of the NAD dependent deacetylases (sirtuins also known as SIRT enzymes), which modulates, obesity, energy metabolism, aging, and longevity [10–12, 57–66]. In fact, the drug resveratrol, an activator of the sirtuin enzymes, has been recently shown to protect animals against high fat (caloric) diet (HFD)-induced obesity, via an increase in energy expenditure [10–11, 19]. In addition, activation of SIRT1 enzymes in mice feed HFD ameliorate pathological effects of obesity (including glucose tolerance), and increase longevity [10]. These data indicates that SIRT1 enzyme not only prevent obesity, but also promote salutary health benefits in HFD feed animals [10, 11, 19]. The effects of SIRT1 on obesity and energy metabolism are, at least in parts, mediated by deacetylation and activation of peroxisome proliferato ractivated receptor α coactivator, PGC1α [10, 11, 19] (Fig. 3). Very strong evidence supports the notion that PGC1α is a key regulator of energy metabolism and mitochondrial biogenesis [67]. However, to date, the intracellular mechanisms that regulate SIRT1 mediated activation of PGC1α, inmammalian cells, have not been elucidate.
Fig. (3).
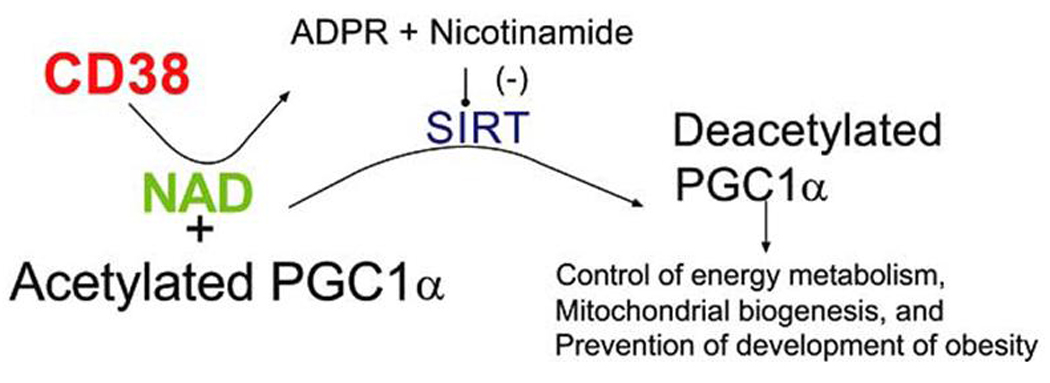
Possible mechanism of regulation of SIRT1, PGC1 pathway, and obesity by CD38 inhibition.
4.5. SIRT1 has Multiple Cellular Targets
In addition to the deacetylation of PGC-1 [1–12], SIRT1deacetylates other diverse substrates including p53, forkheadtranscription factor (FOXO), NF-κβ, Ku70, MyoD, LXR ,and histones. Thus, it influences gene silencing, apoptosis, stress resistance, cellular senescence, fat and glucose metabolism. The combination of these cellular functions might contribute to the physiological effects of SIRT1. Despite the extensive studies of SIRT1 function, the regulation of SIRT1 is poorly understood.
5. CD38 AS A REGULATOR OF NAD AND SIRT1
5.1. CD38 and NAD Metabolism
As discussed above, CD38 is an enzyme that has been implicated on the generation of the second messenger cyclic- ADP-ribose (cADPR) [12–26]. However, its main enzymatic activity is the hydrolysis of NAD to nicotinamide and ADPR. We postulated that CD38 is the major NADase in mammalian cells and that it regulates intracellular NAD and nicotinamide levels. In fact, our recent publications indicate that in CD38 deficient mice, tissue levels of NAD are several folds higher than in wild type animals [17]. In addition, we observed that NADase activity is essentially absent in several tissues from CD38 deficient mice [17].
5.2. CD38, a Regulator of SIRT1 Activity?
We proposed that by modulating availability of NAD and nicotinamide to the SIRT1 enzyme, CD38 regulates SIRT1 activity. These findings have strong implications for the understanding of the basic mechanisms that modulate obesity, metabolic syndrome, energy homeostasis, longevity, and aging.
5.3. CD38 and Obesity
A correlation has been observed between chromosome 4 near marker D4S403, where the CD38 gene is located, and the development of metabolic syndrome that refers to the clustering of disease conditions such as obesity, insulin resistance, hyperinsulinemia, and dyslipidemia [68]. However, to date, except for our studies [17–19], no other studies have been published on the role of CD38 on diet-induced obesity. CD38 regulates SIRT1 via a Non-cADPR mediated mechanism. Although CD38 has been implicated as the enzyme responsible for the generation of the second messenger cADPR [5], CD38 also appears to have cADPR-independent functions. In the case of regulation of NAD, SIRT1 activity, and obesity, our data indicates that CD38 appears to do it via a cADPR independent way, but a SIRT1 dependent mechanism [19]. The regulation of NAD by CD38 and its implication for pharmacological approaches aim at increasing SIRT1 activity. We proposed that by augmenting NAD and decreasing nicotinamide levels, inhibition of CD38 will not only increase SIRT1 activity but also will increase the sensitivity of SIRT1 to its pharmacological agonists such as resveratrol. In fact, we have previously observed that the activation of recombinant SIRT1 by resveratrol is inhibited by the addition of active recombinant CD38 to the reaction media [17].
6. CD38 INHIBITORS
To date a few CD38 inhibitors have been reported including NAD analogs (arabiono-NAD), nicotinamide derivatives (nicotinamide and nicotinic acid), reducing agents (such as dithiothreitol), and other unrelated compounds (Fig. 4; and reference [27, 71]). The compound 2,2'-dihydroxyazobenzene (DAB) has been recently shown to protect against cardiac dysfunction-induced by angiotensin II [69]. At the present time, the search for specific and potent CD38 inhibitors remains elusive. However, a recent report on a new CD38 assays indicates that an intensive search for CD38 inhibitors is going at this time [70], and it maybe a question of time before potent and specific CD38 inhibitors are available atleast for research. In any case, some important aspects of the search for CD38 inhibitors deserve further discussion. First, it is important to say that it is possible that molecules that inhibit CD38 may also inhibit SIRT1 [71]. In fact, SIRT1 and CD38 have several similarities in their enzymatic and catalytical properties [71], and CD38 has been proposed as a model enzyme for the study of the mechanism of SIRT1 catalysis [71]. Both SIRT1 and CD38 degrades NAD to nicotinamide and an ADPR derivative. Furthermore, both enzymes are capable of base-exchange reaction (Fig. 1B). In this regard, inhibitors of CD38 may also have effects upon SIRT1 activity, a potential undesirable “side-effect”. Secondly, as discussed above CD38 have other functions that are mediated by the generation of calcium regulating second messengers such as smooth muscle contraction, cell death, and apoptosis, neural and hormonal signaling, egg fertilization and others [2–9, 21, 69, 72, 73]. In this regard, CD38 inhibitors may have beneficial effects upon conditions, where cellular calcium homeostasis is deregulated such as in hypertension, cardiac ischemia, asthma and dysfunctional labor [2–9, 21, 69, 72, 73]. On the other hand, CD38 has been implicated in the secretion and function of hormones such as oxytocin and ACTH [74, 75], and may modulate maternal and social behavior [75]. These roles indicate that inhibition of CD38 may have potential deleterious effects. Potential immunologic dysfunction may be one of the worst possible “side-effects” of CD38 inhibitors. It has been shown that CD38 plays a key role in the mechanism by which the organism fights bacterial infection [76], and knockout of CD38 leads to increase susceptibility to lethal bacterial infection [76]. Despite these limitations, the search for CD38 inhibitors and the determination of their potential therapeutic roles will generate key new data that will provide new insights on multiple physiological and pathological conditions, and CD38 inhibitors may hold the key to new therapeutic strategies to multiple metabolic and inflammatory conditions.
Fig. (4).
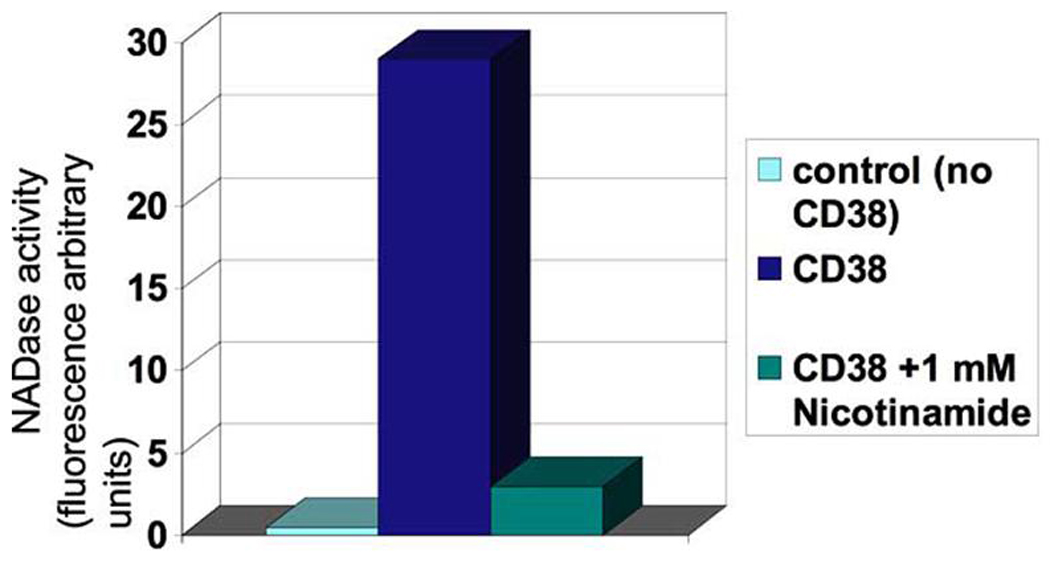
A typical CD38-NADase assay using etheno-NAD and recombinant CD38. Activity is inhibited by the reaction product nicotinamide. NADase acitivity is defined using etheno-NAD as a substrate and adding CD38 in the presence or absence of 1mM nicotinamide. Samples were incubated for 10 min.
7. CONCLUSION
Finally, it is important to discuss the fact that NADases are present in many other organisms such as bacteria and protozoans [77, 78]. Group A streptococci produce several exoproteins that are thought to contribute to the pathogenesis of human infection. One of these proteins is a NAD+-glycohydrolase (NADase). When group A streptococci are bound to the surface of epithelial cells in vitro, pores in the cell membrane are form and bacterial NADase is delivered to the epithelial cell cytoplasm. In vitro, intoxication of keratinocytes with NADase is associated with cytotoxic effects and induction of apoptosis [77]. In this regard, bacterial NADase plays a key role on the virulence of some bacteria [77]. Furthermore, we have recently described that the parasite Toxoplasma gondii has a NADase/ADP-ribosyl cyclase [78, 79]. Furthermore, in toxoplasma generation of cADPR, induced by abscisic acid, plays a key role in the mechanisms of cell invasion, differentiation and egress [78–80]. Inhibition of the NADase/ADP-ribosyl cyclase may be a novel target for pharmacological therapy against parasitic infection [78–80]. It is possible that CD38 inhibitors may cross many species barriers and may also be effective against microorganismal NADases, and maybe use for the treatment of microbiotic infection. In any case, search for specific bacterial and protozoa NADase inhibitors may also be of great importance. The recent development on the understanding of the catalytic properties of CD38 and the development of assays to study its NADase and base-exchange reaction maybe the initial step for the development of CD38 and species specific NADase inhibitors that may have multiple potential therapeutic roles in many human diseases. The future in this field is extremely exciting and provides promises of new and exciting pharmacological tools, let the hunt begin.
ACKNOWLEDGEMENTS
Work on Dr. Chini’s laboratory has been supported by the Mayo Clinic and Foundation, American Heart Association (AHA), American Federation for Aging Research (AFAR), the Federation for Anesthesia Research (FAER), and the NIH subward grant HL0713835-05A2. Dr. Chini is a consultant to Pfizer Pharmaceuticals, and has a pending patent application for the use of CD38 inhibitors.
REFERENCES
- 1.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 2.Clapper DL, Walseth TF, Dargie PJ, Lee HC, et al. Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. J Biol Chem. 1987;262:9561–9568. [PubMed] [Google Scholar]
- 3.Lee HC, Walseth TF, Bratt GT, Hayes RN, Clapper DL. Structural determination of a cyclic metabolite of NAD+ with intracellular Ca2+-mobilizing activity. J Biol Chem. 1989;264:1608–1615. [PubMed] [Google Scholar]
- 4.Galione A, Lee HC, Busa WB. Ca[2+]-induced Ca2+ release in sea urchin egg homogenates: modulation by cyclic ADP-ribose. Science. 1991;261:1143–1146. doi: 10.1126/science.1909457. [DOI] [PubMed] [Google Scholar]
- 5.Lee HC. Mechanisms of calcium signaling by cyclic ADP-ribose and NAADP. Physiol Rev. 1997;77:1133–1164. doi: 10.1152/physrev.1997.77.4.1133. [DOI] [PubMed] [Google Scholar]
- 6.Chini EN, Beers KW, Dousa TP. Nicotinate adenine dinucleotide phosphate [NAADP] triggers a specific calcium release system in sea urchin eggs. J Biol Chem. 1995;270:3216–3223. doi: 10.1074/jbc.270.7.3216. [DOI] [PubMed] [Google Scholar]
- 7.Chini EN, Dousa TP. Nicotinate-adenine dinucleotide phosphateinduced Ca[2+]-release does not behave as a Ca[2+]-induced Ca[2+]-release system. Biochem J. 1996;316:709–711. doi: 10.1042/bj3160709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dousa TP, Chini EN, Beers KW. Adenine nucleotide diphosphates: emerging second messengers acting via intracellular Ca2+ release. Am J Physiol. 1996;271:C1007–C1024. doi: 10.1152/ajpcell.1996.271.4.C1007. [DOI] [PubMed] [Google Scholar]
- 9.Galione A, Patel S, Churchill GC. NAADP-induced calcium release in sea urchin eggs. Biol Cell. 2000;92:197–204. doi: 10.1016/s0248-4900(00)01070-4. [DOI] [PubMed] [Google Scholar]
- 10.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a highcalorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC- 1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Ziegler M, Niere M. NAD+ surfaces again. Biochem J. 2004;382:5–6. doi: 10.1042/BJ20041217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bedalov A, Simon JA. Neuroscience. NAD to the rescue. Science. 2004;305:954–955. doi: 10.1126/science.1102497. [DOI] [PubMed] [Google Scholar]
- 14.Rongvaux A, Andris F, Van Gool F, Leo O. Reconstructing eukaryotic NAD metabolism. Bioassay. 2003;7:683–690. doi: 10.1002/bies.10297. [DOI] [PubMed] [Google Scholar]
- 15.Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Revollo JR, Körner A, Mills KF, Satoh A, Wang T, Garten A, et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aksoy P, White TA, Thompson M, Chini EN. Regulation of intracellular levels of NAD: a novel role for CD38. Biochem Biophys Res Commun. 2005;345:1386–1392. doi: 10.1016/j.bbrc.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 18.Aksoy P, Escande C, White TA, Thompson M, Soares S, Benech JC, et al. Regulation of SIRT 1 mediated NAD dependent deacetylation: a novel role for the multifunctional enzyme CD38. Biochem. Biophys Res Com. 2006;349:353–359. doi: 10.1016/j.bbrc.2006.08.066. [DOI] [PubMed] [Google Scholar]
- 19.Barbosa MT, Soares SM, Novak CM, Sinclair D, Levine JA, Aksoy P, et al. The enzyme CD38 [a NAD glycohydrolase, EC 3.2.2.5] is necessary for the development of diet-induced obesity. FASEB J. 2007;21:3629–3639. doi: 10.1096/fj.07-8290com. [DOI] [PubMed] [Google Scholar]
- 20.de Toledo FGS, Cheng J, Dousa TP. Retinoic acid and triiodothyronine stimulate ADP-ribosyl cyclase activity in rat vascular smooth muscle cells. Biochem Biophys Res Comm. 1997;238:847–850. doi: 10.1006/bbrc.1997.7392. [DOI] [PubMed] [Google Scholar]
- 21.de Toledo FG, Cheng J, Liang M, Chini EN, Dousa TP. ADPRibosyl cyclase in rat vascular smooth muscle cells: properties and regulation. Circ Res. 2000;86:1153–1159. doi: 10.1161/01.res.86.11.1153. [DOI] [PubMed] [Google Scholar]
- 22.Kuemmerle JF, Makhlouf GM. Agonist-stimulated cyclic ADP ribose. Endogenous modulator of Ca[2+]-induced Ca2+ release in intestinal longitudinal muscle. J Biol Chem. 1995;270:25488–25494. doi: 10.1074/jbc.270.43.25488. [DOI] [PubMed] [Google Scholar]
- 23.White TA, Johnson S, Walseth TF, Lee HC, Graeff RM, Munshi CB, et al. Subcellular localization of cyclic ADP-ribosyl cyclase and cyclic ADP-ribose hydrolase activities in porcine airway smooth muscle. Biochim Biophys Acta. 2000;1498:64–71. doi: 10.1016/s0167-4889(00)00077-x. [DOI] [PubMed] [Google Scholar]
- 24.Higashida H, Egorova A, Higashida C, Zhong ZG, Yokoyama S, Noda M, et al. Sympathetic potentiation of cyclic ADP-ribose formation in rat cardiac myocytes. J Biol Chem. 1999;274:33348–33354. doi: 10.1074/jbc.274.47.33348. [DOI] [PubMed] [Google Scholar]
- 25.Clementi E, Riccio M, Sciorati C, Nistico G, Meldolesi J. The type 2 ryanodine receptor of neurosecretory PC12 cells is activated by cyclic ADP-ribose. Role of the nitric oxide/cGMP pathway. J Biol Chem. 1996;271:17739–17745. doi: 10.1074/jbc.271.30.17739. [DOI] [PubMed] [Google Scholar]
- 26.Morita K, Kitayama S, Dohi T. Stimulation of cyclic ADP-ribose synthesis by acetylcholine and its role in catecholamine release in bovine adrenal chromaffin cells. J Biol Chem. 1997;272:21002–21009. doi: 10.1074/jbc.272.34.21002. [DOI] [PubMed] [Google Scholar]
- 27.Beers KW, Chini EN, Dousa TP. All-trans-retinoic acid stimulates synthesis of cyclic ADP-ribose in renal LLC-PK1 cells. J Clin Invest. 1995;95:2385–2390. doi: 10.1172/JCI117932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okamoto H. The CD38-cyclic ADP-ribose signaling system in insulin secretion. Mol Cell Biochem. 1999;193:115–118. [PubMed] [Google Scholar]
- 29.Takahashi K, Kukimoto Tokita K, Inageda K, Inoue S, Kontani K, et al. Accumulation of cyclic ADP-ribose measured by a specific radioimmunoassay in differentiated human leukemic HL-60 cells with all-trans-retinoic acid. FEBS Lett. 1995;371:204–208. doi: 10.1016/0014-5793(95)00914-u. [DOI] [PubMed] [Google Scholar]
- 30.Chini EN, Klener P, Beers KW, Chini CCS, Grande JP, Dousa TP, et al. Cyclic ADP-ribose metabolism in rat kidney: high capacity for synthesis in glomeruli. Kidney Int. 1997;51:1500–1506. doi: 10.1038/ki.1997.206. [DOI] [PubMed] [Google Scholar]
- 31.Guse AH, da Silva CP, Berg I, Skapenko AL, Weber K, Heyer P, et al. Regulation of calcium signalling in T lymphocytes by the second messenger cyclic ADP-ribose. Nature. 1999;398:70–73. doi: 10.1038/18024. [DOI] [PubMed] [Google Scholar]
- 32.Khoo KM, Han MK, Park JB, Chae SW, Kim UH, Lee HC, et al. Localization of the cyclic ADP-ribose-dependent calcium signaling pathway in hepatocyte nucleus. J Biol Chem. 2000;275:24807–24817. doi: 10.1074/jbc.M908231199. [DOI] [PubMed] [Google Scholar]
- 33.Chini EN, de Toledo FGS, Thompson MA, Dousa TP. Effect of estrogen upon cyclic ADP ribose metabolism: beta-estradiol stimulates ADP ribosyl cyclase in rat uterus. Proc Natl Acad Sci USA. 1997;94:5872–5876. doi: 10.1073/pnas.94.11.5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willmott N, Sethi JK, Walseth TF, Lee HC, White AM, Galione A. Nitric oxide-induced mobilization of intracellular calcium via the cyclic ADP-ribose signaling pathway. J Biol Chem. 1996;271:3699–3705. doi: 10.1074/jbc.271.7.3699. [DOI] [PubMed] [Google Scholar]
- 35.Wu Y, Kuzma J, Marechal E, Graeff R, Lee HC, Foster R, et al. Abscisic acid signaling through cyclic ADP-ribose in plants. Science. 1997;278:2126–2130. doi: 10.1126/science.278.5346.2126. [DOI] [PubMed] [Google Scholar]
- 36.Masuda W, Takenaka S, Inageda K, Nishina H, Takahashi K, Katada T, et al. Oscillation of ADP-ribosyl cyclase activity during the cell cycle and function of cyclic ADP-ribose in a unicellular organism, Euglena gracilis. FEBS Lett. 1997;405:104–106. doi: 10.1016/s0014-5793(97)00168-3. [DOI] [PubMed] [Google Scholar]
- 37.Reyes-Harde M, Empson R, Potter BV, Galione A, Stanton PK, et al. Evidence of a role for cyclic ADP-ribose in long-term synaptic depression in hippocampus. Proc Natl Acad Sci USA. 1999;96:4061–4066. doi: 10.1073/pnas.96.7.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hellmich MR, Strumwasser F. Purification and characterization of a molluscan egg-specific NADase, a second-messenger enzyme. Cell Regul. 1991;2:193–202. doi: 10.1091/mbc.2.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee HC, Aarhus R. ADP-ribosyl cyclase: an enzyme that cyclizes NAD+ into a calcium-mobilizing metabolite. Cell Regul. 1991;3:203–209. doi: 10.1091/mbc.2.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graeff RM, Walseth TF, Fryxell K, Branton WD, Lee HC. Enzymatic synthesis and characterizations of cyclic GDP-ribose. A procedure for distinguishing enzymes with ADP-ribosyl cyclase activity. J Biol Chem. 1994;269:30260–30267. [PubMed] [Google Scholar]
- 41.States DJ, Walseth TF, Lee HC. Similarities in amino acid sequences of Aplysia ADP-ribosyl cyclase and human lymphocyte antigen CD38. Trends Biochem Sci. 1992;17:495. doi: 10.1016/0968-0004(92)90337-9. [DOI] [PubMed] [Google Scholar]
- 42.Guse AH. Cyclic ADP-ribose. J Mol Med. 2000;78:26–35. doi: 10.1007/s001090000076. [DOI] [PubMed] [Google Scholar]
- 43.Hirata Y, Kimura N, Sato K, Ohsugi Y, Takasawa S, Okamoto H, et al. ADP ribosyl cyclase activity of a novel bone marrow stromal cell surface molecule, BST-1. FEBS Lett. 1994;356:244–248. doi: 10.1016/0014-5793(94)01279-2. [DOI] [PubMed] [Google Scholar]
- 44.Liang M, Chini EN, Cheng J, Dousa TP. Synthesis of NAADP and cADPR in mitochondria. Arch Biochem Biophys. 1999;37:317–325. doi: 10.1006/abbi.1999.1463. [DOI] [PubMed] [Google Scholar]
- 45.Chini EN, Thompson MA, Dousa TP. Enzymatic synthesis of NAADP by ADP-ribosyl cyclases. FASEB J. 1996;10:A143. [Google Scholar]
- 46.Lee HC. A unified mechanism of enzymatic synthesis of two calcium messengers: cyclic ADP-ribose and NAADP. Biol Chem. 1999;380:785–793. doi: 10.1515/BC.1999.098. [DOI] [PubMed] [Google Scholar]
- 47.Chini EN, Dousa TP. Enzymatic synthesis and degradation of nicotinate adenine dinucleotide phosphate [NAADP], a Ca[2+]- releasing agonist, in rat tissues. Biochem Biophys Res Commun. 1995;205:167–174. doi: 10.1006/bbrc.1995.1485. [DOI] [PubMed] [Google Scholar]
- 48.Chini EN, Chini CC, Kato I, Takasawa S, Okamoto H. CD38 is the major enzyme responsible for synthesis of nicotinic acid-adenine dinucleotide phosphate in mammalian tissues. Biochem J. 2002;362:125–130. doi: 10.1042/0264-6021:3620125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chini EN, de Toledo FGS. Nicotinic acid adenine dinucleotide phosphate: a new intracellular second messenger? Am J Physiol Cell Physiol. 2008;282:C1191–C1198. doi: 10.1152/ajpcell.00475.2001. [DOI] [PubMed] [Google Scholar]
- 50.Soares S, Thompson M, White T, Isbell A, Yamasaki M, Prakash Y, et al. NAADP as a second messenger: neither CD38 nor baseexchange reaction are necessary for in vivo generation of NAADP in myometrial cells. Am J Physiol Cell Physio. 2007;292:C277–C239. doi: 10.1152/ajpcell.00638.2005. [DOI] [PubMed] [Google Scholar]
- 51.Kim BJ, Park KH, Yim CY, Takasawa S, Okamoto H, Im MJ, et al. Generation of nicotinic acid adenine dinucleotide phosphate and cyclic ADP-ribose by glucagon-like peptide-1 evokes Ca2+ signal that is essential for insulin secretion in mouse pancreatic islets. Diabetes. 2008;57:868–878. doi: 10.2337/db07-0443. [DOI] [PubMed] [Google Scholar]
- 52.Palade P. The hunt for an alternate way to generate NAADP. Focus on "NAADP as a second messenger: neither CD38 nor baseexchange reaction are necessary for in vivo generation of NAADP in myometrial cells". Am J Physiol Cell Physiol. 2007;292:C4–C7. doi: 10.1152/ajpcell.00390.2006. [DOI] [PubMed] [Google Scholar]
- 53.Kim H, Jacobson EL, Jacobson MK. Synthesis and degradation of cyclic ADP-ribose by NAD glycohydrolases. Science. 1993;261:1330–1333. doi: 10.1126/science.8395705. [DOI] [PubMed] [Google Scholar]
- 54.Zielinska W, Barata H, Chini EN. Metabolism of cyclic ADPribose: Zinc is an endogenous modulator of the cyclase/NAD glycohydrolase ratio of a CD38-like enzyme from human seminal fluid. Life Sci. 2004;74:1781–1790. doi: 10.1016/j.lfs.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 55.Young GS, Choleris E, Lund FE, Kirkl JB. Decreased cADPR and increased NAD+ in the Cd38−/− mouse. Biochem Biophys Res Commun. 2006;346:188–192. doi: 10.1016/j.bbrc.2006.05.100. [DOI] [PubMed] [Google Scholar]
- 56.Adebanjo OA, Anandatheerthavarada HK, Koval AP, Moonga BS, Biswas G, Sun L, et al. A new function for CD38/ADP-ribosyl cyclase in nuclear Ca2+ homeostasis. Nat Cell Biol. 1999;1:409–414. doi: 10.1038/15640. [DOI] [PubMed] [Google Scholar]
- 57.Leibiger IB, Berggren PO. Sirt1: a metabolic master switch that modulates lifespan. Nat Med. 2006;12:34–36. doi: 10.1038/nm0106-34. [DOI] [PubMed] [Google Scholar]
- 58.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2008;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 59.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ajmo JM, Liang X, Rogers CQ, Pennock B, You M. Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295(4):G833–G842. doi: 10.1152/ajpgi.90358.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen D, Bruno J, Easlon E, Lin SJ, Cheng HL, Alt FW, et al. Tissue- specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008;22:1753–1757. doi: 10.1101/gad.1650608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guarente L, Picard F. Calorie restriction--the SIR2 connection. Cell. 2005;120:473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 63.Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Aging Dev. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 64.Kusama S, Ueda R, Suda T, Nishihara S, Matsuura ET. Involvement of drosophila Sir2-like genes in the regulation of life span. Genes Genet Syst. 2006;81(5):341–348. doi: 10.1266/ggs.81.341. [DOI] [PubMed] [Google Scholar]
- 65.Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283(41):27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liang H, Ward WF. PGC-1ﬡ: key regulator of energy metabolism. Adv Physiol Educ. 2006;30:145–151. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- 68.Cai G, Cole SA, Freeland-Graves JH, MacCluer JW, Blangero J, et al. Principal component for metabolic syndrome risk maps to chromosome 4p in Mexican Americans: the San Antonio Family Heart Study. Hum Biol. 2004;76:651–655. doi: 10.1353/hub.2005.0001. [DOI] [PubMed] [Google Scholar]
- 69.Gul R, Kim SY, Park KH, Kim BJ, Kim SJ, Im MJ, et al. A novel signaling pathway of ADP-ribosyl cyclase activation by angiotensin II in adult rat cardiomyocytes. Am J Physiol Heart Circ Physiol. 2008;295(1):H77–H88. doi: 10.1152/ajpheart.01355.2007. [DOI] [PubMed] [Google Scholar]
- 70.Preugschat F, Tomberlin GH, Porter DJ. The base exchange reaction of NAD(+) glycohydrolase: Identification of novel heterocyclic alternative substrates. Arch Biochem Biophys. 2008;479(2):114–120. doi: 10.1016/j.abb.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 71.Sauve AA, Schramm VL. SIR2: the biochemical mechanism of NAD(+)-dependent protein deacetylation and ADP-ribosyl enzyme intermediates. Curr Med Chem. 2004;11:807–826. doi: 10.2174/0929867043455675. [DOI] [PubMed] [Google Scholar]
- 72.Deshpande DA, White TA, Dogan S, Walseth TF, Panettieri RA, Kannan MS. CD38/cyclic ADP-ribose signaling: role in the regulation of calcium homeostasis in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2005;288:L773–L788. doi: 10.1152/ajplung.00217.2004. [DOI] [PubMed] [Google Scholar]
- 73.Barata H, Thompson M, Zielinska W, Han YS, Mantilla CB, Prakash YS, et al. The role of cyclic-ADP-ribose-signaling pathway in oxytocin-induced Ca2+ transients in human myometrium cells. Endocrinology. 2004;145:881–889. doi: 10.1210/en.2003-0774. [DOI] [PubMed] [Google Scholar]
- 74.Soares SM, Thompson M, Chini EN. Role of the second-messenger cyclic-adenosine 5'-diphosphate-ribose on adrenocorticotropin secretion from pituitary cells. Endocrinology. 2005;146:2186–2192. doi: 10.1210/en.2004-1298. [DOI] [PubMed] [Google Scholar]
- 75.Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446:41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- 76.Partida-Sánchez S, Cockayne DA, Monard S, Jacobson EL, Oppenheimer N, Garvy B, et al. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat Med. 2001;7:1209–1216. doi: 10.1038/nm1101-1209. [DOI] [PubMed] [Google Scholar]
- 77.Bricker AL, Carey VJ, Wessels MR. Role of NADase in virulence in experimental invasive group A streptococcal infection. Infect Immun. 2005;73:6562–6566. doi: 10.1128/IAI.73.10.6562-6566.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chini EN, Nagamune K, Wetzel DM, Sibley LD. Evidence that the cADPR signalling pathway controls calcium-mediated microneme secretion in Toxoplasma gondii. Biochem J. 2005;15:269–277. doi: 10.1042/BJ20041971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nagamune K, Moreno SN, Chini EN, Sibley LD. Calcium regulation and signaling in apicomplexan parasites. Subcell Biochem. 2008;47:70–81. doi: 10.1007/978-0-387-78267-6_5. [DOI] [PubMed] [Google Scholar]
- 80.Nagamune K, Hicks LM, Fux B, Brossier F, Chini EN, Sibley LD. Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii. Nature. 2008;451:207–210. doi: 10.1038/nature06478. [DOI] [PMC free article] [PubMed] [Google Scholar]


