Abstract
In this study, we investigated age- and tissue-dependent changes in the DNA base excision repair (BER) of oxidative lesions in mitochondrial and nuclear extracts by measuring single-nucleotide (SN)- and long-patch (LP)-BER activities in five tissues isolated from 4, 10 and 20 month old mice. Age-dependent SN-BER and LP-BER activity was increased in the mitochondria of liver, kidney and heart, but generally decreased in skeletal muscles. In contrast, no significant changes in repair activity were observed in nuclear extracts of the same tissues, except for quadriceps, where the SN-BER activity was higher in the old animals. Moreover, the BER activities in both the nucleus and mitochondria were significantly lower in skeletal muscles compared to liver or kidney of the same mice. The protein level of three antioxidant enzymes, Mn and Cu/Zn superoxide dismutases (SOD) and catalase, was also significantly lower in skeletal muscle compared to liver or kidney. In addition, we found higher levels of protein carbonylation in the mitochondria of skeletal muscle relative to other tissues. Thus, it appears likely that mouse skeletal muscle is highly susceptible to oxidative stress due to deficiency in both repair of oxidative DNA damage and antioxidant enzymes, contributing to age-dependent muscle loss.
Keywords: mitochondria, base excision repair, aging, oxidative stress, sarcopenia
1. Introduction
Progressive decline in physiological function associated with accumulation of somatic damage is considered the primary cause of biological aging. Among the various etiological agents causing somatic damage, reactive oxygen species (ROS) are generally believed to be the major contributor, and the mitochondria are the predominant source of endogenous ROS (Kowaltowski et al., 2009 and references therein). Age-associated decline in skeletal muscle mass and strength, a condition known as sarcopenia, is strongly associated with elevated oxidative stress. The rate of muscle loss in physically active human subjects has been estimated at 1–2% annually past the age of 50 (Hughes et al., 2002), and both loss of mtDNA and accumulation of oxidative damage in the mitochondrial genome of human skeletal muscle have been observed during aging (Short et al., 2005). In addition, the overall number of quiescent satellite stem cells, whose activation is mainly responsible for replacing damaged skeletal muscle cells, decreases with age (Charge et al., 2002). Heart failure, positively correlated with age, is also associated with loss of cardiomyocytes, a typical postmitotic cell type, possessing only limited regenerative capacity (Nadal-Ginard et al., 2003). Thus oxidative stress is proposed to play a role in the decrease of tissue regenerative capacity with age.
Cellular protection against oxidative damage includes elimination of ROS by antioxidant molecules and ROS-inactivating antioxidant enzymes, removal of damaged macromolecules and the repair of oxidatively damaged DNA. ROS induce a variety of DNA lesions leading to altered cellular signaling, mutagenesis, senescence and cell death. The nuclear and mitochondrial genomes of eukaryotic cells conserve the base excision repair (BER) pathway, the major process for repairing ROS-induced lesions, including oxidatively modified bases and single-strand breaks in DNA. Details of the BER pathway have recently been reviewed (Almeida and Sobol, 2007; Fortini and Dogliotti, 2007; Hegde et al., 2008). Briefly, repair of damaged or abnormal bases is initiated with their excision either by a monofunctional DNA glycosylase or by a bifunctional DNA glycosylase associated with intrinsic AP lyase activity. In the former case, e.g., uracil, abasic endonuclease 1 (APE1) cleaves DNA strand 5’ to the abasic (AP) site generated by monofunctional uracil-DNA glycosylase (UDG). For oxidized bases, bifunctional glycosylases such as 8-oxoguanine-DNA glycosylase (OGG1) cleave the DNA strand after base excision to generate 3’ phosphoribose or 3’ phosphate, which is removed by APE or PNK, respectively. In both cases, the 3’ OH terminus acts as a primer for DNA polymerase. Subsequent gap-filling by DNA polymerase and nick-sealing by DNA ligase can proceed via two subpathways: single-nucleotide (SN)-BER, in which only the damaged base is replaced, or long-patch (LP)-BER, where 2–6 additional nucleotides at the 5’ terminus of the damage are removed. In contrast to the nucleus — where distinct DNA polymerases, DNA ligases and other proteins are assigned to LP- or SN-BER — in the mitochondria DNA polymerase γ and DNA ligase 3 are involved in both replication and repair of mtDNA (Pinz and Bogenhagen, 1998). Unlike the nucleus, in which LP-BER activity has been documented (Frosina et al., 1996), only SN-BER activity has been reported in mitochondrial extracts (Stierum et al., 1999). Recently, we and others independently discovered LP-BER activity in mammalian mitochondria, significantly increasing the number of mutagenic lesions which could be efficiently repaired (Szczesny et al., 2008; Akbari et al., 2008; Liu et al., 2008). Moreover, BER is the only well characterized repair pathway in the mitochondria for repair of oxidized and alkylated bases, uracil and AP sites, and also single strand breaks in DNA.
Although several studies have investigated age-dependent changes in activity of specific BER enzymes, few have measured the impact of aging on overall BER capacity. In most cases, irrespective of tissue, species or strain, BER activity was generally lower in aged animals. Decreased uracil- and 8-oxoG-initiated BER in nuclear extracts of old mouse brain, liver and testes have been reported (Cabelof et al., 2002). Such changes have been posited to be partially due to lower activity of nuclear DNA polymerase β (Intano et al., 2003). Significant age-dependent decreases in the activity of the mitochondrial glycosylases OGG1, UDG and NTH were observed in various brain regions; however, age- or region-specific changes in uracilinitiated total BER activity were not found (Imam et al., 2006). Furthermore, the activities of three nuclear and mitochondrial DNA glycosylases in young mice showed tissue specificity, and activity was generally lower in the mitochondria relative to the nucleus (Karahalil et al., 2002). We have proposed that, during mouse aging and in senescent human fibroblasts, the intracellular distribution of key BER proteins is altered, compromising BER capacity (Szczesny et al., 2004; Szczesny and Mitra, 2005; Szczesny et al., 2003). Moreover, differential regulation of two DNA glycosylases activity, OGG1 and UDG, in both nucleus and mitochondria, of red and white skeletal muscles in old rats was shown (Radak et al., 2007). Increased activity of OGGI in nuclei and mitochondria of red fibers after training was shown in rats (Radak et al., 2007) and human skeletal muscles (Radak et al., 2003). However, comprehensive analysis of mitochondrial SN-and LP-BER during aging has not been conducted.
In the present report, we measured age- and tissue-specific changes in activity in both BER subpathways in the mouse. The mitochondrial and nuclear BER activity were determined by analyzing the total repair synthesis using duplex oligonucleotides containing uracil or tetrahydrofuran (THF), specific lesions for SN- and LP-BER, respectively. We explored possible correlations between DNA repair, oxidative damage and the level of antioxidant enzymes in liver, kidney, heart and skeletal muscle of mice. We conclude that repair of oxidatively damaged DNA is significantly lower, together with antioxidant capacity, in skeletal muscle relative to the liver or kidney. Thus, it appears that skeletal muscles are particularly susceptible to oxidative insult. These results could provide the molecular mechanism for age-associated muscle fiber loss, known as sarcopenia.
2. Materials and Methods
2.1. Materials
Young (3–5 months), middle-aged (10–12 months), and old (20–22 months) BALB/c male mice were purchased from the National Institute on Aging (Bethesda, MD). The maximum lifespan of this strain is 26–27 months (Goodrick, 1975). All animal experiments were performed according to the NIH Guidelines for Care and Use of Laboratory Animals and approved by the UTMB Animal Care and Use Committee (# 09-02-006). Reverse-phase HPLC-purified oligonucleotides were purchased from the Midland Certified Reagent Company, and the sequence of each oligonucleotide is presented in Table 1.
Table 1.
Oligonucleotides used in this study.
| Oligol |
| 5'-GAT CTG ATT CCC CAT CTC CTC AGT TTC ACT U AGT GAA GGC ATG CAC CCT TCT-3’ |
| 3'-CTA GAC TAA GGG GTA GAG GAG TCA AAG TGA G TCA CTT CCG TAC GTG GGA AGA-5' |
| Oligo2 |
| 5'-GAT CTG ATT CCC CAT CTC CTC AGT TTC ACT THF AGT GAA GGC ATG CAC CCT TCT-3’ |
| 3'-CTA GAC TAA GGG GTA GAG GAG TCA AAG TGA C TCA CTT CCG TAC GTG GGA AGA-5' |
| Oligo3 |
| 5' GAT CTG ATT CCC CAT CTC CTC AGT TTC ACT THF CTG CAC CGC ATG 3' |
| 3' CTA GAC TAA GGG GTA GAG GAG TCAAAG TGA C GAC GTG GCG TAC 5 |
2.2. Preparation of the whole cell, mitochondrial and nuclear extracts
Total cell extracts were prepared from fresh tissues by homogenizing in 20 mM Tris-HCl pH 8.8, 100 mM NaCl, 1 mM EDTA, 0.5 % Nonidet P-40, 12 mM Na-deoxycholate, followed by clean-up centrifugation at 20,000×g for 10 min. Mitochondrial extracts were prepared from fresh mouse tissue by differential centrifugation using a Mitochondrial Isolation Kit for Tissue (PIERCE) according to manufacturer’s recommendations. To remove externally adhering contaminants, intact mitochondria (1 mg/ml) were treated with trypsin (10 µg/ml) for 20 min at room temperature, followed by the addition of an equivalent amount of bovine trypsin inhibitor (Invitrogen) to stop proteolytic activity of trypsin (Gordon et al., 2001; Schulke et al., 1999). Pelleted at 8,000×g, mitochondria were washed twice in buffer containing 20 mM HEPES-KOH, pH 7.4, 250 mM sucrose and 1 mM dithiothreitol and finally lyzed in 20 mM HEPES-KOH pH 7.4, 1 mM EDTA, 1 mM DTT, 300 mM KCl, 5 % glycerol and 0.5 % Triton X-100. This procedure allows us to obtain pure mitochondrial extracts as we have previously shown (Szczesny et al., 2003; Szczesny and Mitra, 2005; Szczesny et al., 2008). Nuclear fractions isolated from fresh mouse tissues were obtained by homogenization in 10 mM HEPES pH 7.6, 10 mM KCl, 1.5 mM MgCl2, 2 mM DTT, 0.1 mM EDTA, 0.1 EGTA and 0.1 % NP-40 containing protease inhibitor cocktail (ROCHE), followed by centrifugation at 20,000×g for 1 min and two additional washes with the same buffer. Nuclear proteins were precipitated using a high salt buffer containing 20 mM HEPES pH 7.6, 0.42 M NaCl, 1 mM EDTA, 0.1 mM EGTA, 2 mM DTT, 20 % glycerol and a protease inhibitor cocktail (ROCHE) for 1 h at 4°C with continuous shaking. After centrifugation at 20,000×g for 30 min, the supernatant containing nuclear proteins was used for further studies. Protein concentration was determined using a Bio-Rad kit with bovine serum albumin as the standard.
2.3. DNA repair assay
DNA repair assay was carried out as described earlier with slight modifications (de Souza-Pinto et al., 2004). Briefly, the assay mixture (20 µl) contained 40 mM HEPES pH 7.6, 0.1 mM EDTA, 5 mM MgCl2, 0.2 mg/ml BSA, 50 mM KCl, 1 mM DTT, 2 mM ATP, 3 % glycerol, radiolabeled [α-32P]dCTP for SN-BER or 20 µM of each unlabeled dNTPs and 4 µCi of the [α-32P]-dATP for LP-BER; mitochondrial (15 µg) or nuclear (5 µg) protein extract and duplex DNA substrate (oligo1 or 2 for SN- and LP-BER, respectively; see Table 1). After incubation at 37°C for various times, the reaction was inhibited by addition of 90 % formamide (10 µl), and the substrates and products were separated by electrophoresis in 20 % acrylamide/7M urea gel. The radioactivity in the bands was quantitated by PhosphorImager (Molecular Dynamics) using ImageQuant software. Preliminary enzyme assays were carried out to ensure linearity of the reaction with respect to both time and amount of extract.
2.4. AP-endonuclease activity assay
The APE activity was determined as described earlier (Szczesny and Mitra, 2005) with slight modification. Oligo3 (Table 1) was labeled at the 5’-end with [γ-32P]-ATP and T4 polynucleotide kinase (New England BioLabs) as previously described (Izumi and Mitra, 1998). We measured strand cleavage 5’ to a tetrahydrofuran (THF, a stable AP analog) residue in a 43-mer oligodeoxynucleotide duplex in which the 5’-32P-labeled strand contained THF annealed with an equimolar amount of the complementary oligo with C opposite the THF residue. The enzyme reaction (20 µl) was carried out in 50 mM Tris-HCl pH 8.6, 50 mM KCl, 2mM MgCl2, 0.2 pmol 32P-labeled oligo3, 50 nM unlabeled oligo3 substrate and 2 µg of mitochondrial extract. After incubation at 37°C for 10 min, 10 µl of loading solution (90 % formamide, 0.05 % bromophenol blue and 0.05 % xylene cyanol) was added to the assay mixture, which was then electrophoresed in 20 % polyacrylamide gel containing 7 M urea to separate denaturated substrate and product (32P-labeled 43 and 30-mer, respectively). Radioactivity was then quantitated by PhosphorImager (Molecular Dynamics) using ImageQuant software. Preliminary enzyme assays were carried out to ensure linearity of the reaction with respect to both time and amount of extract.
2.5. Western blot analysis
Western blot analysis was performed as described previously (Ramana et al., 1998). After electroblotting, the membranes were sequentially probed with antibodies against MnSOD, Cu/ZnSOD (Upstate), Catalase (Abcam), GAPDH (Cell Signaling), the 70kDa subunit of complex II and the β subunit of complex V (Molecular Probes). The primary antibodies were quantitated by chemiluminescence using a horseradish peroxidase (HRP)-conjugated secondary antibody (anti-mouse or anti-rabbit IgG-HRP; Amersham). The membranes were washed and incubated in ECL reagent (Amersham) and visualized and analyzed using Gel Logic 2200 (Kodak) and Kodak Molecular Imaging Software (Kodak) for quantification, respectively.
2.6. Measurement of protein carbonylation
The amount of protein carbonyl groups was determined using the slot blot technique and an OxyBlot Protein Oxidation Detection Kit (Millipore), according to manufacturer’s recommendation. To ensure equal loading, whole-cell or mitochondrial extract was separated by SDS-PAGE and analyzed by Western blot using an antibody against GAPDH (Cell Signaling) for whole-cell extract or a 70kDa subunit of complex II (encoded in nuclear genome) and β subunit of complex V (encoded in mitochondrial genome) (Molecular Probes) for mitochondrial extract. The obtained signals were used as an internal control for equal protein loading. Preliminary experiments ensuring linearity in amount of analyzed proteins extracts were performed.
2.7. Activity of Citrate Synthase
The specific activity of citrate synthase was analyzed using a Citrate Synthase Assay Kit (SIGMA) according to manufacturer’s recommendations. Briefly, citrate synthase catalyzes the reaction between acetyl coenzyme A and oxaloacetic acid to form citric acid, thus formed thiol group of acetyl coenzyme A react with DTNB to form TNB (5-thio-2-nitrobenzoic acid) which was measured spectrophotometrically at 412nm. The results obtained were used to calculate the total mitochondrial volume in tissues of all ages (Holloszy et al., 1970). Calculated mitochondrial volumes were used throughout manuscript as normalizing factor for activities of APE, SN and LP-BER in mitochondria as well as for protein level of MnSOD (defined as arbitrary units).
2.8. Statistical analysis
At least six animals from each age group were used for experiments carried out in duplicate. The results are presented as mean ± standard deviation. Obtained results from middle-aged and old animals were compared individually to young animals, and differences between ages were tested for statistical significance with student t-test (p<0.05, indicated by asterisks). For tissue-specific changes in young animals, the results from kidney, heart, pectorals and quadriceps were compared individually to liver, and the differences between ages were tested for statistical significance with student t-test (p<0.05, indicated by asterisks).
3. Results
3.1. Age-dependent changes in DNA base excision repair pathway
Total DNA BER capacity in mitochondrial and nuclear extracts of five tissues and three mouse age groups were analyzed to evaluate changes in repair activity during aging. Total repair synthesis, rather than activity of individual BER enzymes, reflected the age- and tissue-specific changes in coordinated DNA repair. We measured total repair activity using a duplex oligonucleotide containing uracil opposite guanine (Oligo1, Table 1) and incorporation of [α32P]-dCMP for SN-BER or duplex oligonucleotide containing tetrahydrofuran (THF) opposite cytosine (Oligo2, Table 1) and incorporation of [α32P]-dAMP for LP-BER, as reported earlier (Szczesny et al., 2008). As a control, non-damage containing oligonucleotide was used (data not shown) as reported before (Szczesny et al., 2008). For objective comparison, we normalized the repair activity to that in the corresponding tissue of young mice (set as 1) to show age associated changes in particular tissue.
Mitochondrial BER
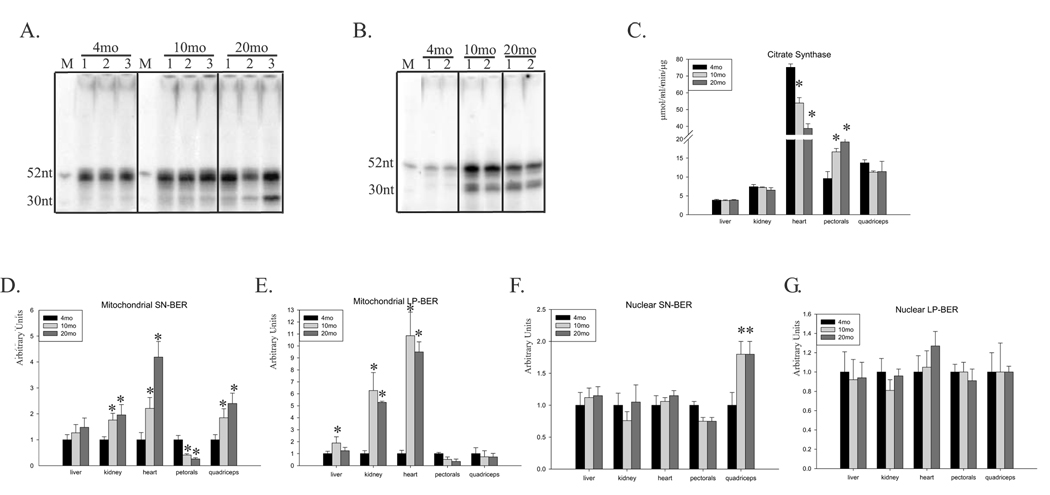
Fig.1A shows a representation of total repair activity with mitochondrial extracts from young, middle-aged and old mouse livers using uracil containing oligo indicating SN-BER. A representative gel of age-dependent changes in LP-BER activity of kidneys’ mitochondrial extracts with THF containing oligo is shown in Fig.1B. The increase of 52nt long radioactive signal indicates an increase in total repair activity with age. The presence of the faster migrating band (31nt) indicates the intermediate unligated product of repair synthesis. In order to normalize activities of mitochondrial BER, we measured specific activity of citrate synthase, a normalizing factor used to correct for variations in mitochondrial volume (Holloszy et al., 1970; Karahalil et al., 2002). Tissue- and age-dependent changes in the activity of citrate synthase are shown in Fig.1C. The highest mitochondrial volume was detected in the heart, which had 10 times the specific activity of the liver and kidney. Furthermore, the activity of citrate synthase in the heart decreased with age, in contrast to the liver, kidney and quadriceps, where no significant changes were detected (Fig.1C). The only tissue in which mitochondrial volume increased with age was the pectorals (1-fold increase). Therefore, in order to normalize the obtained radioactive signals for SN-and LP-BER we use the calculated mitochondrial volume as the normalizing factor and for easier comparison, set result of each young tissue as 1. An age-dependent increase in mitochondrial SN-BER activity was apparent, with 70–100 % increase in the liver and kidney and 4- and 2.5-fold increase in the heart and quadriceps, respectively (Fig.1D). In contrast, a 70 % decrease in activity was observed in the pectorals. Different results were obtained with the same extracts using a THF-containing oligonucleotide, a common substrate for measuring LP-BER activity (Oligo2, Table 1). We observed with age a slight increase in activity in the liver, and 6- to 10-fold increase in the kidney and heart (Fig.1E). However, mitochondrial LP-BER activity decreased in both the pectorals and quadriceps (Fig.1E). Interestingly, in liver, kidney and heart, the highest increase was observed in middle-aged animals, suggesting that repair capacity peaks in middle-age and then declines. Generally, in most tissues, an age-dependent increase in both mitochondrial BER pathways was observed, except in skeletal muscle where BER decreased with age, with the exception of SN-BER in quadriceps.
Fig.1.
Age-dependent changes in BER activity in the mitochondria and nucleus of mouse tissue. (A) A representative radiogram of total SN-BER activity assayed with three mitochondrial extracts of 4-, 10-, and 20-month old mouse liver using uracil containing oligo. M, marker (52 and 30nt). (B) A representative radiogram of total LP-BER activity assayed with two mitochondrial extracts of 4-, 10-, and 20-month old mouse kidney using THF containing oligo. M, marker (52 and 30nt). (C) Age-dependent changes in citrate synthase activity in extracts of mouse liver, kidney, heart, pectorals and quadriceps. (D) and (E) Age-dependent changes in SN-and LP-BER activity, respectively, in mitochondrial extracts of mouse liver, kidney, heart, pectorals and quadriceps. (F) and (G) Age-dependent changes in SN- and LP-BER, respectively, in nuclear extracts of the same mouse tissue. Extracts from 6–7 animals were used. The activity in each 4-month-old mouse tissue was set as 1 for easier comparison. Activity of mtBER pathways were normalized to activity of citrate synthase. Error bars represent standard errors, and asterisk denotes p<0.05 relative to young mice (4-month old).
Nuclear BER
In contrast to the mitochondria, nuclear SN- and LP-BER activity showed no significant age-dependent change in most tissues, except for pectorals and quadriceps, where we observed 30 % decrease and 100 % increase in SN-BER activity, respectively (Fig.1F). We also observed 20 % increase in nuclear LP-BER in the heart (Fig.1G). It needs to be mentioned that oligonucleotide containing THF based assay for nuclear extracts favor LP-BER activity with DNA polymerase beta since the replicative DNA polymerases will require PCNA, for which plasmid based assay is necessary. These results suggest that mitochondrial and nuclear BER networks are differentially regulated. Interestingly, in skeletal muscles, similar trends of changes during aging of mitochondrial and nuclear SN- and LP-BER pathways were observed.
APE activity in mitochondria
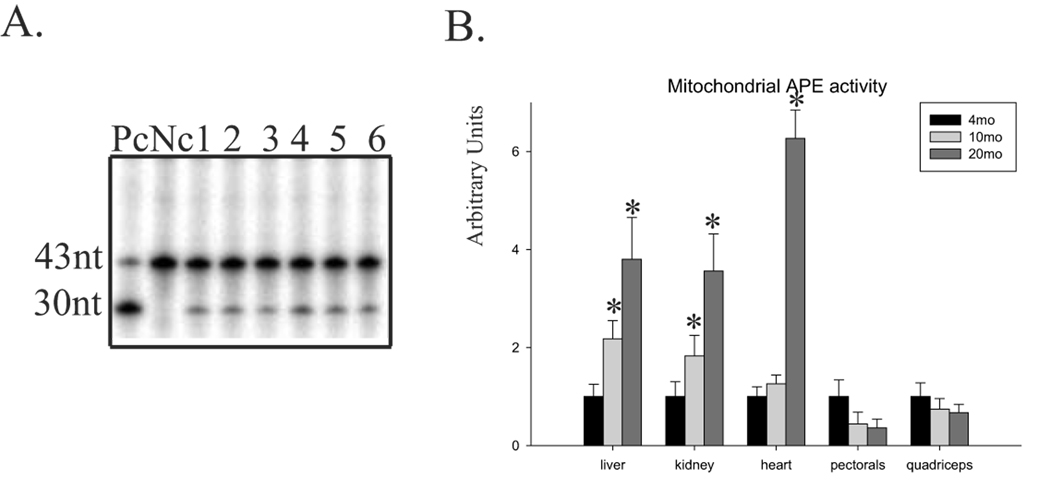
APE1, a key component of BER, is believed to be rate-limiting in the repair of oxidative DNA damage (Moore et al., 2000; Fan et al., 2003). We tested this possibility by analyzing age-dependent changes in specific APE activity in mitochondrial extracts using duplex [γ32P] 5’ labeled oligo3 (Table 1) as previously described (Szczesny and Mitra, 2005). As an example, the APE activity in six mitochondrial extracts of young pectorals is shown in Fig.2A. To show age-dependent changes in individual tissue, the APE activity was normalized to specific activity of citrate synthase, and the obtained results in young tissue were set as 1 for easier comparison. Mitochondrial-specific APE activity increased in the liver (4-fold), kidney (3.5-fold) and heart (6-fold) during aging, but not in skeletal muscles, where it decreased (Fig.2B). Moreover, we compared mitochondrial-specific APE activity with total BER activity in an age-dependent manner. Mitochondrial APE activity was positively correlated with SN-BER in most tissues except for quadriceps, where we found increased SN-BER but decreased APE activity (Fig.1D and Fig.2B). In addition, mitochondrial LP-BER positively correlated with the activity of mitochondrial APE in young versus middle-aged animals in all tissues. However, decreased mitochondrial LP-BER activity in old relative to middle-aged mice was observed in the liver, kidney and heart, which did not correlate with increasing APE activity. This finding suggests that even an increase in APE1 activity could not increase total LP-BER repair capacity in the mitochondria, and together with the different relative rates of age-dependent change in APE and total BER activity, these results indicate that APE1 is not the rate-limiting factor for mitochondrial BER pathways.
Fig.2.
Age-dependent changes in mitochondrial APE activity. (A) A representative mitochondrial APE activity assay performed by cleavage of 5’-end labeled oligo3 containing THF using six different mitochondrial extracts from the pectoral muscle of a 4-month old mouse. Pc, positive control, reaction with recombinant APE1. Nc, negative control, reaction without protein. (B) Age-dependent changes of mitochondrial APE activity in mouse liver, kidney, heart, pectorals and quadriceps. Extracts from 6–7 animals were used except for heart, where 9 animals were used. Activity in the 4–month old mouse was set at 1. Activities of mtAPE were normalized to activity of citrate synthase. Error bars represent standard errors, and asterisk denotes p<0.05 relative to young mice (4-month old).
3.2. Tissue-specific changes in mitochondrial and nuclear BER and levels of antioxidant enzymes in young mice
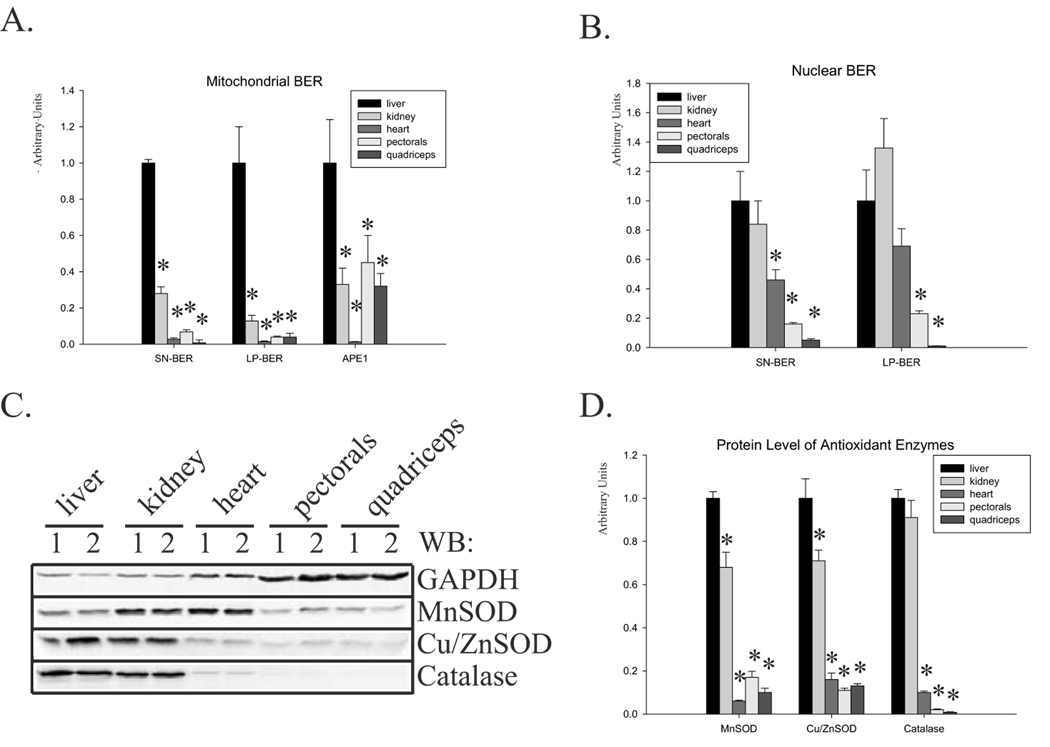
We also compared tissue-specific BER activity in young mice. Normalized to citrate synthase, mitochondrial BER and APE activity revealed the highest SN- and LP-BER and APE specific activity in the liver (Fig.3A). SN- and LP-BER specific activity in the mitochondria of the heart, pectorals and quadriceps was more than 10-fold lower than in the liver. Similarly, the activity of mitochondrial APE was highest in the liver, but differences with other tissues were smaller: 50 % less in the pectorals and 40 % less in the kidney and quadriceps (Fig.3A). Specific APE activity in young heart was 10-fold lower than in the liver, due to higher mitochondrial volume in this particular tissue as measured by activity of citrate synthase (Fig.1C). Those results further support the observation that mtAPE is not a rate limiting factor for mtBERs. Activity of the nuclear BER pathways in young mice showed a different pattern (Fig.3B). Nuclear SN-BER activity was very similar in the liver and kidney, and 50 % lower in the heart. The lowest activity was found in both skeletal muscles (Fig.3B). Similar results were obtained for nuclear LP-BER; however, the highest activity was measured in the kidney (40 % higher than in the liver, Fig.3B). We also compared protein levels of antioxidant enzymes by Western blot analysis; the highest level of all three enzymes was found in the liver and kidney (Fig.3C, D). The level of MnSOD was normalized to activity of citrate synthase and Cu/ZnSOD and catalase to the level of GAPDH, since they localized in mitochondria and cytoplasm, respectively. The lowest level of all three enzymes was again found in the heart and the skeletal muscles (Fig.3D). In summary, skeletal muscle contains the lowest level of nuclear and mitochondrial BER activity and the lowest level of antioxidant enzymes.
Fig.3.
Comparison of mitochondrial and nuclear BER and protein levels of antioxidant enzymes in young mouse tissues. (A) Normalized to citrate synthase, activities of SN-, LP-BER and APE in mitochondrial extracts isolated from young mice. (B) SN- and LP-BER activities in nuclear extracts of a 4-month old mice. (C) A representative Western blot with protein level of all three antioxidant enzymes. Total cell extracts of two young animals are presented. (D) The relative protein levels of the antioxidant enzymes MnSOD, Cu/ZnSOD and catalase were quantified in young mice tissue. Extracts from 6–7 animals were used. For easier comparison, repair activity and protein levels in the young mouse liver were set at 1. The level of MnSOD was further normalized to activity of citrate synthase. Error bars represent standard errors, and asterisk denotes p<0.05 when compared to young (4-month old) mice.
3.3. Age-dependent changes in proteins level of antioxidant enzymes
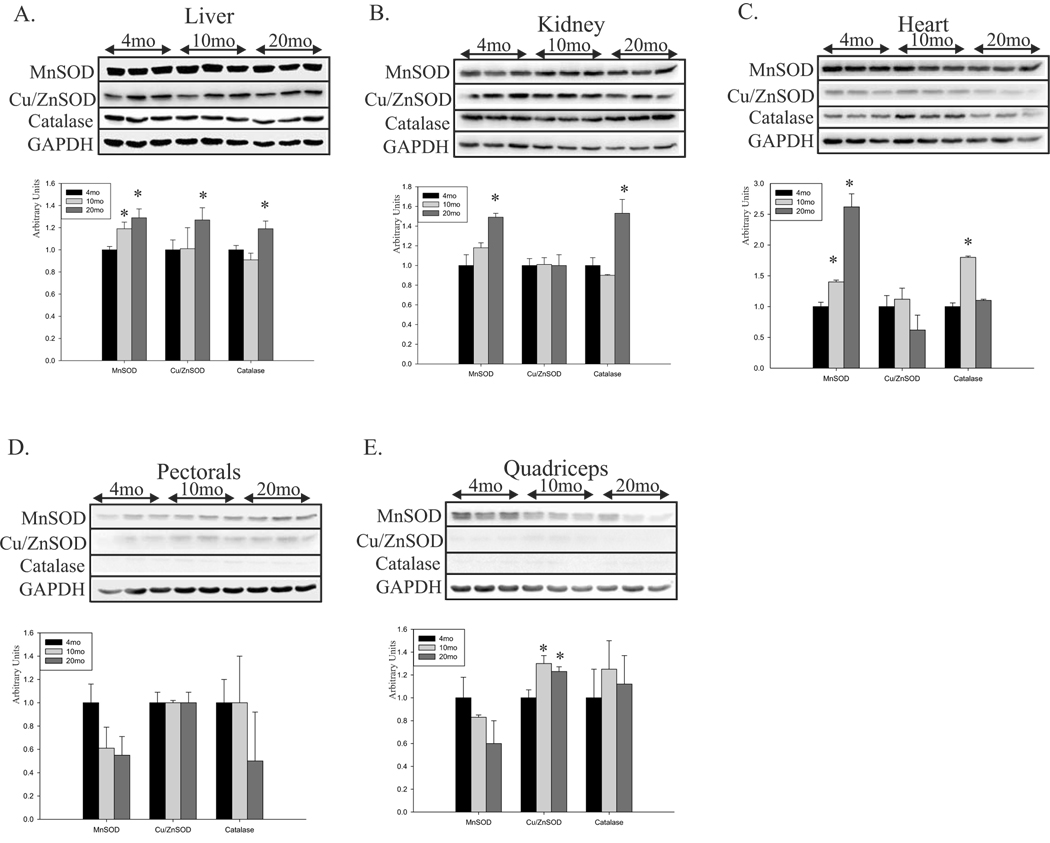
Cellular defense against oxidative damage is provided by antioxidant enzymes, which convert the highly reactive ROS to less reactive or non-reactive species. The few reports of age-dependent changes in the level and activity of antioxidant enzymes have produced inconsistent results (Cristiano et al., 1995; Rikans et al., 1991; Carrillo et al., 1992). Despite the large amount of data published on the expression, activity and level of antioxidant enzymes, we investigated the possibility that low BER activity, especially in skeletal muscle, could be partially compensated for by an age-associated increase in the level of antioxidant enzymes and compared protein levels of MnSOD, CuZn-SOD and catalase in whole cell extracts by age (Fig.4). As a control, we used GAPDH, one of the housekeeping proteins, whose expression does not change in tissue during aging (Raue et al., 2007; Chua et al., 2007; Touchberry et al., 2006). In addition, since MnSOD are mitochondrial proteins, we normalized the level of MnSOD by the specific activity of citrate synthase. In the liver, kidney and heart, protein levels of most of the analyzed antioxidant enzymes increased with age; however, no change or even decrease (MnSOD in quadriceps) was detected in both skeletal muscles (Fig.4). These results indicate that the mitochondria, especially in skeletal muscles, are more vulnerable to oxidative stress because of low BER activity as well as low levels of SODs and catalase.
Fig.4.
Age-dependent changes in the level of antioxidant enzymes in mouse tissue. Total cell extracts of 4-, 10-, and 20-month-old mouse liver (A), kidney (B), heart (C), pectorals (D), and quadriceps (E) were immunoblotted against the antioxidant enzymes MnSOD, Cu/ZnSOD, and catalase; GAPDH was used as the control (upper panels). Lower panels show the quantified level of antioxidant enzymes normalized to GAPDH, with each young tissue set at 1, except for MnSOD where mitochondrial volume was used. Extracts from 6–7 animals were used. The level of MnSOD was normalized to activity of citrate synthase. Error bars represent standard errors, and asterisks denote p<0.05 when compared to young (4-month old) animals.
3.4. Protein carbonylation and aging
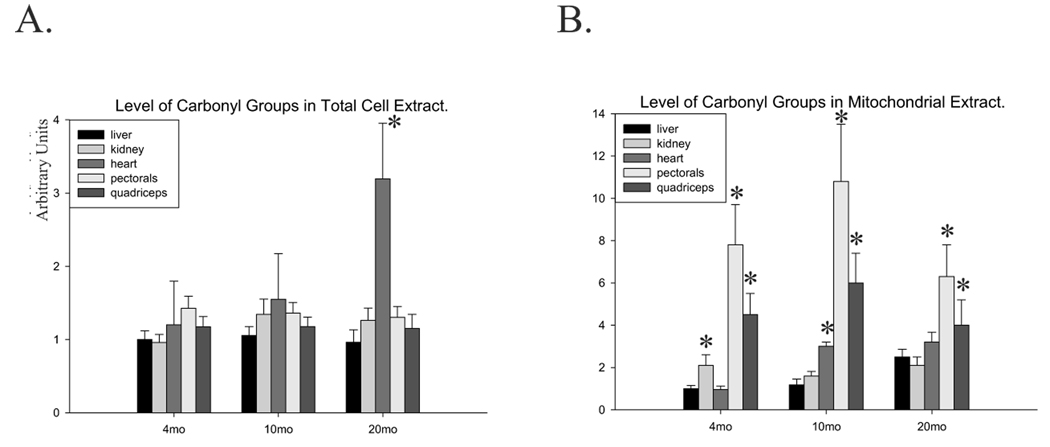
Carbonylation of proteins is a hallmark of oxidative stress; therefore, we measured the level of total protein carbonyls, normalized to that in the young liver, to evaluate the state of oxidative stress in various tissues (described in Materials and Methods). We detected no significant age-dependent changes in the level of carbonyl groups in the liver, kidney, pectorals or quadriceps (Fig.5A). However, 3-fold more carbonyl groups were observed in the heart of old animals. Because the mitochondria are not only the source of ROS, but also their immediate target, we analyzed oxidative damage to mitochondrial proteins. With aging, oxidative damage to mitochondrial proteins increased in all tissues (Fig.5B). The level of carbonyl groups in the mitochondrial extracts of skeletal muscles, particularly pectorals, was about 8–10-fold higher compared to mitochondrial extracts from the liver, kidney and heart (Fig.5B), further supporting our observation of low levels of antioxidant enzyme in mitochondrial extracts, particularly in skeletal muscles.
Fig.5.
Age-dependent changes of oxidatively damaged proteins in the total and mitochondrial extracts of mouse tissues. The levels of carbonyl groups in total (A) and mitochondrial extracts (B) from 4-, 10-, and 20-month old mouse liver, kidney, heart, pectorals, and quadriceps are shown. Extracts from 6–7 animals were used with the exception of heart where 9 animals were used. Results for the 4-month old mouse liver were set at 1 for easier comparison. Amount of carbonyl groups in mitochondrial extracts were normalized using 70kDa subunit of complex II and subunit β of complex V as described in Materials and Methods. Error bars represent standard errors, and asterisk denotes p<0.05 when compared to young (4-month old) animals.
4. Discussion
We here investigated age-dependent changes in the mitochondrial and nuclear BER activity in several mouse tissues. To the best of our knowledge, this is the first such comprehensive analysis of repair capacity including separate measurement of SN- and LP-BER activity for oxidative damage in the mitochondria and nucleus of mouse tissue and their age-dependent modulations. When comparing changes between different tissues, several considerations need to be taken into account. Comparing mitochondrial BER activity is relatively straightforward, and citrate synthase can be used as an internal control and to measure total mitochondrial volume (Holloszy et al., 1970). Levels of antioxidant enzymes were compared after normalization to that of a housekeeping protein, GAPDH, commonly used in studies of age-dependent changes (Raue et al., 2007; Chua et al., 2007; Touchberry et al., 2006).
Activities of both BER pathways increased with age in mitochondrial extracts of the liver, kidney and heart, in contrast to skeletal muscle, where reduction in both activities was observed. On the other hand, analysis of the nuclear extracts indicated no significant age-dependent changes in most tissues analyzed, with the exception of quadriceps, which showed significantly increased SN-BER activity. Although the activity of several proteins involved in BER during aging have been analyzed previously, few studies have measured age-dependent changes in total repair activity in mitochondrial or nuclear extracts. We could not detect any significant age-dependent changes in BER in nuclear extracts of mouse liver, in contrast to the observations of others (Intano et al., 2003; Cabelof et al., 2002). This discrepancy could be explained by a difference in methodology in assessing DNA repair activity. It is well known that repair of uracil may occur through two pathways: SN- and LP-BER (Akbari et al., 2004). Therefore, we performed separate assays for SN-BER in the presence of only one nucleotide instead of all four, to measure true SN-BER instead of total BER. We measured the activity of LP-BER using a reduced AP site, THF, due to the inability of DNA polymerase β or γ to catalyze the lyase reaction, which requires the presence of deoxyribose aldehyde (Hegde et al., 2008). However, while the SN-BER pathway can repair most base damage, the LP-BER may be important in repairing oxidized AP-sites. Thus, analyzing SN- and LP-BER separately should provide a better estimate of changes by age and tissue type. The AP site and its oxidation products are the most abundant producers of genotoxic damage, and approximately 50,000–200,000 AP sites can be generated in a single mammalian cell per day (Nakamura and Swenberg, 1999). In fact, more than 70 % of these could be oxidized AP sites, which are LP-BER substrates (Sung and Demple, 2006). Mitochondria are considered the major cellular source of ROS. Although the number of oxidized AP sites in the mitochondrial genome has not been quantitated, it is likely very high because of the amount of ROS. Therefore, mitochondrial LP-BER, which we (Szczesny et al., 2008) and others (Akbari et al., 2008; Liu et al., 2008) recently discovered, could be crucial for maintaining mitochondrial genome fidelity in the face of a large burden of oxidized AP sites.
Our studies showed that mitochondrial BER activity generally increased with age in all tissues, with the exception of skeletal muscle. We used two different skeletal muscles, pectorals and quadriceps, because of their aerobic vs anaerobic physiological characteristics. The pectoral is an adductor, internal rotator and flexor of the shoulder; its fibers, which are slow twitch (type I), have high myoglobin levels to improve oxygen delivery and high mitochondrial content; they are primarily red muscle and highly aerobic. On the other hand, the quadriceps contain predominantly fast-twitch type II fibers with fewer mitochondria and less myoglobin. The large stores of glycogen and high levels of glycolytic enzymes enable these fibers to respire anaerobically (Choksi et al., 2008). In fact, the specific activity of citrate synthase reflecting mitochondrial volume in old animals was significantly higher in pectorals vs quadriceps, which further supports this observation. A low BER capacity in terminally differentiated muscle cells has been previously reported by analyzing BER activity in nuclear extracts of mouse skeletal muscle cell line during the process of differentiation (Narciso et al., 2007). Our results detail the differences in nuclear and mitochondrial BER pathways in fully differentiated skeletal muscle and their direct comparison to other tissues together with age-dependent modulation.
Oxidative stress occurs in a variety of conditions, causing increased ROS production or a decline in antioxidant activity. Tissue-specific changes in the level of antioxidant enzymes during aging have been previously reported (Cristiano et al., 1995; Muradian et al., 2002; Sinitsyna et al., 2006). However, most of these studies measured age-dependent changes in particular tissues. Our results showed low levels of MnSOD, Cu/ZnSOD and catalase in skeletal muscle, compared to liver or kidney. In muscle cells after injury, satellite cells are stimulated to replace dead cells by division, but this reservoir may be depleted with aging or pathological conditions (Marzetti and Leeuwenburgh, 2006; Hawke and Garry, 2001). During aging, ROS production may dramatically increase because of altered function of the respiratory chain and insufficient cellular antioxidant defenses. Such oxidative insult, combined with a less efficient BER system which enhances oxidative stress, could play a key role in both the age-related decrease of muscle performance and mass (sarcopenia), and disorders associated with free radical overproduction. It has become clear, from studies at our laboratory and elsewhere, that DNA repair could be crucial in this regard, particularly that of the mitochondrial genome. In fact, susceptibility to apoptosis in skeletal muscle has been proposed (Chabi et al., 2008), and our results could explain the molecular mechanism of this process.
We speculate that a low level of mitochondrial DNA repair, together with a low level of antioxidant enzymes (which results in higher oxidative damage mainly to mitochondrial proteins) could explain the observed age-dependent decline in mitochondrial activity, particularly in the skeletal muscle. Several studies have shown that mitochondrial DNA in skeletal muscle accumulates oxidative damage in an age-dependent manner (Hayakawa et al., 1991; Katayama et al., 1991; Lee et al., 1993, Herbst et al., 2007). The persistence of this damage would be expected to cause a high mutation rate in the mitochondrial genome. Thus, it is not surprising that mitochondrial mutations such as deletions, duplications and point mutations have been shown to accumulate in an age-dependent manner, leading to a mosaic pattern of respiratory chain deficiencies in pre- and post-mitotic tissues. It was recently proposed that progressive accumulation of oxidative damage in the mitochondrial genome of skeletal muscles during aging could result in loss of muscle fibers (Hiona and Leeuwenburgh, 2008). In summary, low levels of BER activity and antioxidant enzymes, particularly in skeletal muscles, are likely to contribute to age-associated muscle loss and emphasize the importance of DNA repair, particularly in the mitochondrial genome.
Acknowledgements
This work is supported by UTMB Claude D. Pepper Older Americans Independence Center NIH Grant # P30 AG024832 (pilot grant to B.Sz.) and NIH P01 AG10514 and R01 CA53791 (to S. M.). We thank Sarah Toombs Smith for her suggestions in editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akbari M, Otterlei M, Pena-Diaz J, Aas PA, Kavli B, Liabakk NB, Hagen L, Imai K, Durandy A, Slupphaug G, Krokan HE. Repair of U/G and U/A in DNA by UNG2-associated repair complexes takes place predominantly by short-patch repair both in proliferating and growth-arrested cells. Nucleic Acids Res. 2004;32:5486–5498. doi: 10.1093/nar/gkh872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari M, Visnes T, Krokan HE, Otterlei M. Mitochondrial base excision repair of uracil and AP sites takes place by single-nucleotide insertion and long-patch DNA synthesis. DNA Repair (Amst) 2008;7:605–616. doi: 10.1016/j.dnarep.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Almeida KH, Sobol RW. A unified view of base excision repair: lesion-dependent protein complexes regulated by post-translational modification. DNA Repair (Amst) 2007;6:695–711. doi: 10.1016/j.dnarep.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabelof DC, Raffoul JJ, Yanamadala S, Ganir C, Guo Z, Heydari AR. Attenuation of DNA polymerase beta-dependent base excision repair and increased DMS-induced mutagenicity in aged mice. Mutat Res. 2002;500:135–145. doi: 10.1016/s0027-5107(02)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo MC, Kanai S, Sato Y, Kitani K. Age-related changes in antioxidant enzyme activities are region and organ, as well as sex, selective in the rat. Mech Ageing Dev. 1992;65:187–198. doi: 10.1016/0047-6374(92)90035-c. [DOI] [PubMed] [Google Scholar]
- Chabi B, Ljubicic V, Menzies KJ, Huang JH, Saleem A, Hood DA. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell. 2008;7:2–12. doi: 10.1111/j.1474-9726.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- Charge SB, Brack AS, Hughes SM. Aging-related satellite cell differentiation defect occurs prematurely after Ski-induced muscle hypertrophy. Am J Physiol Cell Physiol. 2002;283:C1228–C1241. doi: 10.1152/ajpcell.00206.2002. [DOI] [PubMed] [Google Scholar]
- Choksi KB, Nuss JE, Deford JH, Papaconstantinou J. Age-related alterations in oxidatively damaged proteins of mouse skeletal muscle mitochondrial electron transport chain complexes. Free Radic Biol Med. 2008;45:826–838. doi: 10.1016/j.freeradbiomed.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua WC, Liu L, Mansfield KJ, Vaux KJ, Moore KH, Millard RJ, Burcher E. Age-related changes of P2X(1) receptor mRNA in the bladder detrusor from men with and without bladder outlet obstruction. Exp Gerontol. 2007;42:686–692. doi: 10.1016/j.exger.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Cristiano F, de Haan JB, Iannello RC, Kola I. Changes in the levels of enzymes which modulate the antioxidant balance occur during aging and correlate with cellular damage. Mech Ageing Dev. 1995;80:93–105. doi: 10.1016/0047-6374(94)01561-y. [DOI] [PubMed] [Google Scholar]
- de Souza-Pinto NC, Harris CC, Bohr VA. p53 functions in the incorporation step in DNA base excision repair in mouse liver mitochondria. Oncogene. 2004;23:6559–6568. doi: 10.1038/sj.onc.1207874. [DOI] [PubMed] [Google Scholar]
- Fan Z, Beresford PJ, Zhang D, Xu Z, Novina CD, Yoshida A, Pommier Y, Lieberman J. Cleaving the oxidative repair protein Ape1 enhances cell death mediated by granzyme A. Nat Immunol. 2003;4:145–153. doi: 10.1038/ni885. [DOI] [PubMed] [Google Scholar]
- Fortini P, Dogliotti E. Base damage and single-strand break repair: mechanisms and functional significance of short- and long-patch repair subpathways. DNA Repair (Amst) 2007;6:398–409. doi: 10.1016/j.dnarep.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Frosina G, Fortini P, Rossi O, Carrozzino F, Raspaglio G, Cox LS, Lane DP, Abbondandolo A, Dogliotti E. Two pathways for base excision repair in mammalian cells. J Biol Chem. 1996;271:9573–9578. doi: 10.1074/jbc.271.16.9573. [DOI] [PubMed] [Google Scholar]
- Goodrick CL. Life-span and the inheritance of longevity of inbred mice. J Gerontol. 1975;30:257–263. doi: 10.1093/geronj/30.3.257. [DOI] [PubMed] [Google Scholar]
- Gordon DM, Wang J, Amutha B, Pain D. Self-association and precursor protein binding of Saccharomyces cerevisiae Tom40p, the core component of the protein translocation channel of the mitochondrial outer membrane. Biochem J. 2001;356:207–215. doi: 10.1042/0264-6021:3560207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- Hayakawa M, Torii K, Sugiyama S, Tanaka M, Ozawa T. Age-associated accumulation of 8-hydroxydeoxyguanosine in mitochondrial DNA of human diaphragm. Biochem Biophys Res Commun. 1991;179:1023–1029. doi: 10.1016/0006-291x(91)91921-x. [DOI] [PubMed] [Google Scholar]
- Hegde ML, Hazra TK, Mitra S. Early steps in the DNA base excision/single-strand interruption repair pathway in mammalian cells. Cell Res. 2008;18:27–47. doi: 10.1038/cr.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst A, Pak JW, McKenzie D, Bua E, Bassiouni M, Aiken JM. Accumulation of mitochondrial DNA deletion mutations in aged muscle fibers: evidence for a causal role in muscle fiber loss. J Gerontol A Biol Sci Med Sci. 2007;62:235–245. doi: 10.1093/gerona/62.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiona A, Leeuwenburgh C. The role of mitochondrial DNA mutations in aging and sarcopenia: implications for the mitochondrial vicious cycle theory of aging. Exp Gerontol. 2008;43:24–33. doi: 10.1016/j.exger.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy JO, Oscai LB, Don IJ, Mole PA. Mitochondrial citric acid cycle and related enzymes: adaptive response to exercise. Biochem Biophys Res Commun. 1970;40:1368–1373. doi: 10.1016/0006-291x(70)90017-3. [DOI] [PubMed] [Google Scholar]
- Hughes VA, Frontera WR, Roubenoff R, Evans WJ, Singh MA. Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr. 2002;76:473–481. doi: 10.1093/ajcn/76.2.473. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Karahalil B, Hogue BA, Souza-Pinto NC, Bohr VA. Mitochondrial and nuclear DNA-repair capacity of various brain regions in mouse is altered in an age-dependent manner. Neurobiol Aging. 2006;27:1129–1136. doi: 10.1016/j.neurobiolaging.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Intano GW, Cho EJ, McMahan CA, Walter CA. Age-related base excision repair activity in mouse brain and liver nuclear extracts. J Gerontol A Biol Sci Med Sci. 2003;58:205–211. doi: 10.1093/gerona/58.3.b205. [DOI] [PubMed] [Google Scholar]
- Izumi T, Mitra S. Deletion analysis of human AP-endonuclease: minimum sequence required for the endonuclease activity. Carcinogenesis. 1998;19:525–527. doi: 10.1093/carcin/19.3.525. [DOI] [PubMed] [Google Scholar]
- Karahalil B, Hogue BA, de Souza-Pinto NC, Bohr VA. Base excision repair capacity in mitochondria and nuclei: tissue-specific variations. Faseb J. 2002;16:1895–1902. doi: 10.1096/fj.02-0463com. [DOI] [PubMed] [Google Scholar]
- Katayama M, Tanaka M, Yamamoto H, Ohbayashi T, Nimura Y, Ozawa T. Deleted mitochondrial DNA in the skeletal muscle of aged individuals. Biochem Int. 1991;25:47–56. [PubMed] [Google Scholar]
- Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med. 2009;47:333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Lee CM, Chung SS, Kaczkowski JM, Weindruch R, Aiken JM. Multiple mitochondrial DNA deletions associated with age in skeletal muscle of rhesus monkeys. J Gerontol. 1993;48:B201–B205. doi: 10.1093/geronj/48.6.b201. [DOI] [PubMed] [Google Scholar]
- Liu P, Qian L, Sung JS, de Souza-Pinto NC, Zheng L, Bogenhagen DF, Bohr VA, Wilson DM, 3rd, Shen B, Demple B. Removal of Oxidative DNA Damage via FEN1-Dependent Long-Patch Base Excision Repair in Human Cell Mitochondria. Mol Cell Biol. 2008 doi: 10.1128/MCB.00457-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol. 2006;41:1234–1238. doi: 10.1016/j.exger.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Moore DH, Michael H, Tritt R, Parsons SH, Kelley MR. Alterations in the expression of the DNA repair/redox enzyme APE/ref-1 in epithelial ovarian cancers. Clin Cancer Res. 2000;6:602–609. [PubMed] [Google Scholar]
- Muradian KK, Utko NA, Fraifeld V, Mozzhukhina TG, Pishel IN, Litoshenko AY. Superoxide dismutase, catalase and glutathione peroxidase activities in the liver of young and old mice: linear regression and correlation. Arch Gerontol Geriatr. 2002;35:205–214. doi: 10.1016/s0167-4943(02)00025-0. [DOI] [PubMed] [Google Scholar]
- Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Myocyte death, growth, and regeneration in cardiac hypertrophy and failure. Circ Res. 2003;92:139–150. doi: 10.1161/01.res.0000053618.86362.df. [DOI] [PubMed] [Google Scholar]
- Nakamura J, Swenberg JA. Endogenous apurinic/apyrimidinic sites in genomic DNA of mammalian tissues. Cancer Res. 1999;59:2522–2526. [PubMed] [Google Scholar]
- Narciso L, Fortini P, Pajalunga D, Franchitto A, Liu P, Degan P, Frechet M, Demple B, Crescenzi M, Dogliotti E. Terminally differentiated muscle cells are defective in base excision DNA repair and hypersensitive to oxygen injury. Proc Natl Acad Sci U S A. 2007;104:17010–17015. doi: 10.1073/pnas.0701743104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinz KG, Bogenhagen DF. Efficient repair of abasic sites in DNA by mitochondrial enzymes. Mol Cell Biol. 1998;18:1257–1265. doi: 10.1128/mcb.18.3.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radak Z, Apor P, Pucsok J, Berkes I, Ogonovszky H, Pavlik G, Nakamoto H, Goto S. Marathon running alters the DNA base excision repair in human skeletal muscle. Life Sci. 2003;72:1627–1633. doi: 10.1016/s0024-3205(02)02476-1. [DOI] [PubMed] [Google Scholar]
- Radak Z, Kumagai S, Nakamoto H, Goto S. 8-Oxoguanosine and uracil repair of nuclear and mitochondrial DNA in red and white skeletal muscle of exercise-trained old rats. J Appl Physiol. 2007;102:1696–1701. doi: 10.1152/japplphysiol.01051.2006. [DOI] [PubMed] [Google Scholar]
- Ramana CV, Boldogh I, Izumi T, Mitra S. Activation of apurinic/apyrimidinic endonuclease in human cells by reactive oxygen species and its correlation with their adaptive response to genotoxicity of free radicals. Proc Natl Acad Sci U S A. 1998;95:5061–5066. doi: 10.1073/pnas.95.9.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Proteolytic gene expression differs at rest and after resistance exercise between young and old women. J Gerontol A Biol Sci Med Sci. 2007;62:1407–1412. doi: 10.1093/gerona/62.12.1407. [DOI] [PubMed] [Google Scholar]
- Rikans LE, Moore DR, Snowden CD. Sex-dependent differences in the effects of aging on antioxidant defense mechanisms of rat liver. Biochim Biophys Acta. 1991;1074:195–200. doi: 10.1016/0304-4165(91)90061-k. [DOI] [PubMed] [Google Scholar]
- Schulke N, Sepuri NB, Gordon DM, Saxena S, Dancis A, Pain D. A multisubunit complex of outer and inner mitochondrial membrane protein translocases stabilized in vivo by translocation intermediates. J Biol Chem. 1999;274:22847–22854. doi: 10.1074/jbc.274.32.22847. [DOI] [PubMed] [Google Scholar]
- Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinitsyna O, Krysanova Z, Ishchenko A, Dikalova AE, Stolyarov S, Kolosova N, Vasunina E, Nevinsky G. Age-associated changes in oxidative damage and the activity of antioxidant enzymes in rats with inherited overgeneration of free radicals. J Cell Mol Med. 2006;10:206–215. doi: 10.1111/j.1582-4934.2006.tb00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stierum RH, Dianov GL, Bohr VA. Single-nucleotide patch base excision repair of uracil in DNA by mitochondrial protein extracts. Nucleic Acids Res. 1999;27:3712–3719. doi: 10.1093/nar/27.18.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JS, Demple B. Roles of base excision repair subpathways in correcting oxidized abasic sites in DNA. Febs J. 2006;273:1620–1629. doi: 10.1111/j.1742-4658.2006.05192.x. [DOI] [PubMed] [Google Scholar]
- Szczesny B, Bhakat KK, Mitra S, Boldogh I. Age-dependent modulation of DNA repair enzymes by covalent modification and subcellular distribution. Mech Ageing Dev. 2004;125:755–765. doi: 10.1016/j.mad.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Szczesny B, Hazra TK, Papaconstantinou J, Mitra S, Boldogh I. Age-dependent deficiency in import of mitochondrial DNA glycosylases required for repair of oxidatively damaged bases. Proc Natl Acad Sci U S A. 2003;100:10670–10675. doi: 10.1073/pnas.1932854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesny B, Mitra S. Effect of aging on intracellular distribution of abasic (AP) endonuclease 1 in the mouse liver. Mech Ageing Dev. 2005;126:1071–1078. doi: 10.1016/j.mad.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Szczesny B, Tann AW, Longley MJ, Copeland WC, Mitra S. Long patch base excision repair in mammalian mitochondrial genomes. J Biol Chem. 2008 doi: 10.1074/jbc.M803491200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchberry CD, Wacker MJ, Richmond SR, Whitman SA, Godard MP. Age-related changes in relative expression of real-time PCR housekeeping genes in human skeletal muscle. J Biomol Tech. 2006;17:157–162. [PMC free article] [PubMed] [Google Scholar]