Abstract
Purpose of review
The role of SGK1 in renal physiology and pathophysiology is reviewed with particular emphasis of recent advances.
Recent findings
The mammalian target of rapamycin complex 2 (mTORC2) has been shown to phosphorylate SGK1 at Ser422 (the so-called hydrophobic motif). Ser397 and Ser401 are two additional SGK1-phosphorylation sites required for maximal SGK1-activity. A 5′ variant alternate transcript of human Sgk1 has been identified that is widely expressed and shows improved stability, enhanced membrane association, and greater stimulation of epithelial Na+ transport. SGK1 is essential for optimal processing of the epithelial sodium channel and also regulates the expression of the Na+-Cl− cotransporter. With regard to pathophysiology, SGK1 participates in the stimulation of renal tubular glucose transport in diabetes, the renal profibrotic effect of both angiotensin II and aldosterone, and in fetal programming of arterial hypertension.
Summary
The outlined recent findings advanced our understanding of the molecular regulation of SGK1 as well as the role of the kinase in renal physiology and the pathophysiology of renal disease and hypertension. Future studies using pharmacological inhibitors of SGK1 will reveal the utility of the kinase as a new therapeutic target.
Keywords: Metabolic syndrome, hypertension, fibrosis, inflammation, coagulation
Introduction
The serum- and glucocorticoid-inducible kinase 1 (SGK1) was originally cloned as an immediate early gene transcriptionally stimulated by serum and glucocorticoids in rat mammary tumor cells [1]. The human isoform has been discovered as a gene upregulated by cell shrinkage [2]. As listed in Table 1, SGK1 is under transcriptional control of a variety of further stimuli.
Table 1.
Regulation of SGK1 transcription (due to space limits, only recent original papers are cited, for previous citations see the cited reviews)
| Stimulators | References |
|---|---|
| glucocorticoids | [1,3-7] |
| mineralocorticoids | [7-9] |
| gonadotropins | [7] |
| progestin | [10] |
| medroxyprogesterone | [11] |
| 1,25-dyhydroxyvitamin D3 (1,25(OH)2D3) | [7] |
| transforming growth factor β (TGFβ) | [7] |
| interleukin 6 | [12] |
| fibroblast and platelet-derived growth factor | [7] |
| thrombin | [13] |
| endothelin | [14] |
| advanced glycation end products (AGE) | [15] |
| further cytokines | [7] |
| activation of peroxisome proliferator-activated receptor γ |
[7] |
| cell shrinkage | [7,16] |
| chelation of Ca2+ | [17] |
| A6 cell swelling | [17] |
| excessive glucose concentrations | [7,18] |
| metabolic acidosis | [19] |
| salt loading of spontaneously hypertensive mice | [20] |
| heat shock, UV radiation and oxidative stress | [21,22] |
| DNA damage | [7] |
| ischemia | [23] |
| neuronal injury | [7] |
| neuronal excitotoxicity | [7] |
| neuronal challenge by exposure to microgravity | [24] |
| fear conditioning | [25] |
| plus maze exposure | [26] |
| enrichment training | [7] |
| amphetamine | [7] |
| lysergic acid dimethylamide LSD | [7] |
| electroconvulsive therapy | [27] |
| sleep deprivation | [27] |
| fluoxetine | [27] |
| Rett syndrome | [28] |
| organ rejection | [29] |
| dialysis | [30] |
| wound healing | [7] |
| diabetic nephropathy | [7,31] |
| glomerulonephritis | [7] |
| liver cirrhosis | [7] |
| fibrosing pancreatitis | [7] |
| Crohn’s disease | [7] |
| lung fibrosis | [7] |
| cardiac fibrosis | [32] |
| Inhibitors | |
| heparin | [7] |
| mutations in the gene MECP2 | [7,28] |
| dietary iron | [7] |
| nucleosides | [33] |
| Signaling molecules | |
| protein kinase C | [7] |
| protein kinase Raf | [7] |
| mitogen-activated protein kinase (BMK1) | [7] |
| mitogen-activated protein kinase (MKK1) | [7] |
| stress-activated protein kinase-2 (SAPK2, p38 kinase) |
[7] |
| Nuclear factor of activated T cells (NFAT) 5 | [16] |
| phosphatidylinositol-(PI)-3-kinase | [7] |
| cyclic AMP | [7,10] |
| extracellular signal-regulated kinase (ERK1/2) | [7] |
| P53 | [7] |
| cytosolic Ca2+ | [7] |
| nitric oxide | [7,34] |
| EWS/NOR1(NR4A3) fusion protein | [35] |
The gene encoding human SGK1 has been localised to chromosome 6q23 [36]. Distinct translational isoforms of SGK1 have been disclosed differing in regulation of expression, subcellular localization and function [37-39]. The kinase forms dimers by two intermolecular disulfide bonds between Cys258 in the activation loop and Cys193 [40]. SGK kinases were detected in a variety of species including shark and Caenorhabditis elegans [7]. Yeast express two orthologues, Ypk1 and Ypk2, kinases involved in the regulation of endocytosis and required for survival [7].
Regulation of SGK1 expression and activity
SGK1 expression is virtually ubiquitous [2], but varies between different cell types, as observed in brain [7,41,42], eye [7], inner ear [3,4,43], semicircular canal duct epithelium [4], lung [7,44-48], kidney [7,49], liver [7], intestine [7], pancreas [7] and ovary [7]. Moreover, typical expression patterns are found during embryonic as well as postnatal development [41,50-52]. The subcellular localisation of SGK1 may depend on the functional state of the cell. Activation of SGK1 following exposure of cells to serum has been suggested to trigger importin-alpha mediated entry of SGK1 into the nucleus [7] whereas activation by hyperosmotic shock or glucocorticoids enhances cytosolic localization of the kinase [1]. SGK1 may further localize to the mitochondrial membrane [53,54].
SGK1 transcription is rapidly regulated by a wide variety of stimulators and inhibitors (Table 1). Transcription factor binding sites have been identified in the promoter of the rat SGK1 gene for the glucocorticoid receptor (GR), the mineralocorticoid receptor (MR), the progesterone receptor (PR), the vitamin D receptor (VDR), the retinoid X receptor (RXR), the farnesoid X receptor (FXR), the sterol regulatory element binding protein (SREBP), peroxisome proliferator-activated receptor γ (PPARγ), the cAMP response element binding protein (CREB), the p53 tumor suppressor protein, the Sp1 transcription factor, the activating protein 1 (AP1), the activating transcription factor 6 (ATF6), the heat shock factor (HSF), reticuloendotheliosis viral oncogene homolog (c-Rel), nuclear factor κB (NFκB), signal transducers and activators of transcription (STAT), TGFβ dependent transcription factors SMAD3 and SMAD4, and fork-head activin signal transducer (FAST) [1].
SGK1 is activated by the phosphatidylinositol-3-kinase (PI3-kinase) pathway involving the 3-phosphoinositide (PIP3)-dependent kinase PDK1 [7]. PIP3 is degraded and thus SGK1 activation discontinued by the phosphatase and tensin homolog PTEN [55]. SGK1 activation by PDK1 may involve the scaffold protein Na+/H+ exchanger regulating factor 2 (NHERF2), which mediates the assembly of SGK1 and PDK1 via its PDZ domains and PIF consensus sequence [7]. Activation of SGK1 by PDK1 may further involve the mammalian target of rapamycin mTOR [56-59] and the serine/threonine kinase WNK1 (with no lysine kinase 1) [60-62].
PI3-kinase pathway dependent activation of SGK1 is triggered by insulin, IGF1 , hepatic growth factor (HGF), and follicle stimulating hormone (FSH) [7]. SGK1 can further be activated by bone marrow kinase/extracellular signal-regulated kinase 5 (BK/ERK5) or by p38α [7], by feeding [63], by an increase of cytosolic Ca2+ activity with subsequent activation of calmodulin-dependent protein kinase kinase (CaMKK) [7], and by the small G-protein Rac1 [7]. SGK1 is further activated by neuronal depolarization, cAMP, lithium, oxidation and adhesion to fibronectin [7].
SGK1 is degraded with a half-life of 30 minutes [7]. SGK1 may be ubiquitinated [64,65] by the ubiquitin ligase Nedd4-2 (neuronal precursor cells expressed developmentally downregulated) [66].
SGK1 dependent regulation of cellular functions
The SGK1 kinase consensus sequence R-X-R-X-X-(S/T)-phi (X stands for any amino acid, R for arginine and phi indicates a hydrophobic amino acid) is shared by other kinases [7] and the only exclusive SGK1 targets known are the N-myc downregulated genes NDRG1 and NDRG2 [7,67]. Thus, most SGK1 sensitive functions are similarly regulated by SGK and protein kinase B isoforms or other related kinases. As listed in Table 2, SGK1 regulates a wide variety of channels and carriers.
Table 2.
Regulation of carriers and transporters by SGK1 (due to space limits, only recent original papers are cited, for previous citations see the cited reviews)
| Channel | Function | References |
|---|---|---|
| ENaC | Epithelial Na+ channel | [7,68-77] |
| ROMK1 | Renal outer medullary K+ channel |
[7] |
| TRPV5 | Ca2+ channel | [7] |
| TRPV6 | Ca2+ channel | [78] |
| ClCKa/barttin | Cl− channel | [7,79] |
| ClC2 | Cl− channel | [7] |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
[80,81] |
| VSOAC | volume-sensitive osmolyte and anion channel |
[7] |
| SCN5A | Na+ channel | [7] |
| KCNE1/KCNQ1 | K+ channel | [7,82] |
| KCNQ4 | K+ channel | [83] |
| Kv1.3 | K+ channel | [7,84] |
| Kv1.5 | K+ channel | [85,86] |
| Kv4.3 | K+ channel | [87] |
| ASIC1 | acid sensing ion channel | [88] |
| GluR6 | glutamate receptor | [89] |
| 4F2/LAT | cation channel | [7] |
| NCC | NaCl cotransport | [90] |
| NKCC2 | Na+,K+,2Cl- cotransport | [7] |
| NHE3 | Na+/H+ exchanger | [7,91-93] |
| SGLT1 | glucose transporters | [7,94,95] |
| GLUT1 | glucose transporter | [96] |
| GLUT4 | glucose transporter | [97] |
| ASCT2 | amino acid transporter | [97] |
| SN1 | glutamine transporter | [7] |
| EAAT1 | glutamate transporter | [7] |
| EAAT2 | glutamate transporter | [7,98] |
| EAAT3 | glutamate transporter | [7] |
| EAAT4 | glutamate transporter | [7,99] |
| EAAT5 | glutamate transporter | [100] |
| PepT2 | peptide transporter | [101] |
| NaDC-1 | Na+,dicarboxylate cotransporter | [7] |
| CreaT | creatine transporter | [102,103] |
| SMIT | Na+, myoinositol cotransporter | [104] |
| NaPiIIb | phosphate carrier | [7,105,106] |
| Na+/K+-ATPase | Na+/K+-pump | [7,107] |
SGK1 regulates the channels and carriers partially by phosphorylating the target proteins [7]. Alternatively, SGK1 phosphorylates the ubiquitin ligase Nedd4-2 [7], which otherwise ubiquitinates channel and transport proteins thus preparing them for clearance from the cell membrane and subsequent degradation [7]. Phosphoprylated Nedd4-2 is bound to 14-3-3, and is thus unable to ubiquitinate its targets [108-111]. The P355LNedd4-2 variant, found in patients with end-stage renal disease (ESRD) suffering from arterial hypertension, was shown to be more sensitive to phosphorylation by SGK1 and, accordingly, to exert a weaker negative effect on ENaC [7]. Furthermore, SGK1 may phosphorylate WNK4, a kinase inhibiting ENaC activity [112], and inhibit inducible nitric oxide synthase, and the decreased formation of nitric oxide may then disinhibit ENaC [114]. SGK1 may also be effective through modification of channel or carrier expression [113]. SGK1 may further regulate carriers and channels through activation of the phosphatidylinositol-3-phosphate-5-kinase PIKfyve and subsequent formation of PIP2 [95,115,116].
SGK1 phosphorylates and thus inhibits several enzymes, including the ubiquitin ligase Nedd4-2 [7,66], the mitogen-activated protein kinase/ERK kinase kinase 3 (MEKK3) [7], the kinase SEK1 [21], the B-Raf kinase [7], the phosphomannose mutase 2 [117], inducible nitric oxide (NO) synthase [114] and the glycogen synthase kinase 3 GSK3 [7,118], which, however, may be regulated by PKB/Akt rather than SGK1 [7].
SGK1 regulates several transcription factors, including the cAMP responsive element binding protein (CREB) [119], nuclear factor kappa B (NFκB) [32,120,121], and the forkhead transcription factor FKHR-L1 (FOXO3a) [7,122].
SGK1 phosphorylates a variety of further proteins such as the type A natriuretic peptide receptor (NPR-A) [7], Ca2+ regulated heat-stable protein of apparent molecular mass 24 kDa CRHSP24 [123], the adaptor precursor (APP) Fe65 [72], NDRG1 and NDRG2 [67], mosinVc [124], filamin C [7], microtubule-associated protein tau [125] and huntingtin [7]. In most cases, the functional significance of SGK1 dependent phorphorylation of those proteins is still elusive. A wide variety of functions have, however, been described, which are regulated by SGK1 (Table 3). Most of those functions have been reviewed previously [7].
Table 3.
SGK1 sensitive functions (due to space limits, only recent original papers are cited, for previous citations see the cited reviews)
| Function | References |
|---|---|
| Cell volume | [7,126] |
| Cell survival | [7,127,128] |
| Cell proliferation | [1,7] |
| Aldosterone release | [1,7] |
| Insulin release | [86,86,129] |
| Glucose metabolism | [130] |
| Function of decidualizing cells | [10,10] |
| Gastric acid secretion | [131] |
| Intestinal transport | [94] |
| Renal transport | [7,16,90,132] |
| Proteinuria & nephrotic syndrome | [133-135] |
| Blood pressure regulation | [7,136,137] |
| Coagulation | [13] |
| Pulmonary hypertension | [13] |
| Cardiac excitation | [7,82,138] |
| Cardiac hypertrophy | [139,140] |
| Memory consolidation | [7,24,25,125,141,142] |
| Pain perception | [143] |
| Tumor growth and metastasis | [7,39,144-146] |
| Inflammation and Fibrosis | [7,32,147-150] |
In the following the role of SGK1 in the regulation of electrolyte metabolism and blood pressure will be discussed in more detail.
The role of SGK1 in renal function and salt appetite
SGK1 stimulates a variety of renal tubular ion channels and transporters (Table 2) and thus participates in the regulation of renal electrolyte excretion (for earlier references see [7,151-154]).
Specifically, SGK1 participates in the regulation of renal Na+ excretion by aldosterone, insulin and IGF1 [91,155]. The effect of aldosterone is only partially dependent on the presence of SGK1 and effects of aldosterone and SGK1 are additive. In contrast, antidiuretic hormone (ADH) or insulin do not further stimulate ENaC in cells expressing active SGK1 [156]. Experiments in SGK1-knockout (sgk1−/−) mice indeed revealed subtle but relevant impairment of renal salt retention [90]. In salt replete conditions arterial blood pressure and renal salt output is similar in sgk1−/− mice and their wild-type littermates (sgk1+/+). The maintenance of salt balance and blood pressure requires, however, increased plasma aldosterone levels in sgk1−/− mice [7]. Under NaCl-deficient diet, NaCl excretion decreases in both sgk1−/− and sgk1+/+ mice, the renal NaCl retention remains, however, insufficient in sgk1−/− mice, despite an increase of plasma aldosterone concentration, decrease of arterial blood pressure, decrease of glomerular filtration rate and enhanced proximal tubular Na+ reabsorption [7]. The renal salt loss of sgk1−/− mice may not be only due to decreased stimulation of ENaC as enhanced ENaC activity has been observed in salt depleted sgk1−/− mice [90], presumably as a result of stimulation of ENaC expression by hyperaldosteronism [90]. Similarly, despite the lack of SGK1 ENaC activity is enhanced in the colon of sgk1−/− mice, which is again likely due to hyperaldosteronism [157]. The enhanced renal salt loss despite increased ENaC activity may be due to decreased expression of the NaCl cotransport in early distal tubule [90]. Inhibition of ENaC by triamterene leads to excessive, eventually lethal salt loss in sgk1−/− but not in sgk1+/+ mice, an observation again pointing to a renal transport defect other than ENaC in sgk1−/− mice [158]. SGK1 is particularly important for the antinatriuretic effect of insulin, which is significantly blunted in sgk1−/− mice [136]. Renal salt loss does not appear to be more pronounced in mice lacking both SGK1 and SGK3 [159].
In dehydration, the enhanced medullary osmolarity upregulates the expression of SGK1, which in turn stimulates the expression of the natriuretic peptide receptor type A thus sensitizing the epithelial cells to natriuretic peptides and triggering natriuresis [16]. The renal loss of Na+ counteracts hyperosomolarity during water deprivation [16].
Lack of SGK1 further impairs the ability of the kidney to excrete a K+ load. Despite enhanced plasma levels of aldosterone, which should increase renal K+ excretion, sgk1−/− mice do not adequately increase the renal K+ excretion following an acute K+ load [7]. Following a chronic K+ load, sgk1−/− mice enhance renal K+ excretion only upon marked increase of plasma K+ and aldosterone concentrations [7]. SGK1 is further critically important for the effect of insulin on cellular K+ uptake [160].
The sgk1−/− mice express significantly less TRPV5 Ca2+ channels [132], an observation consistent with a stimulating effect of SGK1 on TRPV5 [7]. However, despite decreased TRPV5 expression, the renal Ca2+ excretion is decreased in sgk1−/− mice [132]. The extracellular volume contraction following renal salt loss due to impaired stimulation of NaCl cotransport and ENaC enhances Na+ [7] and presumably Ca2+ reabsorption in the proximal tubule and in the thick ascending limb of the loop of Henle, thus decreasing renal Ca2+ excretion. It is noteworthy that inhibition of NaCl cotransport by thiazide diuretics regularly leads to anticalciuria [161] by a mechanism that involves upregulation of proximal tubular Ca2+ reabsorption [162].
In normal kidneys, the proximal tubular SGK1 expression is low [7] and SGK1 is thus not considered to participate in the regulation of proximal tubular transport. In diabetic nephropathy, however, SGK1 is expressed throughout the kidney including proximal renal tubules and thus stimulates renal tubular glucose transport [163]. The effect may be due to stimulation of SGLT1 [7] or due to activation of KCNQ1/KCNE1 [7], which maintains the electrical driving force for electrogenic glucose transport [164].
SGK1 is expressed in glomerular podocytes [22,165] and is upregulated in those cells by aldosterone and oxidative stress [20,22]. Thus, SGK1 may participate in the development of proteinuria during mineralocorticoid excess and inflammation. As a matter of fact, the proteinuria following DOCA treatment is significantly blunted in SGK1 knockout mice [133]. The sgk1−/− mice are, however, not protected against doxorubicin induced glomerular injury [135]. SGK1-dependent renal salt retention favor the development of edema during treatment with PPARγ agonists [166] or in nephrotic syndrome [135]. Furthermore, increased SGK1 expression has been found during ascites formation in cirrhotic rats [167].
SGK1 does not only participate in the regulation of salt excretion but contributes to the regulation of salt intake [168]. Treatment of animals with the mineralocorticoid DOCA is followed by excessive salt intake in sgk1+/+ but not in sgk1−/− mice. Thus, SGK1 plays a dual role in mineralocorticoid-regulated NaCl homeostasis, stimulating both, intake and renal retention of salt.
Role of SG1 in hypertension, obesity and metabolic syndrome
The effect of SGK1 on renal salt excretion and salt intake is expected to impact on blood pressure control. As a matter of fact, a certain variant of the SGK1 gene (the combined presence of distinct polymorphisms in intron 6 [I6CC] and in exon 8 [E8CC/CT]) is associated with moderately enhanced blood pressure [7]. The gene variant is common, affecting 3-5 % of a Caucasian population [7]. While one study failed to detect a correlation of the gene variant with blood pressure in patients with renal failure [7], a subsequent study on more than four thousand individuals confirmed the association of the gene variant with increased blood pressure [169]. This latter study revealed a particularly strong correlation between insulinemia and blood pressure in individuals carrying the SGK1 gene variant unravelling the decisive role of SGK1 in the hypertension paralleling hyperinsulinemia [169]. Hyperinsulinemia by pretreatment with a high-fructose diet [136] or a high fat diet [137] sensitizes arterial blood pressure to high-salt intake in sgk1+/+ but not in sgk1−/− mice. This observation underscores the role of SGK1 in insulin induced antinatriuresis and blood pressure control. Moreover, SGK1 may participate in the hypertensive effects of glucocorticoids [170]. Lack of Nedd4-2 leads to hypertension in mice thus mimicking overactivity of SGK1 [171]. Beyond the contribution of SGK1 to blood pressure increase in a given individual, maternal SGK1 appears to be critical for the fetal programming of hypertension in the offspring by stress of the mother [172].
As SGK1 is a powerful stimulator of the Na+ coupled glucose transporter SGLT1 [7], the SGK1 gene variant may further accelerate the intestinal glucose absorption. Enhanced SGLT1 activity accelerates intestinal glucose absorption leading to excessive insulin release, fat deposition, and subsequent decrease of plasma glucose concentration triggering repeated glucose uptake and thus obesity [7]. Conversely, inhibitors of SGLT1 have been shown to counteract obesity [7]. Accordingly, the same SGK1 gene variant associated with enhanced blood pressure proved to be associated with increased body mass index [7]. Moreover, this SGK1 gene variant is more prevalent in patients with type 2 diabetes than in individuals without family history of diabetes [173]
Hypertension, obesity and susceptibility to develop type II diabetes are hallmarks of metabolic syndrome, which is associated with enhanced morbidity and mortality from cardiovascular disease [7]. Metabolic syndrome and Cushing’s syndrome share common clinical features , but the plasma cortisol levels are not typically elevated in metabolic syndrome [7]. The disorder is rather caused by inappropriate activity of a downstream signaling element. To the extend that SGK1 mediates effects of glucocorticoids on blood pressure and body weight, a SGK1 gene variant leading to increased SGK1 activity would trigger glucocorticoid actions without enhanced plasma glucocorticoid concentrations. Metabolic syndrome is further typically associated with enhanced coagulation [7], which is again stimulated by SGK1 [13]. Taken together, compelling evidence suggests a participation of SGK1 in the pathophysiology of metabolic syndrome.
Conclusions
The serum- and glucocorticoid-inducible kinase SGK1 is a powerful regulator of metabolism, transport, transcription and enzyme activity and thus participates in the regulation of a wide variety of physiological functions including epithelial transport, excitability, cell proliferation and apoptosis. The phenotype of SGK1 knockout mice is mild and obviously, the function of this kinase is involved in but not critically important for the maintenance of house keeping functions. However, following appropriate challenges the lack of SGK1 becomes obvious and physiologically or pathophysiologically relevant. SGK1 plays a particularly important role in electrolyte balance, extracellular volume regulation, metabolic syndrome, tumor growth, inflammation, and fibrosing disease.
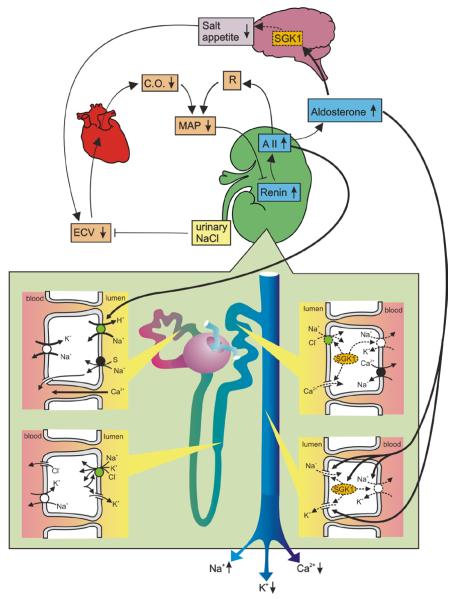
Fig. 1. Renal function in SGK1 deficient mice.
SGK1 deficiency decreases NCC and ENaC activity and blunts the stimulation of renal Na+ reabsorption by insulin and aldosterone as well as the stimulation of salt appetite by mineralocorticoids. The extracellular fluid volume (ECV) contraction decreases cardiac output (C.O.) thus compromising maintenance of mean arterial pressure (MAP) with subsequent stimulation of the renin-angiotensin and aldosterone system. The hyperaldosteronism at least partially reverses the decrease of ENaC, ROMK and Na+/K+ ATPase activity in principle cells. Nevertheless, SGK1 deficiency impairs stimulation of renal K+ excretion during K+ excess. Despite its stimulation of the distal tubular Ca2+ channel TRPV5, lack of SGK1 leads to anticalciuria due compensatory stimulation of Na+ (and Ca2+) reabsorption in proximal tubules. (A II = angiotensin II; R= total peripheral vascular resistance).
Footnotes
Disclosure: The study in the laboratories of the authors is funded by the Deutsche Forschungsgemeinschaft (DFG, IRTG to F.L.) and the National Institutes of Health (NIH-R01DK56248 and NIH-P30DK079337 to V.V.). The authors declare that they have no conflict of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Firestone GL, Giampaolo JR, O’Keeffe BA. Stimulus-dependent regulation of the serum and glucocorticoid inducible protein kinase (Sgk) transcription, subcellular localization and enzymatic activity. Cell Physiol Biochem. 2003;13:1–12. doi: 10.1159/000070244. [DOI] [PubMed] [Google Scholar]
- 2.Waldegger S, Barth P, Raber G, et al. Cloning and characterization of a putative human serine/threonine protein kinase transcriptionally modified during anisotonic and isotonic alterations of cell volume. Proc Natl Acad Sci U S A. 1997;94:4440–4445. doi: 10.1073/pnas.94.9.4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SH, Kim KX, Raveendran NN, et al. Regulation of Epithelial Sodium Channel (ENaC)-Mediated Sodium Transport by Glucocorticoids in Reissner’s Membrane Epithelium. Am J Physiol Cell Physiol. 2009 doi: 10.1152/ajpcell.00338.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pondugula SR, Raveendran NN, Ergonul Z, et al. Glucocorticoid regulation of genes in the amiloride-sensitive sodium transport pathway by semicircular canal duct epithelium of neonatal rat. Physiol Genomics. 2006;24:114–123. doi: 10.1152/physiolgenomics.00006.2005. [DOI] [PubMed] [Google Scholar]
- 5.van Gemert NG, Meijer OC, Morsink MC, et al. Effect of brief corticosterone administration on SGK1 and RGS4 mRNA expression in rat hippocampus. Stress. 2006;9:165–170. doi: 10.1080/10253890600966169. [DOI] [PubMed] [Google Scholar]
- 6.Yaylaoglu MB, Agbemafle BM, Oesterreicher TJ, et al. Diverse patterns of cell-specific gene expression in response to glucocorticoid in the developing small intestine. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1041–G1050. doi: 10.1152/ajpgi.00139.2006. [DOI] [PubMed] [Google Scholar]
- 7.Lang F, Bohmer C, Palmada M, et al. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151–1178. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- 8.Bertog M, Cuffe JE, Pradervand S, et al. Aldosterone responsiveness of the epithelial sodium channel (ENaC) in colon is increased in a mouse model for Liddle’s syndrome. J Physiol. 2008;586:459–475. doi: 10.1113/jphysiol.2007.140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fakitsas P, Adam G, Daidie D, et al. Early aldosterone-induced gene product regulates the epithelial sodium channel by deubiquitylation. J Am Soc Nephrol. 2007;18:1084–1092. doi: 10.1681/ASN.2006080902. [DOI] [PubMed] [Google Scholar]
- 10.Feroze-Zaidi F, Fusi L, Takano M, et al. Role and regulation of the serum- and glucocorticoid-regulated kinase 1 in fertile and infertile human endometrium. Endocrinology. 2007;148:5020–5029. doi: 10.1210/en.2007-0659. [DOI] [PubMed] [Google Scholar]
- 11.Thomas CP, Liu KZ, Vats HS. Medroxyprogesterone acetate binds the glucocorticoid receptor to stimulate alpha-ENaC and sgk1 expression in renal collecting duct epithelia. Am J Physiol Renal Physiol. 2006;290:F306–F312. doi: 10.1152/ajprenal.00062.2005. [DOI] [PubMed] [Google Scholar]
- 12.Meng F, Yamagiwa Y, Taffetani S, et al. IL-6 activates serum and glucocorticoid kinase via p38alpha mitogen-activated protein kinase pathway. Am J Physiol Cell Physiol. 2005;289:C971–C981. doi: 10.1152/ajpcell.00081.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belaiba RS, Djordjevic T, Bonello S, et al. The serum- and glucocorticoid-inducible kinase Sgk-1 is involved in pulmonary vascular remodeling: role in redox-sensitive regulation of tissue factor by thrombin. Circ Res. 2006;98:828–836. doi: 10.1161/01.RES.0000210539.54861.27. [DOI] [PubMed] [Google Scholar]
- 14.Wolf SC, Schultze M, Risler T, et al. Stimulation of serum- and glucocorticoid-regulated kinase-1 gene expression by endothelin-1. Biochem Pharmacol. 2006;71:1175–1183. doi: 10.1016/j.bcp.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Chang CT, Wu MS, Tian YC, et al. Enhancement of epithelial sodium channel expression in renal cortical collecting ducts cells by advanced glycation end products. Nephrol Dial Transplant. 2007;22:722–731. doi: 10.1093/ndt/gfl668. [DOI] [PubMed] [Google Scholar]
- 16.Chen S, Grigsby CL, Law CS, et al. Tonicity-dependent induction of Sgk1 expression has a potential role in dehydration-induced natriuresis in rodents. J Clin Invest. 2009;119:1647–1658. doi: 10.1172/JCI35314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfau A, Grossmann C, Freudinger R, et al. Ca2+ but not H2O2 modulates GRE-element activation by the human mineralocorticoid receptor in HEK cells. Mol Cell Endocrinol. 2007;264:35–43. doi: 10.1016/j.mce.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Hills CE, Bland R, Bennett J, et al. High glucose up-regulates ENaC and SGK1 expression in HCD-cells. Cell Physiol Biochem. 2006;18:337–346. doi: 10.1159/000097611. [DOI] [PubMed] [Google Scholar]
- 19.Faroqui S, Sheriff S, Amlal H. Metabolic acidosis has dual effects on sodium handling by rat kidney. Am J Physiol Renal Physiol. 2006;291:F322–F331. doi: 10.1152/ajprenal.00338.2005. [DOI] [PubMed] [Google Scholar]
- 20.Nagase M, Yoshida S, Shibata S, et al. Enhanced aldosterone signaling in the early nephropathy of rats with metabolic syndrome: possible contribution of fat-derived factors. J Am Soc Nephrol. 2006;17:3438–3446. doi: 10.1681/ASN.2006080944. [DOI] [PubMed] [Google Scholar]
- 21.Kim MJ, Chae JS, Kim KJ, et al. Negative regulation of SEK1 signaling by serum- and glucocorticoid-inducible protein kinase 1. EMBO J. 2007;26:3075–3085. doi: 10.1038/sj.emboj.7601755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibata S, Nagase M, Yoshida S, et al. Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension. 2007;49:355–364. doi: 10.1161/01.HYP.0000255636.11931.a2. [DOI] [PubMed] [Google Scholar]
- 23.Feng Y, Wang Y, Xiong J, et al. Enhanced expression of serum and glucocorticoid-inducible kinase-1 in kidneys of L-NAME-treated rats. Kidney Blood Press Res. 2006;29:94–99. doi: 10.1159/000093461. [DOI] [PubMed] [Google Scholar]
- 24.David S, Stegenga SL, Hu P, et al. Expression of serum- and glucocorticoid-inducible kinase is regulated in an experience-dependent manner and can cause dendrite growth. J Neurosci. 2005;25:7048–7053. doi: 10.1523/JNEUROSCI.0006-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee CT, Ma YL, Lee EH. Serum- and glucocorticoid-inducible kinase1 enhances contextual fear memory formation through down-regulation of the expression of Hes5. J Neurochem. 2007;100:1531–1542. doi: 10.1111/j.1471-4159.2006.04284.x. [DOI] [PubMed] [Google Scholar]
- 26.Koya E, Spijker S, Homberg JR, et al. Molecular reactivity of mesocorticolimbic brain areas of high and low grooming rats after elevated plus maze exposure. Brain Res Mol Brain Res. 2005;137:184–192. doi: 10.1016/j.molbrainres.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Conti B, Maier R, Barr AM, et al. Region-specific transcriptional changes following the three antidepressant treatments electro convulsive therapy, sleep deprivation and fluoxetine. Mol Psychiatry. 2007;12:167–189. doi: 10.1038/sj.mp.4001897. [DOI] [PubMed] [Google Scholar]
- 28.Nuber UA, Kriaucionis S, Roloff TC, et al. Up-regulation of glucocorticoid-regulated genes in a mouse model of Rett syndrome. Hum Mol Genet. 2005;14:2247–2256. doi: 10.1093/hmg/ddi229. [DOI] [PubMed] [Google Scholar]
- 29.Velic A, Gabriels G, Hirsch JR, et al. Acute rejection after rat renal transplantation leads to downregulation of NA+ and water channels in the collecting duct. Am J Transplant. 2005;5:1276–1285. doi: 10.1111/j.1600-6143.2005.00890.x. [DOI] [PubMed] [Google Scholar]
- 30.Friedrich B, Alexander D, Aicher WK, et al. Influence of standard haemodialysis treatment on transcription of human serum- and glucocorticoid-inducible kinase SGK1 and taurine transporter TAUT in blood leukocytes. Nephrol Dial Transplant. 2005;20:768–774. doi: 10.1093/ndt/gfh697. [DOI] [PubMed] [Google Scholar]
- 31.Wang Q, Zhang A, Li R, et al. High glucose promotes the CTGF expression in human mesangial cells via serum and glucocorticoid-induced kinase 1 pathway. J Huazhong Univ Sci Technolog Med Sci. 2008;28:508–512. doi: 10.1007/s11596-008-0504-z. [DOI] [PubMed] [Google Scholar]
- 32.Vallon V, Wyatt AW, Klingel K, et al. SGK1-dependent cardiac CTGF formation and fibrosis following DOCA treatment. J Mol Med. 2006;84:396–404. doi: 10.1007/s00109-005-0027-z. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Wingo CS, Xia SL. Downregulation of SGK1 by nucleotides in renal tubular epithelial cells. Am J Physiol Renal Physiol. 2007;293:F1751–F1757. doi: 10.1152/ajprenal.00091.2007. [DOI] [PubMed] [Google Scholar]
- 34.Turpaev K, Bouton C, Diet A, et al. Analysis of differentially expressed genes in nitric oxide-exposed human monocytic cells. Free Radic Biol Med. 2005;38:1392–1400. doi: 10.1016/j.freeradbiomed.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Poulin H, Filion C, Ladanyi M, et al. Serum- and glucocorticoid-regulated kinase 1 (SGK1) induction by the EWS/NOR1(NR4A3) fusion protein. Biochem Biophys Res Commun. 2006;346:306–313. doi: 10.1016/j.bbrc.2006.05.134. [DOI] [PubMed] [Google Scholar]
- 36.Waldegger S, Erdel M, Nagl UO, et al. Genomic organization and chromosomal localization of the human SGK protein kinase gene. Genomics. 1998;51:299–302. doi: 10.1006/geno.1998.5258. [DOI] [PubMed] [Google Scholar]
- 37.Arteaga MF, Alvarez dlR, Alvarez JA, et al. Multiple translational isoforms give functional specificity to serum- and glucocorticoid-induced kinase 1. Mol Biol Cell. 2007;18:2072–2080. doi: 10.1091/mbc.E06-10-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raikwar NS, Snyder PM, Thomas CP. An evolutionarily conserved N-terminal Sgk1 variant with enhanced stability and improved function. Am J Physiol Renal Physiol. 2008;295:F1440–F1448. doi: 10.1152/ajprenal.90239.2008. ** A 5′ variant alternate transcript of human SGK1 was identified that is widely expressed, is conserved from rodent to humans, and is predicted to encode an SGK1 isoform, SGK1_i2, with a different NH2 terminus. This variant shows improved stability, enhanced membrane association, and greater stimulation of epithelial Na+ transport in a heterologous expression system.
- 39.Simon P, Schneck M, Hochstetter T, et al. Differential regulation of serum- and glucocorticoid-inducible kinase 1 (SGK1) splice variants based on alternative initiation of transcription. Cell Physiol Biochem. 2007;20:715–728. doi: 10.1159/000110432. [DOI] [PubMed] [Google Scholar]
- 40.Zhao B, Lehr R, Smallwood AM, et al. Crystal structure of the kinase domain of serum and glucocorticoid-regulated kinase 1 in complex with AMP PNP. Protein Sci. 2007;16:2761–2769. doi: 10.1110/ps.073161707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keller-Wood M, Powers MJ, Gersting JA, et al. Genomic analysis of neuroendocrine development of fetal brain-pituitary-adrenal axis in late gestation. Physiol Genomics. 2006;24:218–224. doi: 10.1152/physiolgenomics.00176.2005. [DOI] [PubMed] [Google Scholar]
- 42.Stichel CC, Schoenebeck B, Foguet M, et al. sgk1, a member of an RNA cluster associated with cell death in a model of Parkinson’s disease. Eur J Neurosci. 2005;21:301–316. doi: 10.1111/j.1460-9568.2005.03859.x. [DOI] [PubMed] [Google Scholar]
- 43.Zhong SX, Liu ZH. Expression patterns of Nedd4 isoforms and SGK1 in the rat cochlea. Acta Otolaryngol. 2008:1–5. doi: 10.1080/00016480802552501. [DOI] [PubMed] [Google Scholar]
- 44.Aoi W, Niisato N, Sawabe Y, et al. Aldosterone-induced abnormal regulation of ENaC and SGK1 in Dahl salt-sensitive rat. Biochem Biophys Res Commun. 2006;341:376–381. doi: 10.1016/j.bbrc.2005.12.194. [DOI] [PubMed] [Google Scholar]
- 45.Brown SG, Gallacher M, Olver RE, et al. The regulation of selective and nonselective Na+ conductances in H441 human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L942–L954. doi: 10.1152/ajplung.00240.2007. [DOI] [PubMed] [Google Scholar]
- 46.Inglis SK, Gallacher M, Brown SG, et al. SGK1 activity in Na(+) absorbing airway epithelial cells monitored by assaying NDRG1-Thr(346/356/366) phosphorylation. Pflugers Arch. 2008 doi: 10.1007/s00424-008-0587-1. [DOI] [PubMed] [Google Scholar]
- 47.Keller-Wood M, Wood CE, Hua Y, et al. Mineralocorticoid receptor expression in late-gestation ovine fetal lung. J Soc Gynecol Investig. 2005;12:84–1. doi: 10.1016/j.jsgi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 48.Wirbelauer J, Schmidt B, Klingel K, et al. Serum and glucocorticoid-inducible kinase in pulmonary tissue of preterm fetuses exposed to chorioamnionitis. Neonatology. 2008;93:257–262. doi: 10.1159/000111531. [DOI] [PubMed] [Google Scholar]
- 49.Uawithya P, Pisitkun T, Ruttenberg BE, et al. Transcriptional profiling of native inner medullary collecting duct cells from rat kidney. Physiol Genomics. 2008;32:229–253. doi: 10.1152/physiolgenomics.00201.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cobb J, Duboule D. Comparative analysis of genes downstream of the Hoxd cluster in developing digits and external genitalia. Development. 2005;132:3055–3067. doi: 10.1242/dev.01885. [DOI] [PubMed] [Google Scholar]
- 51.Huber SM, Friedrich B, Klingel K, et al. Protein and mRNA expression of serum and glucocorticoid-dependent kinase 1 in metanephrogenesis. Dev Dyn. 2001;221:464–469. doi: 10.1002/dvdy.1155. [DOI] [PubMed] [Google Scholar]
- 52.Lee E, Lein ES, Firestone GL. Tissue-specific expression of the transcriptionally regulated serum and glucocorticoid-inducible protein kinase (Sgk) during mouse embryogenesis. Mech Dev. 2001;103:177–181. doi: 10.1016/s0925-4773(01)00351-3. [DOI] [PubMed] [Google Scholar]
- 53.Cordas E, Naray-Fejes-Toth A, Fejes-Toth G. Subcellular location of serum- and glucocorticoid-induced kinase-1 in renal and mammary epithelial cells. Am J Physiol Cell Physiol. 2007;292:C1971–C1981. doi: 10.1152/ajpcell.00399.2006. [DOI] [PubMed] [Google Scholar]
- 54.Engelsberg A, Kobelt F, Kuhl D. The N-terminus of the serum- and glucocorticoid-inducible kinase Sgk1 specifies mitochondrial localization and rapid turnover. Biochem J. 2006;399:69–76. doi: 10.1042/BJ20060386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lian Z, Di Cristofano A. Class reunion: PTEN joins the nuclear crew. Oncogene. 2005;24:7394–7400. doi: 10.1038/sj.onc.1209089. [DOI] [PubMed] [Google Scholar]
- 56.Dunlop EA, Tee AR. Mammalian target of rapamycin complex 1: Signaling inputs, substrates and feedback mechanisms. Cell Signal. 2009 doi: 10.1016/j.cellsig.2009.01.012. in press. [DOI] [PubMed] [Google Scholar]
- 57.Garcia-Martinez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Biochem J. 2008;416:375–385. doi: 10.1042/BJ20081668. ** The identity of the protein kinase(s) responsible for phosphorylation of SGK1 at Ser422 (the so-called hydrophobic motif) that promotes activation of the kinase by PDK1, was unclear. This study revealed the identity of a ‘PDK2′ kinase that catalyses Ser422 phosphorylation as mTORC2 (mammalian target of rapamycin complex 2), a multiprotein kinase that phosphorylates a similar site in PKB.
- 58.Hong F, Larrea MD, Doughty C, et al. mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol Cell. 2008;30:701–711. doi: 10.1016/j.molcel.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 59.Yan L, Mieulet V, Lamb RF. mTORC2 is the hydrophobic motif kinase for SGK1. Biochem J. 2008;416:e19–e21. doi: 10.1042/BJ20082202. [DOI] [PubMed] [Google Scholar]
- 60.Chen W, Chen Y, Xu BE, et al. Regulation of a Third Conserved Phosphorylation Site in SGK1. J Biol Chem. 2009;284:3453–3460. doi: 10.1074/jbc.M807502200. ** This study identified two new phosphorylation sites, Ser397 and Ser401, that both are required for maximum SGK1 activity induced by extracellular agents or by coexpression with other protein kinases, with the largest loss of activity from mutation of Ser397. Coexpression with active Akt1 increased the phosphorylation of Ser397 and thereby SGK1 kinase activity, revealing further complexity underlying the regulation of SGK1 activity.
- 61.Xu BE, Stippec S, Chu PY, et al. WNK1 activates SGK1 to regulate the epithelial sodium channel. Proc Natl Acad Sci U S A. 2005;102:10315–10320. doi: 10.1073/pnas.0504422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu BE, Stippec S, Lazrak A, et al. WNK1 activates SGK1 by a phosphatidylinositol 3-kinase-dependent and non-catalytic mechanism. J Biol Chem. 2005;280:34218–34223. doi: 10.1074/jbc.M505735200. [DOI] [PubMed] [Google Scholar]
- 63.Bayascas JR, Wullschleger S, Sakamoto K, et al. Mutation of the PDK1 PH domain inhibits protein kinase B/Akt, leading to small size and insulin resistance. Mol Cell Biol. 2008;28:3258–3272. doi: 10.1128/MCB.02032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arteaga MF, Wang L, Ravid T, et al. An amphipathic helix targets serum and glucocorticoid-induced kinase 1 to the endoplasmic reticulum-associated ubiquitin-conjugation machinery. Proc Natl Acad Sci U S A. 2006;103:11178–1113. doi: 10.1073/pnas.0604816103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bogusz AM, Brickley DR, Pew T, et al. A novel N-terminal hydrophobic motif mediates constitutive degradation of serum- and glucocorticoid-induced kinase-1 by the ubiquitin-proteasome pathway. FEBS J. 2006;273:2913–2928. doi: 10.1111/j.1742-4658.2006.05304.x. [DOI] [PubMed] [Google Scholar]
- 66.Zhou R, Snyder PM. Nedd4-2 phosphorylation induces serum and glucocorticoid-regulated kinase (SGK) ubiquitination and degradation. J Biol Chem. 2005;280:4518–4523. doi: 10.1074/jbc.M411053200. [DOI] [PubMed] [Google Scholar]
- 67.Murray JT, Cummings LA, Bloomberg GB, et al. Identification of different specificity requirements between SGK1 and PKBalpha. FEBS Lett. 2005;579:991–994. doi: 10.1016/j.febslet.2004.12.069. [DOI] [PubMed] [Google Scholar]
- 68.De Seigneux S, Leroy V, Ghzili H, et al. NF-kappaB inhibits sodium transport via down-regulation of SGK1 in renal collecting duct principal cells. J Biol Chem. 2008;283:25671–25681. doi: 10.1074/jbc.M803812200. [DOI] [PubMed] [Google Scholar]
- 69.Edinger RS, Lebowitz J, Li H, et al. Functional regulation of the epithelial Na+ channel by IkappaB kinase-beta occurs via phosphorylation of the ubiquitin ligase Nedd4-2. J Biol Chem. 2009;284:150–157. doi: 10.1074/jbc.M807358200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hills CE, Squires PE, Bland R. Serum and glucocorticoid regulated kinase and disturbed renal sodium transport in diabetes. J Endocrinol. 2008;199:343–349. doi: 10.1677/JOE-08-0295. [DOI] [PubMed] [Google Scholar]
- 71.Lee IH, Dinudom A, Sanchez-Perez A, et al. Akt mediates the effect of insulin on epithelial sodium channels by inhibiting Nedd4-2. J Biol Chem. 2007;282:29866–29873. doi: 10.1074/jbc.M701923200. [DOI] [PubMed] [Google Scholar]
- 72.Lee IH, Campbell CR, Cook DI, et al. Regulation of epithelial Na+ channels by aldosterone: role of Sgk1. Clin Exp Pharmacol Physiol. 2008;35:235–241. doi: 10.1111/j.1440-1681.2007.04844.x. [DOI] [PubMed] [Google Scholar]
- 73.Naray-Fejes-Toth A, Boyd C, Fejes-Toth G. Regulation of epithelial sodium transport by promyelocytic leukemia zinc finger protein. Am J Physiol Renal Physiol. 2008;295:F18–F26. doi: 10.1152/ajprenal.00573.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pao AC, McCormick JA, Li H, et al. NH2 terminus of serum and glucocorticoid-regulated kinase 1 binds to phosphoinositides and is essential for isoform-specific physiological functions. Am J Physiol Renal Physiol. 2007;292:F1741–F1750. doi: 10.1152/ajprenal.00027.2007. [DOI] [PubMed] [Google Scholar]
- 75.Vasquez MM, Castro R, Seidner SR, et al. Induction of serum- and glucocorticoid-induced kinase-1 (SGK1) by cAMP regulates increases in alpha-ENaC. J Cell Physiol. 2008;217:632–642. doi: 10.1002/jcp.21534. [DOI] [PubMed] [Google Scholar]
- 76.Wang J, Knight ZA, Fiedler D, et al. Activity of the p110-alpha subunit of phosphatidylinositol-3-kinase is required for activation of epithelial sodium transport. Am J Physiol Renal Physiol. 2008;295:F843–F850. doi: 10.1152/ajprenal.90348.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wielputz MO, Lee IH, Dinudom A, et al. (NDRG2) stimulates amiloride-sensitive Na+ currents in Xenopus laevis oocytes and fisher rat thyroid cells. J Biol Chem. 2007;282:28264–28273. doi: 10.1074/jbc.M702168200. [DOI] [PubMed] [Google Scholar]
- 78.Bohmer C, Palmada M, Kenngott C, et al. Regulation of the epithelial calcium channel TRPV6 by the serum and glucocorticoid-inducible kinase isoforms SGK1 and SGK3. FEBS Lett. 2007;581:5586–5590. doi: 10.1016/j.febslet.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 79.Bergler T, Stoelcker B, Jeblick R, et al. High osmolality induces the kidney-specific chloride channel CLC-K1 by a serum and glucocorticoid-inducible kinase 1 MAPK pathway. Kidney Int. 2008;74:1170–1177. doi: 10.1038/ki.2008.312. ** This study indicates a link between high osmolality and the induction of SGK1 and the subsequent increase of CLC-K1/barttin expression in distal renal tubular cells in vivo and in vitro, which may relate to a role of the kinase in urinary concentration and dilution.
- 80.Sato JD, Chapline MC, Thibodeau R, et al. Regulation of human cystic fibrosis transmembrane conductance regulator (CFTR) by serum- and glucocorticoid-inducible kinase (SGK1) Cell Physiol Biochem. 2007;20:91–8. doi: 10.1159/000104157. [DOI] [PubMed] [Google Scholar]
- 81.Shaw JR, Sato JD, VanderHeide J, et al. The role of SGK and CFTR in acute adaptation to seawater in Fundulus heteroclitus. Cell Physiol Biochem. 2008;22:69–78. doi: 10.1159/000149784. [DOI] [PubMed] [Google Scholar]
- 82.Seebohm G, Strutz-Seebohm N, Ureche ON, et al. Long QT syndrome-associated mutations in KCNQ1 and KCNE1 subunits disrupt normal endosomal recycling of IKs channels. Circ Res. 2008;103:1451–1457. doi: 10.1161/CIRCRESAHA.108.177360. [DOI] [PubMed] [Google Scholar]
- 83.Seebohm G, Strutz-Seebohm N, Baltaev R, et al. Regulation of KCNQ4 potassium channel prepulse dependence and current amplitude by SGK1 in Xenopus oocytes. Cell Physiol Biochem. 2005;16:255–262. doi: 10.1159/000089851. [DOI] [PubMed] [Google Scholar]
- 84.Shumilina E, Lampert A, Lupescu A, et al. Deranged Kv channel regulation in fibroblasts from mice lacking the serum and glucocorticoid inducible kinase SGK1. J Cell Physiol. 2005;204:87–98. doi: 10.1002/jcp.20267. [DOI] [PubMed] [Google Scholar]
- 85.Boehmer C, Laufer J, Jeyaraj S, et al. Modulation of the voltage-gated potassium channel Kv1.5 by the SGK1 protein kinase involves inhibition of channel ubiquitination. Cell Physiol Biochem. 2008;22:591–600. doi: 10.1159/000185543. [DOI] [PubMed] [Google Scholar]
- 86.Ullrich S, Berchtold S, Ranta F, et al. Serum- and glucocorticoid-inducible kinase 1 (SGK1) mediates glucocorticoid-induced inhibition of insulin secretion. Diabetes. 2005;54:1090–1099. doi: 10.2337/diabetes.54.4.1090. [DOI] [PubMed] [Google Scholar]
- 87.Baltaev R, Strutz-Seebohm N, Korniychuk G, et al. Regulation of cardiac shal-related potassium channel Kv 4.3 by serum- and glucocorticoid-inducible kinase isoforms in Xenopus oocytes. Pflugers Arch. 2005;450:26–33. doi: 10.1007/s00424-004-1369-z. [DOI] [PubMed] [Google Scholar]
- 88.Arteaga MF, Coric T, Straub C, et al. A brain-specific SGK1 splice isoform regulates expression of ASIC1 in neurons. Proc Natl Acad Sci U S A. 2008;105:4459–4464. doi: 10.1073/pnas.0800958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Strutz-Seebohm N, Seebohm G, Shumilina E, et al. Glucocorticoid adrenal steroids and glucocorticoid-inducible kinase isoforms in the regulation of GluR6 expression. J Physiol. 2005;565:391–401. doi: 10.1113/jphysiol.2004.079624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fejes-Toth G, Frindt G, Naray-Fejes-Toth A, et al. Epithelial Na+ channel activation and processing in mice lacking SGK1. Am J Physiol Renal Physiol. 2008;294:F1298–F1305. doi: 10.1152/ajprenal.00579.2007. ** This study implicates that SGK1 contributes to the regulation of the expression of the Na+-Cl− cotransporter under Na-depleted conditions and that the kinase is essential for optimal processing of ENaC.
- 91.Fuster DG, Bobulescu IA, Zhang J, et al. Characterization of the regulation of renal Na+/H+ exchanger NHE3 by insulin. Am J Physiol Renal Physiol. 2007;292:F577–F585. doi: 10.1152/ajprenal.00240.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang D, Zhang H, Lang F, et al. Acute activation of NHE3 by dexamethasone correlates with activation of SGK1 and requires a functional glucocorticoid receptor. Am J Physiol Cell Physiol. 2007;292:C396–C404. doi: 10.1152/ajpcell.00345.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yun CC, Chen Y, Lang F. Glucocorticoid activation of Na(+)/H(+) exchanger isoform 3 revisited. The roles of SGK1 and NHERF2. J Biol Chem. 2002;277:7676–7683. doi: 10.1074/jbc.M107768200. [DOI] [PubMed] [Google Scholar]
- 94.Grahammer F, Artunc F, Sandulache D, et al. Renal function of gene targeted mice lacking both SGK1 and SGK3. 2006. in press. [DOI] [PubMed]
- 95.Shojaiefard M, Strutz-Seebohm N, Tavare JM, et al. Regulation of the Na(+), glucose cotransporter by PIKfyve and the serum and glucocorticoid inducible kinase SGK1. Biochem Biophys Res Commun. 2007;359:843–847. doi: 10.1016/j.bbrc.2007.05.111. [DOI] [PubMed] [Google Scholar]
- 96.Palmada M, Boehmer C, Akel A, et al. SGK1 kinase upregulates GLUT1 activity and plasma membrane expression. Diabetes. 2006;55:421–47. doi: 10.2337/diabetes.55.02.06.db05-0720. [DOI] [PubMed] [Google Scholar]
- 97.Jeyaraj S, Boehmer C, Lang F, et al. Role of SGK1 kinase in regulating glucose transport via glucose transporter GLUT4. Biochem Biophys Res Commun. 2007;356:629–635. doi: 10.1016/j.bbrc.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 98.Boehmer C, Palmada M, Rajamanickam J, et al. Post-translational regulation of EAAT2 function by co-expressed ubiquitin ligase Nedd4-2 is impacted by SGK kinases. J Neurochem. 2006;97:911–921. doi: 10.1111/j.1471-4159.2006.03629.x. [DOI] [PubMed] [Google Scholar]
- 99.Rajamanickam J, Palmada M, Lang F, et al. EAAT4 phosphorylation at the SGK1 consensus site is required for transport modulation by the kinase. J Neurochem. 2007;102:858–866. doi: 10.1111/j.1471-4159.2007.04585.x. [DOI] [PubMed] [Google Scholar]
- 100.Boehmer C, Rajamanickam J, Schniepp R, et al. Regulation of the excitatory amino acid transporter EAAT5 by the serum and glucocorticoid dependent kinases SGK1 and SGK3. Biochem Biophys Res Commun. 2005;329:738–742. doi: 10.1016/j.bbrc.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 101.Boehmer C, Palmada M, Klaus F, et al. The peptide transporter PEPT2 is targeted by the protein kinase SGK1 and the scaffold protein NHERF2. Cell Physiol Biochem. 2008;22:705–714. doi: 10.1159/000185554. [DOI] [PubMed] [Google Scholar]
- 102.Shojaiefard M, Christie DL, Lang F. Stimulation of the creatine transporter SLC6A8 by the protein kinases SGK1 and SGK3. Biochem Biophys Res Commun. 2005;334:742–746. doi: 10.1016/j.bbrc.2005.06.164. [DOI] [PubMed] [Google Scholar]
- 103.Shojaiefard M, Christie DL, Lang F. Stimulation of the creatine transporter SLC6A8 by the protein kinase mTOR. Biochem Biophys Res Commun. 2006;341:945–949. doi: 10.1016/j.bbrc.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 104.Klaus F, Palmada M, Lindner R, et al. Up-regulation of hypertonicity-activated myo-inositol transporter SMIT1 by the cell volume-sensitive protein kinase SGK1. J Physiol. 2008;586:1539–1547. doi: 10.1113/jphysiol.2007.146191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Palmada M, Dieter M, Speil A, et al. Regulation of intestinal phosphate cotransporter NaPi IIb by ubiquitin ligase Nedd4-2 and by serum- and glucocorticoid-dependent kinase 1. Am J Physiol Gastrointest Liver Physiol. 2004;287:G143–G150. doi: 10.1152/ajpgi.00121.2003. [DOI] [PubMed] [Google Scholar]
- 106.Shojaiefard M, Lang F. Stimulation of the intestinal phosphate transporter SLC34A2 by the protein kinase mTOR. Biochem Biophys Res Commun. 2006;345:1611–1614. doi: 10.1016/j.bbrc.2006.05.067. [DOI] [PubMed] [Google Scholar]
- 107.Ullrich S, Zhang Y, Avram D, et al. Dexamethasone increases Na+/K+ ATPase activity in insulin secreting cells through SGK1. Biochem Biophys Res Commun. 2007;352:662–667. doi: 10.1016/j.bbrc.2006.11.065. [DOI] [PubMed] [Google Scholar]
- 108.Bhalla V, Daidie D, Li H, et al. Serum- and glucocorticoid-regulated kinase 1 regulates ubiquitin ligase neural precursor cell-expressed, developmentally down-regulated protein 4-2 by inducing interaction with 14-3-3. Mol Endocrinol. 2005;19:3073–3084. doi: 10.1210/me.2005-0193. [DOI] [PubMed] [Google Scholar]
- 109.Ichimura T, Yamamura H, Sasamoto K, et al. 14-3-3 proteins modulate the expression of epithelial Na+ channels by phosphorylation-dependent interaction with Nedd4-2 ubiquitin ligase. J Biol Chem. 2005;280:13187–13194. doi: 10.1074/jbc.M412884200. [DOI] [PubMed] [Google Scholar]
- 110.Liang X, Peters KW, Butterworth MB, et al. 14-3-3 isoforms are induced by aldosterone and participate in its regulation of epithelial sodium channels. J Biol Chem. 2006;281:16323–16332. doi: 10.1074/jbc.M601360200. [DOI] [PubMed] [Google Scholar]
- 111.Nagaki K, Yamamura H, Shimada S, et al. 14-3-3 Mediates phosphorylation-dependent inhibition of the interaction between the ubiquitin E3 ligase Nedd4-2 and epithelial Na+ channels. Biochemistry. 2006;45:6733–6740. doi: 10.1021/bi052640q. [DOI] [PubMed] [Google Scholar]
- 112.Ring AM, Leng Q, Rinehart J, et al. An SGK1 site in WNK4 regulates Na+ channel and K+ channel activity and has implications for aldosterone signaling and K+ homeostasis. Proc Natl Acad Sci U S A. 2007;104:4025–409. doi: 10.1073/pnas.0611728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang W, Xia X, Reisenauer MR, et al. Aldosterone-induced Sgk1 relieves Dot1a-Af9-mediated transcriptional repression of epithelial Na+ channel alpha. J Clin Invest. 2007;117:773–783. doi: 10.1172/JCI29850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Helms MN, Yu L, Malik B, et al. Role of SGK1 in nitric oxide inhibition of ENaC in Na+-transporting epithelia. Am J Physiol Cell Physiol. 2005;289:C717–C726. doi: 10.1152/ajpcell.00006.2005. [DOI] [PubMed] [Google Scholar]
- 115.Klaus F, Laufer J, Czarkowski K, et al. PIKfyve-dependent regulation of the Cl(−) channel ClC-2. Biochem Biophys Res Commun. 2009 doi: 10.1016/j.bbrc.2009.02.053. [DOI] [PubMed] [Google Scholar]
- 116.Strutz-Seebohm N, Shojaiefard M, Christie D, et al. PIKfyve in the SGK1 mediated regulation of the creatine transporter SLC6A8. Cell Physiol Biochem. 2007;20:729–734. doi: 10.1159/000110433. [DOI] [PubMed] [Google Scholar]
- 117.Menniti M, Iuliano R, Amato R, et al. Serum and glucocorticoid-regulated kinase Sgk1 inhibits insulin-dependent activation of phosphomannomutase 2 in transfected COS-7 cells. Am J Physiol Cell Physiol. 2005;288:C148–C155. doi: 10.1152/ajpcell.00284.2004. [DOI] [PubMed] [Google Scholar]
- 118.Wyatt AW, Hussain A, Amann K, et al. DOCA-induced phosphorylation of glycogen synthase kinase 3beta. Cell Physiol Biochem. 2006;17:137–144. doi: 10.1159/000092075. [DOI] [PubMed] [Google Scholar]
- 119.David S, Kalb RG. Serum/glucocorticoid-inducible kinase can phosphorylate the cyclic AMP response element binding protein, CREB. FEBS Lett. 2005;579:1534–1538. doi: 10.1016/j.febslet.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 120.Leroy V, De Seigneux S, Agassiz V, et al. Aldosterone activates NF-kappaB in the collecting duct. J Am Soc Nephrol. 2009;20:131–144. doi: 10.1681/ASN.2008020232. * The studies performed in both cultured cells and freshly isolated rat cortical collecting ducts indicate that aldosterone activates the canonical NF-kappaB pathway in principal cells of the cortical collecting duct by activating the mineralocorticoid receptor and by inducing SGK1.
- 121.Tai DJ, Su CC, Ma YL, et al. SGK1 Phosphorylation of I{kappa}B Kinase {alpha} and p300 Up-regulates NF-{kappa}B Activity and Increases N-Methyl-D-aspartate Receptor NR2A and NR2B Expression. J Biol Chem. 2009;284:4073–4089. doi: 10.1074/jbc.M805055200. [DOI] [PubMed] [Google Scholar]
- 122.Dehner M, Hadjihannas M, Weiske J, et al. Wnt signaling inhibits Forkhead box O3a-induced transcription and apoptosis through up-regulation of serum- and glucocorticoid-inducible kinase 1. J Biol Chem. 2008;283:19201–19210. doi: 10.1074/jbc.M710366200. [DOI] [PubMed] [Google Scholar]
- 123.Auld GC, Campbell DG, Morrice N, et al. Identification of calcium-regulated heat-stable protein of 24 kDa (CRHSP24) as a physiological substrate for PKB and RSK using KESTREL. Biochem J. 2005;389:775–783. doi: 10.1042/BJ20050733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Martel JA, Michael D, Fejes-Toth G, et al. Melanophilin, a novel aldosterone-induced gene in mouse cortical collecting duct cells. Am J Physiol Renal Physiol. 2007;293:F904–F913. doi: 10.1152/ajprenal.00365.2006. [DOI] [PubMed] [Google Scholar]
- 125.Yang YC, Lin CH, Lee EH. Serum- and glucocorticoid-inducible kinase 1 (SGK1) increases neurite formation through microtubule depolymerization by SGK1 and by SGK1 phosphorylation of tau. Mol Cell Biol. 2006;26:8357–8370. doi: 10.1128/MCB.01017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Taruno A, Niisato N, Marunaka Y. Intracellular calcium plays a role as the second messenger of hypotonic stress in gene regulation of SGK1 and ENaC in renal epithelial A6 cells. Am J Physiol Renal Physiol. 2008;294:F177–F186. doi: 10.1152/ajprenal.00250.2007. [DOI] [PubMed] [Google Scholar]
- 127.Amato R, Menniti M, Agosti V, et al. IL-2 signals through Sgk1 and inhibits proliferation and apoptosis in kidney cancer cells. J Mol Med. 2007;85:707–721. doi: 10.1007/s00109-007-0205-2. [DOI] [PubMed] [Google Scholar]
- 128.Shanmugam I, Cheng G, Terranova PF, et al. Serum/glucocorticoid-induced protein kinase-1 facilitates androgen receptor-dependent cell survival. Cell Death Differ. 2007;14:2085–2094. doi: 10.1038/sj.cdd.4402227. [DOI] [PubMed] [Google Scholar]
- 129.Friedrich B, Weyrich P, Stancakova A, et al. Variance of the SGK1 gene is associated with insulin secretion in different European populations: results from the TUEF, EUGENE2, and METSIM studies. PLoS ONE. 2008;3:e3506. doi: 10.1371/journal.pone.0003506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Boini KM, Hennige AM, Huang DY, et al. The serum and glucocorticoid inducible kinase SGK1 mediates salt sensitivity of glucose tolerance. 2006. submitted. [DOI] [PubMed]
- 131.Sandu C, Artunc F, Grahammer F, et al. Role of the serum and glucocorticoid inducible kinase SGK1 in glucocorticoid stimulation of gastric acid secretion. Pflugers Arch. 2007;455:493–503. doi: 10.1007/s00424-007-0305-4. [DOI] [PubMed] [Google Scholar]
- 132.Sandulache D, Grahammer F, Artunc F, et al. Renal Ca2+ handling in sgk1 knockout mice. Pflugers Arch. 2006;452:444–452. doi: 10.1007/s00424-005-0021-x. [DOI] [PubMed] [Google Scholar]
- 133.Artunc F, Amann K, Nasir O, et al. Blunted DOCA/high salt induced albuminuria and renal tubulointerstitial damage in gene-targeted mice lacking SGK1. J Mol Med. 2006;84:737–746. doi: 10.1007/s00109-006-0082-0. [DOI] [PubMed] [Google Scholar]
- 134.Quinkler M, Zehnder D, Eardley KS, et al. Increased expression of mineralocorticoid effector mechanisms in kidney biopsies of patients with heavy proteinuria. Circulation. 2005;112:1435–1443. doi: 10.1161/CIRCULATIONAHA.105.539122. [DOI] [PubMed] [Google Scholar]
- 135.Artunc F, Nasir O, Amann K, et al. Serum- and glucocorticoid-inducible kinase 1 in doxorubicin-induced nephrotic syndrome. Am J Physiol Renal Physiol. 2008;295:F1624–F1634. doi: 10.1152/ajprenal.00032.2008. [DOI] [PubMed] [Google Scholar]
- 136.Huang DY, Boini KM, Friedrich B, et al. Blunted hypertensive effect of combined fructose and high-salt diet in gene-targeted mice lacking functional serum- and glucocorticoid-inducible kinase SGK1. Am J Physiol Regul Integr Comp Physiol. 2006;290:R935–R944. doi: 10.1152/ajpregu.00382.2005. [DOI] [PubMed] [Google Scholar]
- 137.Huang DY, Boini KM, Osswald H, et al. Resistance of mice lacking the serum- and glucocorticoid-inducible kinase SGK1 against salt-sensitive hypertension induced by a high-fat diet. Am J Physiol Renal Physiol. 2006;291:F1264–F1273. doi: 10.1152/ajprenal.00299.2005. [DOI] [PubMed] [Google Scholar]
- 138.Busjahn A, Seebohm G, Maier G, et al. Association of the serum and glucocorticoid regulated kinase (sgk1) gene with QT interval. Cell Physiol Biochem. 2004;14:135–142. doi: 10.1159/000078105. [DOI] [PubMed] [Google Scholar]
- 139.Aoyama T, Matsui T, Novikov M, et al. Serum and glucocorticoid-responsive kinase-1 regulates cardiomyocyte survival and hypertrophic response. Circulation. 2005;111:1652–1659. doi: 10.1161/01.CIR.0000160352.58142.06. [DOI] [PubMed] [Google Scholar]
- 140.Lister K, Autelitano DJ, Jenkins A, et al. Cross talk between corticosteroids and alpha-adrenergic signalling augments cardiomyocyte hypertrophy: a possible role for SGK1. Cardiovasc Res. 2006;70:555–565. doi: 10.1016/j.cardiores.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 141.Chao CC, Ma YL, Lee EH. Protein kinase CK2 impairs spatial memory formation through differential cross talk with PI-3 kinase signaling: activation of Akt and inactivation of SGK1. J Neurosci. 2007;27:6243–6248. doi: 10.1523/JNEUROSCI.1531-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tyan SW, Tsai MC, Lin CL, et al. Serum- and glucocorticoid-inducible kinase 1 enhances zif268 expression through the mediation of SRF and CREB1 associated with spatial memory formation. J Neurochem. 2008;105:820–832. doi: 10.1111/j.1471-4159.2007.05186.x. [DOI] [PubMed] [Google Scholar]
- 143.Geranton SM, Morenilla-Palao C, Hunt SP. A role for transcriptional repressor methyl-CpG-binding protein 2 and plasticity-related gene serum- and glucocorticoid-inducible kinase 1 in the induction of inflammatory pain states. J Neurosci. 2007;27:6163–6173. doi: 10.1523/JNEUROSCI.1306-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Segditsas S, Sieber O, Deheragoda M, et al. Putative direct and indirect Wnt targets identified through consistent gene expression changes in APC-mutant intestinal adenomas from humans and mice. Hum Mol Genet. 2008;17:3864–3875. doi: 10.1093/hmg/ddn286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sherk AB, Frigo DE, Schnackenberg CG, et al. Development of a small-molecule serum- and glucocorticoid-regulated kinase-1 antagonist and its evaluation as a prostate cancer therapeutic. Cancer Res. 2008;68:7475–7483. doi: 10.1158/0008-5472.CAN-08-1047. ** To explore the utility of SGK1 as a therapeutic target, a small-molecule competitive inhibitor of this enzyme was developed, GSK650394. The study shows that the compound quantitatively blocks the effect of androgens on growth of a prostate cancer cell line.
- 146.Yoon JW, Gilbertson R, Iannaccone S, et al. Defining a role for Sonic hedgehog pathway activation in desmoplastic medulloblastoma by identifying GLI1 target genes. Int J Cancer. 2009;124:109–119. doi: 10.1002/ijc.23929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hussain A, Wyatt AW, Wang K, et al. SGK1-dependent upregulation of connective tissue growth factor by angiotensin II. Kidney Blood Press Res. 2008;31:80–86. doi: 10.1159/000119703. ** Using human kidney fibroblasts and mouse lung fibroblasts from gene-targeted mice lacking SGK1, evidence is provided that angiotensin II stimulates the expression of SGK1, which is in turn required for the stimulating effect of angiotensin II on the expression of CTGF. These studies implicate a role for SGK1 in the profibrotic effect of angiotensin II.
- 148.Nagase M, Matsui H, Shibata S, et al. Salt-induced nephropathy in obese spontaneously hypertensive rats via paradoxical activation of the mineralocorticoid receptor: role of oxidative stress. Hypertension. 2007;50:877–883. doi: 10.1161/HYPERTENSIONAHA.107.091058. [DOI] [PubMed] [Google Scholar]
- 149.Nishimura H, Ito Y, Mizuno M, et al. Mineralocorticoid receptor blockade ameliorates peritoneal fibrosis in new rat peritonitis model. Am J Physiol Renal Physiol. 2008;294:F1084–F1093. doi: 10.1152/ajprenal.00565.2007. [DOI] [PubMed] [Google Scholar]
- 150.Terada Y, Kuwana H, Kobayashi T, et al. Aldosterone-stimulated SGK1 activity mediates profibrotic signaling in the mesangium. J Am Soc Nephrol. 2008;19:298–309. doi: 10.1681/ASN.2007050531. ** Using primary cultures of rat mesangial cells and aldosterone-treatment of uninephrectomized rats, evidence is presented that aldosterone stimulates ICAM-1 and CTGF transcription via the activation of SGK1 and NF-kappaB, effects that may contribute to the progression of aldosterone-induced mesangial fibrosis and inflammation.
- 151.Loffing J, Flores SY, Staub O. Sgk kinases and their role in epithelial transport. Annu Rev Physiol. 2006;68:461–490. doi: 10.1146/annurev.physiol.68.040104.131654. [DOI] [PubMed] [Google Scholar]
- 152.Stockand JD. Preserving salt: in vivo studies with Sgk1-deficient mice define a modern role for this ancient protein. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1–R3. doi: 10.1152/ajpregu.00659.2004. [DOI] [PubMed] [Google Scholar]
- 153.Vallon V, Lang F. New insights into the role of serum- and glucocorticoid-inducible kinase SGK1 in the regulation of renal function and blood pressure. Curr Opin Nephrol Hypertens. 2005;14:59–66. doi: 10.1097/00041552-200501000-00010. [DOI] [PubMed] [Google Scholar]
- 154.Vallon V, Wulff P, Huang DY, et al. Role of Sgk1 in salt and potassium homeostasis. Am J Physiol Regul Integr Comp Physiol. 2005;288:R4–10. doi: 10.1152/ajpregu.00369.2004. [DOI] [PubMed] [Google Scholar]
- 155.Gonzalez-Rodriguez E, Gaeggeler HP, Rossier BC. IGF-1 vs insulin: respective roles in modulating sodium transport via the PI-3 kinase/Sgk1 pathway in a cortical collecting duct cell line. Kidney Int. 2007;71:116–125. doi: 10.1038/sj.ki.5002018. [DOI] [PubMed] [Google Scholar]
- 156.Arteaga MF, Canessa CM. Functional specificity of Sgk1 and Akt1 on ENaC activity. Am J Physiol Renal Physiol. 2005;289:F90–F96. doi: 10.1152/ajprenal.00390.2004. [DOI] [PubMed] [Google Scholar]
- 157.Rexhepaj R, Artunc F, Grahammer F, et al. SGK1 is not required for regulation of colonic ENaC activity. Pflugers Arch. 2006;453:97–105. doi: 10.1007/s00424-006-0111-4. [DOI] [PubMed] [Google Scholar]
- 158.Artunc F, Ebrahim A, Siraskar B, et al. Responses to diuretic treatment in gene-targeted mice lacking serum- and glucocorticoid-inducible kinase 1 (SGK1) Kidney & Blood Press Res. 2009 doi: 10.1159/000214439. in press. [DOI] [PubMed] [Google Scholar]
- 159.Grahammer F, Henke G, Sandu C, et al. Intestinal function of gene targeted mice lacking the Serum and Glucocorticoid inducible kinase SGK1. 2006. submitted. [DOI] [PubMed]
- 160.Boini KM, Graf D, Kuhl D, et al. SGK1 dependence of insulin induced hypokalemia. Pflugers Arch. 2009;457:955–961. doi: 10.1007/s00424-008-0559-5. [DOI] [PubMed] [Google Scholar]
- 161.Stier CT, Jr., Itskovitz HD. Renal calcium metabolism and diuretics. Annu Rev Pharmacol Toxicol. 1986;26:101–116. doi: 10.1146/annurev.pa.26.040186.000533. [DOI] [PubMed] [Google Scholar]
- 162.Nijenhuis T, Vallon V, van der Kemp AW, et al. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest. 2005;115:1651–1658. doi: 10.1172/JCI24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ackermann TF, Boini KM, Volkl H, et al. SGK1-sensitive renal tubular glucose reabsorption in diabetes. Am J Physiol Renal Physiol. 2009 doi: 10.1152/ajprenal.90238.2008. ** This study used crossbreeding of diabetic Akita mice with gene-targeted mice lacking SGK1 to provide the first evidence that SGK1 participates in the stimulation of renal tubular glucose transport in diabetic kidneys.
- 164.Vallon V, Grahammer F, Volkl H, et al. KCNQ1-dependent transport in renal and gastrointestinal epithelia. Proc Natl Acad Sci U S A. 2005;102:17864–17869. doi: 10.1073/pnas.0505860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nagase M, Fujita T. Aldosterone and glomerular podocyte injury. Clin Exp Nephrol. 2008;12:233–242. doi: 10.1007/s10157-008-0034-9. [DOI] [PubMed] [Google Scholar]
- 166.Artunc F, Sandulache D, Nasir O, et al. Lack of the serum and glucocorticoid-inducible kinase SGK1 attenuates the volume retention after treatment with the PPARgamma agonist pioglitazone. Pflugers Arch. 2008;456:425–436. doi: 10.1007/s00424-007-0401-5. [DOI] [PubMed] [Google Scholar]
- 167.Ackermann D, Mordasini D, Cheval L, et al. Sodium retention and ascites formation in a cholestatic mice model: role of aldosterone and mineralocorticoid receptor? Hepatology. 2007;46:173–179. doi: 10.1002/hep.21699. [DOI] [PubMed] [Google Scholar]
- 168.Vallon V, Huang DY, Grahammer F, et al. SGK1 as a determinant of kidney function and salt intake in response to mineralocorticoid excess. Am J Physiol Regul Integr Comp Physiol. 2005;289:R395–R401. doi: 10.1152/ajpregu.00731.2004. [DOI] [PubMed] [Google Scholar]
- 169.von Wowern F, Berglund G, Carlson J, et al. Genetic variance of SGK-1 is associated with blood pressure, blood pressure change over time and strength of the insulin-diastolic blood pressure relationship. Kidney Int. 2005;68:2164–2172. doi: 10.1111/j.1523-1755.2005.00672.x. [DOI] [PubMed] [Google Scholar]
- 170.Boini KM, Nammi S, Grahammer F, et al. Role of serum- and glucocorticoid-inducible kinase SGK1 in glucocorticoid regulation of renal electrolyte excretion and blood pressure. Kidney Blood Press Res. 2008;31:280–289. doi: 10.1159/000151666. [DOI] [PubMed] [Google Scholar]
- 171.Shi PP, Cao XR, Sweezer EM, et al. Salt-sensitive hypertension and cardiac hypertrophy in mice deficient in the ubiquitin ligase Nedd4-2. Am J Physiol Renal Physiol. 2008;295:F462–F470. doi: 10.1152/ajprenal.90300.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rexhepaj R, Boini KM, Huang DY, et al. Role of maternal glucocorticoid inducible kinase SGK1 in fetal programming of blood pressure in response to prenatal diet. Am J Physiol Regul Integr Comp Physiol. 2008;294:R2008–R2013. doi: 10.1152/ajpregu.00737.2007. * In this study mice were mated with either the male or female being a SGK1 knockout mouse, resulting in both cases in heterozygotic offspring. First evidence is provided that maternal signals mediated by SGK1 may play a decisive role in fetal programming of hypertension induced by prenatal protein restriction.
- 173.Schwab M, Lupescu A, Mota M, et al. Association of SGK1 gene polymorphisms with type 2 diabetes. Cell Physiol Biochem. 2008;21:151–160. doi: 10.1159/000113757. [DOI] [PubMed] [Google Scholar]