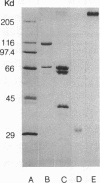
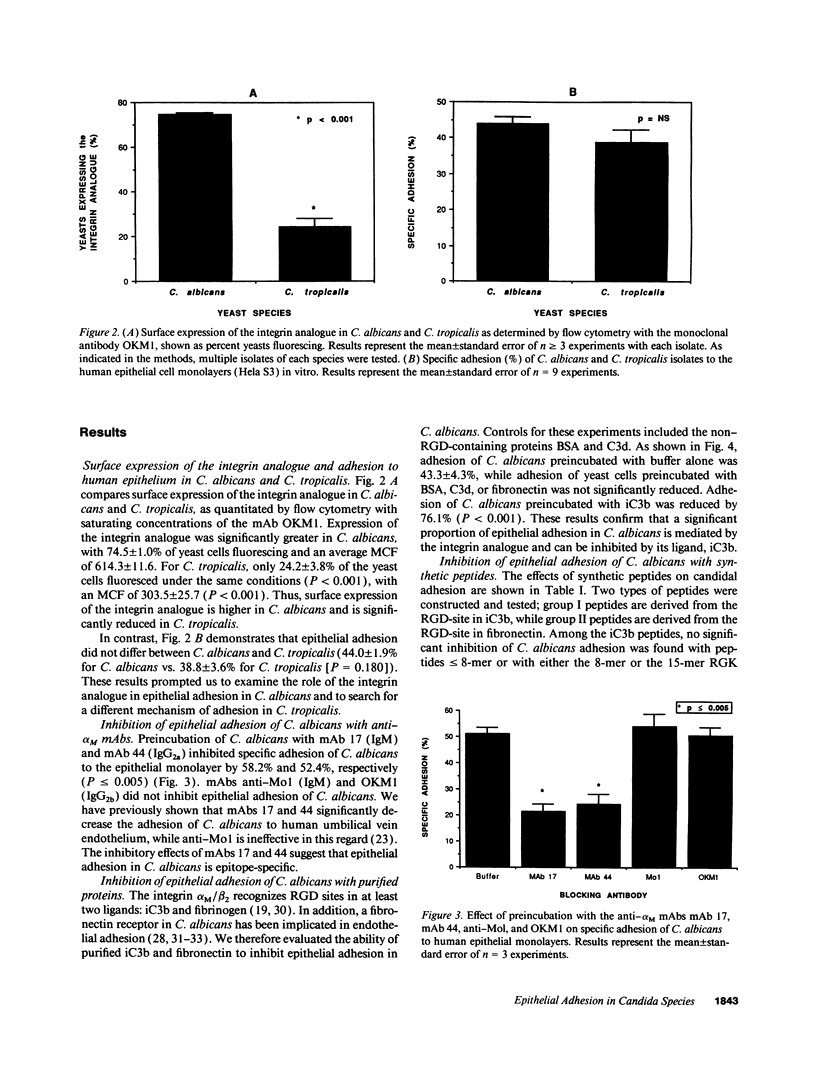
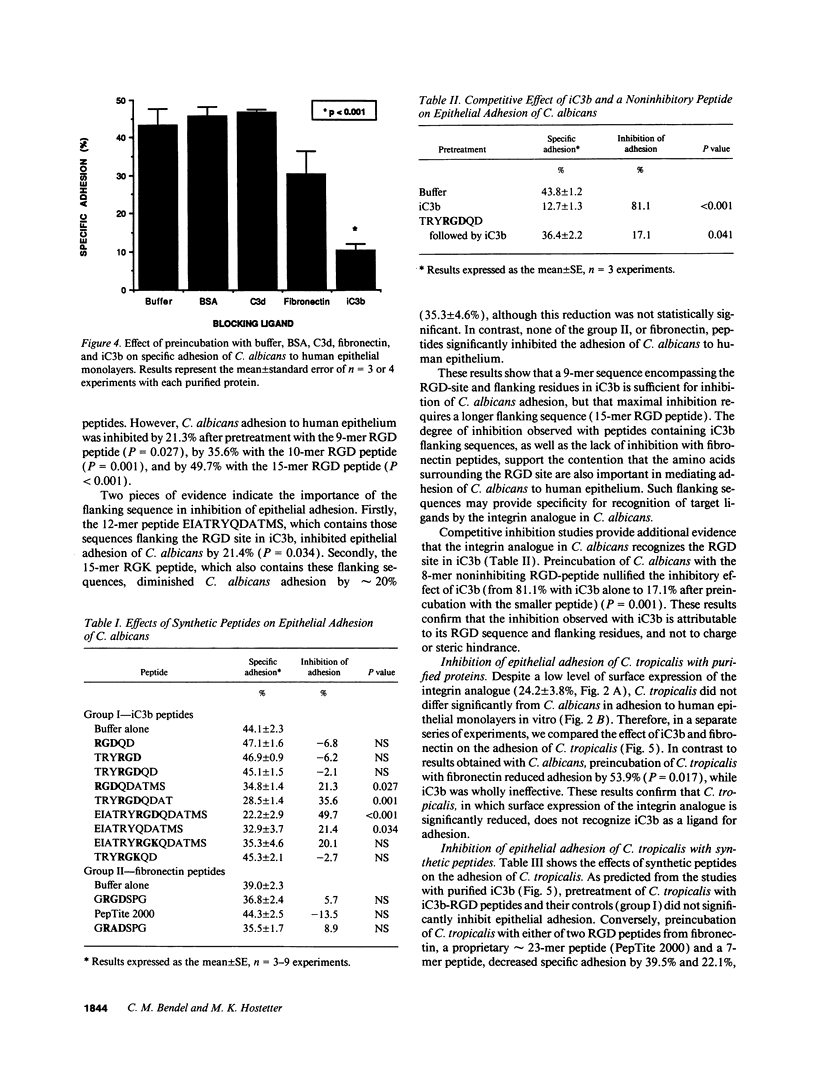
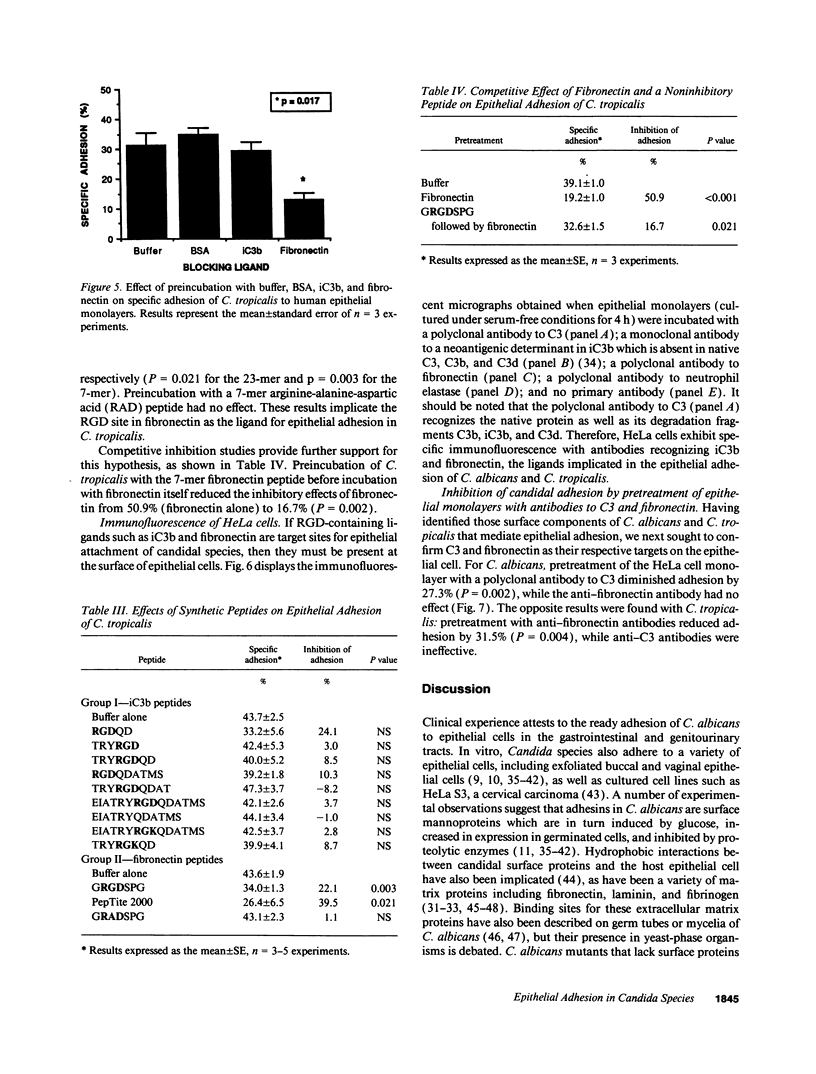
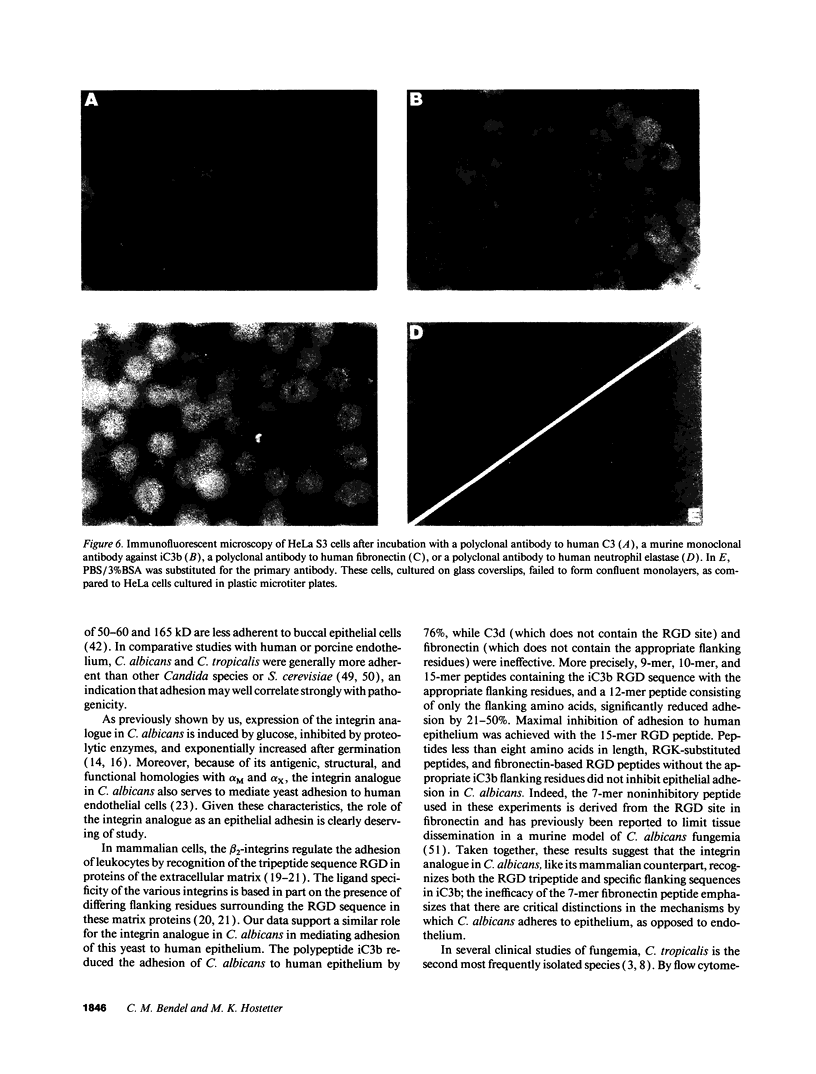
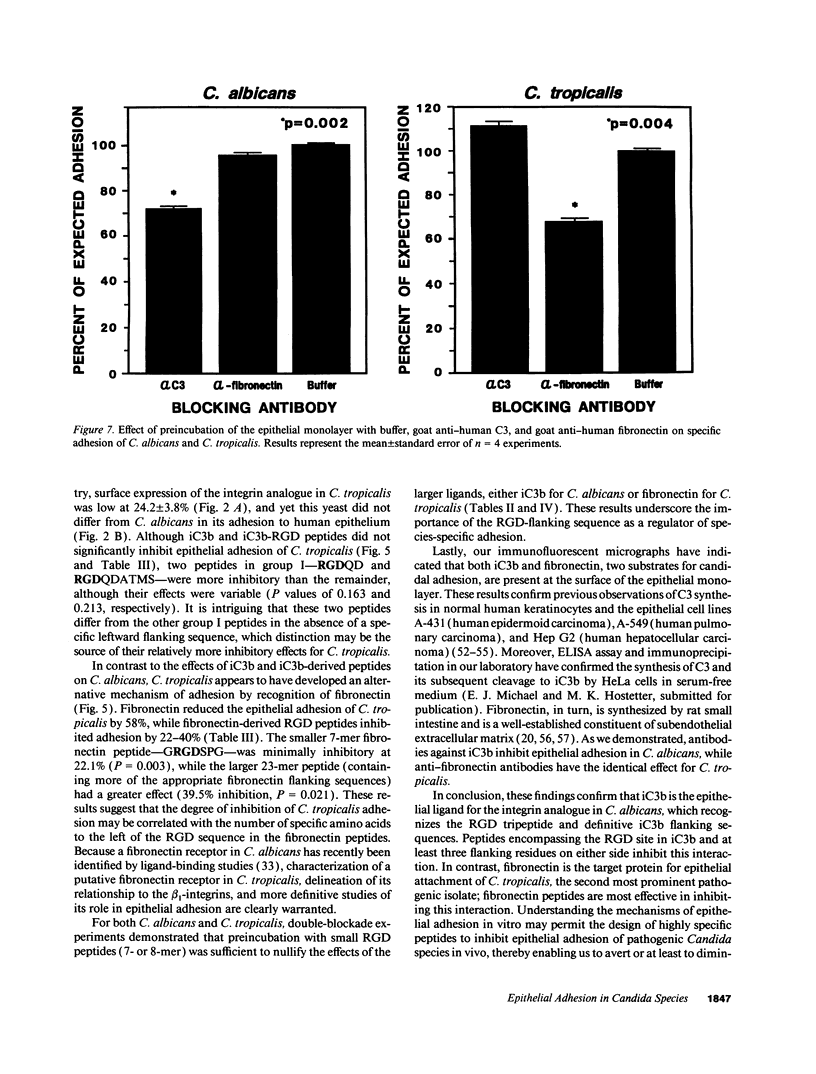
Abstract
The yeast Candida albicans is the leading cause of disseminated fungal infection in neonates, immunocompromised hosts, diabetics, and postoperative patients; Candida tropicalis is the second most frequent isolate. Because the integrin analogue in C. albicans shares antigenic, structural, and functional homologies with the beta 2-integrin subunits alpha M and alpha X, we investigated the role of integrin analogues in epithelial adhesion of C. albicans and C. tropicalis. On flow cytometry with the monoclonal antibody (mAb) OKM1, surface fluorescence was highest for C. albicans and significantly reduced for C. tropicalis (P < 0.001). However, adhesion to the human epithelial cell line HeLa S3 did not differ for these two candidal species: specific adhesion was highest for C. albicans at 44.0 +/- 1.8%, and only slightly lower for C. tropicalis at 38.8 +/- 3.6% (P = NS). The disparity between expression of the integrin analogue and epithelial adhesion suggested distinct mechanisms for this process in C. albicans versus C. tropicalis. Preincubation of C. albicans with anti-alpha M mAbs, with purified iC3b (the RGD ligand for the integrin analogue), or with 9-15-mer RGD peptides from iC3b all inhibited epithelial adhesion significantly (P < 0.001-0.04). Purified fibronectin or fibronectin-RGD peptides failed to block C. albicans adhesion. In contrast, epithelial adhesion of C. tropicalis was significantly inhibited by purified fibronectin and its RGD peptides (P < or = 0.021), but not by iC3b nor the iC3b-RGD peptides. Both iC3b and fibronectin were identified on the surface of epithelial cells after growth in serum-free medium. A polyclonal antibody to C3 inhibited C. albicans adhesion while a control antibody to fibronectin was ineffective; the converse was true for C. tropicalis. These results indicate that the pathogenic yeasts C. albicans and C. tropicalis recognize distinct RGD ligands present at the surface of the epithelial cell and that these interactions can be differentially inhibited by defined RGD peptides containing appropriate flanking sequences.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basset-Séguin N., Caughman S. W., Yancey K. B. A-431 cells and human keratinocytes synthesize and secrete the third component of complement. J Invest Dermatol. 1990 Dec;95(6):621–625. doi: 10.1111/1523-1747.ep12513524. [DOI] [PubMed] [Google Scholar]
- Bouali A., Robert R., Tronchin G., Senet J. M. Characterization of binding of human fibrinogen to the surface of germ-tubes and mycelium of candida albicans. J Gen Microbiol. 1987 Mar;133(3):545–551. doi: 10.1099/00221287-133-3-545. [DOI] [PubMed] [Google Scholar]
- Bouchara J. P., Tronchin G., Annaix V., Robert R., Senet J. M. Laminin receptors on Candida albicans germ tubes. Infect Immun. 1990 Jan;58(1):48–54. doi: 10.1128/iai.58.1.48-54.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler K. M., Baker C. J. Candida: an increasingly important pathogen in the nursery. Pediatr Clin North Am. 1988 Jun;35(3):543–563. doi: 10.1016/s0031-3955(16)36471-9. [DOI] [PubMed] [Google Scholar]
- Calderone R. A., Scheld W. M. Role of fibronectin in the pathogenesis of candidal infections. Rev Infect Dis. 1987 Jul-Aug;9 (Suppl 4):S400–S403. doi: 10.1093/clinids/9.supplement_4.s400. [DOI] [PubMed] [Google Scholar]
- Cooper E. R., Pelton S. I., LeMay M. Acquired immunodeficiency syndrome: a new population of children at risk. Pediatr Clin North Am. 1988 Dec;35(6):1365–1387. doi: 10.1016/s0031-3955(16)36589-0. [DOI] [PubMed] [Google Scholar]
- Dana N., Styrt B., Griffin J. D., Todd R. F., 3rd, Klempner M. S., Arnaout M. A. Two functional domains in the phagocyte membrane glycoprotein Mo1 identified with monoclonal antibodies. J Immunol. 1986 Nov 15;137(10):3259–3263. [PubMed] [Google Scholar]
- Edwards J. E., Jr, Gaither T. A., O'Shea J. J., Rotrosen D., Lawley T. J., Wright S. A., Frank M. M., Green I. Expression of specific binding sites on Candida with functional and antigenic characteristics of human complement receptors. J Immunol. 1986 Dec 1;137(11):3577–3583. [PubMed] [Google Scholar]
- Eigentler A., Schulz T. F., Larcher C., Breitwieser E. M., Myones B. L., Petzer A. L., Dierich M. P. C3bi-binding protein on Candida albicans: temperature-dependent expression and relationship to human complement receptor type 3. Infect Immun. 1989 Feb;57(2):616–622. doi: 10.1128/iai.57.2.616-622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng R. H., Drehmel R., Smith S. M., Goldstein E. J. Saccharomyces cerevisiae infections in man. Sabouraudia. 1984;22(5):403–407. [PubMed] [Google Scholar]
- Fukayama M., Calderone R. A. Adherence of cell surface mutants of Candida albicans to buccal epithelial cells and analyses of the cell surface proteins of the mutants. Infect Immun. 1991 Apr;59(4):1341–1345. doi: 10.1128/iai.59.4.1341-1345.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore B. J., Retsinas E. M., Lorenz J. S., Hostetter M. K. An iC3b receptor on Candida albicans: structure, function, and correlates for pathogenicity. J Infect Dis. 1988 Jan;157(1):38–46. doi: 10.1093/infdis/157.1.38. [DOI] [PubMed] [Google Scholar]
- Gordon D. L., Johnson G. M., Hostetter M. K. Characteristics of iC3b binding to human polymorphonuclear leucocytes. Immunology. 1987 Apr;60(4):553–558. [PMC free article] [PubMed] [Google Scholar]
- Gordon D. L., Johnson G. M., Hostetter M. K. Ligand-receptor interactions in the phagocytosis of virulent Streptococcus pneumoniae by polymorphonuclear leukocytes. J Infect Dis. 1986 Oct;154(4):619–626. doi: 10.1093/infdis/154.4.619. [DOI] [PubMed] [Google Scholar]
- Gustafson K. S., Vercellotti G. M., Bendel C. M., Hostetter M. K. Molecular mimicry in Candida albicans. Role of an integrin analogue in adhesion of the yeast to human endothelium. J Clin Invest. 1991 Jun;87(6):1896–1902. doi: 10.1172/JCI115214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich F., Dierich M. P. Candida albicans and Candida stellatoidea, in contrast to other Candida species, bind iC3b and C3d but not C3b. Infect Immun. 1985 Nov;50(2):598–600. doi: 10.1128/iai.50.2.598-600.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Wong B., Kiehn T. E., Armstrong D. Fungemia in a cancer hospital: changing frequency, earlier onset, and results of therapy. Rev Infect Dis. 1985 Sep-Oct;7(5):646–655. doi: 10.1093/clinids/7.5.646. [DOI] [PubMed] [Google Scholar]
- Hostetter M. K., Lorenz J. S., Preus L., Kendrick K. E. The iC3b receptor on Candida albicans: subcellular localization and modulation of receptor expression by glucose. J Infect Dis. 1990 Apr;161(4):761–768. doi: 10.1093/infdis/161.4.761. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Kiehn T. E., Edwards F. F., Armstrong D. The prevalence of yeasts in clinical specimens from cancer patients. Am J Clin Pathol. 1980 Apr;73(4):518–521. doi: 10.1093/ajcp/73.4.518. [DOI] [PubMed] [Google Scholar]
- Kimura L. H., Pearsall N. N. Relationship between germination of Candida albicans and increased adherence to human buccal epithelial cells. Infect Immun. 1980 May;28(2):464–468. doi: 10.1128/iai.28.2.464-468.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. D., Lee J. C., Morris A. L. Adherence of Candida albicans and other Candida species to mucosal epithelial cells. Infect Immun. 1980 Feb;27(2):667–674. doi: 10.1128/iai.27.2.667-674.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz S. A., Drutz D. J., Harrison J. L., Huppert M. Adherence and penetration of vascular endothelium by Candida yeasts. Infect Immun. 1983 Oct;42(1):374–384. doi: 10.1128/iai.42.1.374-384.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz S. A., Maca R. D. Endothelial cell contraction increases Candida adherence to exposed extracellular matrix. Infect Immun. 1988 Sep;56(9):2495–2498. doi: 10.1128/iai.56.9.2495-2498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz S. A., Smith R. L. A fibronectin receptor on Candida albicans mediates adherence of the fungus to extracellular matrix. J Infect Dis. 1991 Mar;163(3):604–610. doi: 10.1093/infdis/163.3.604. [DOI] [PubMed] [Google Scholar]
- Lee J. C., King R. D. Characterization of Candida albicans adherence to human vaginal epithelial cells in vitro. Infect Immun. 1983 Sep;41(3):1024–1030. doi: 10.1128/iai.41.3.1024-1030.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S. K., Van Seventer G. A., Levin S. M., Wright S. D. Two leukocyte receptors (CD11a/CD18 and CD11b/CD18) mediate transient adhesion to endothelium by binding to different ligands. J Immunol. 1989 Nov 15;143(10):3325–3329. [PubMed] [Google Scholar]
- Marsh P. K., Tally F. P., Kellum J., Callow A., Gorbach S. L. Candida infections in surgical patients. Ann Surg. 1983 Jul;198(1):42–47. doi: 10.1097/00000658-198307000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCourtie J., Douglas L. J. Relationship between cell surface composition, adherence, and virulence of Candida albicans. Infect Immun. 1984 Jul;45(1):6–12. doi: 10.1128/iai.45.1.6-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier-Carpentier F., Kiehn T. E., Armstrong D. Fungemia in the immunocompromised host. Changing patterns, antigenemia, high mortality. Am J Med. 1981 Sep;71(3):363–370. doi: 10.1016/0002-9343(81)90162-5. [DOI] [PubMed] [Google Scholar]
- Morris K. M., Goldberger G., Colten H. R., Aden D. P., Knowles B. B. Biosynthesis and processing of a human precursor complement protein, pro-C3, in a hepatoma-derived cell line. Science. 1982 Jan 22;215(4531):399–400. doi: 10.1126/science.7199205. [DOI] [PubMed] [Google Scholar]
- Quaroni A., Isselbacher K. J., Ruoslahti E. Fibronectin synthesis by epithelial crypt cells of rat small intestine. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5548–5552. doi: 10.1073/pnas.75.11.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayfield E. J., Ault M. J., Keusch G. T., Brothers M. J., Nechemias C., Smith H. Infection and diabetes: the case for glucose control. Am J Med. 1982 Mar;72(3):439–450. doi: 10.1016/0002-9343(82)90511-3. [DOI] [PubMed] [Google Scholar]
- Regnault V., Rivat C., Stoltz J. F. Affinity purification of human plasma fibronectin on immobilized gelatin. J Chromatogr. 1988 Nov 18;432:93–102. doi: 10.1016/s0378-4347(00)80636-2. [DOI] [PubMed] [Google Scholar]
- Rotrosen D., Edwards J. E., Jr, Gibson T. R., Moore J. C., Cohen A. H., Green I. Adherence of Candida to cultured vascular endothelial cells: mechanisms of attachment and endothelial cell penetration. J Infect Dis. 1985 Dec;152(6):1264–1274. doi: 10.1093/infdis/152.6.1264. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E., Hayman E. G. Fibronectin: current concepts of its structure and functions. Coll Relat Res. 1981;1(1):95–128. doi: 10.1016/s0174-173x(80)80011-2. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- SICARD A. M. A NEW SYNTHETIC MEDIUM FOR DIPLOCOCCUS PNEUMONIAE, AND ITS USE FOR THE STUDY OF RECIPROCAL TRANSFORMATIONS AT THE AMIA LOCUS. Genetics. 1964 Jul;50:31–44. doi: 10.1093/genetics/50.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranayake L. P., MacFarlane T. W. The adhesion of the yeast Candida albicans to epithelial cells of human origin in vitro. Arch Oral Biol. 1981;26(10):815–820. doi: 10.1016/0003-9969(81)90178-3. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin R. L., Rogers A. L., Patterson R. J., Beneke E. S. Evidence for mannose-mediated adherence of Candida albicans to human buccal cells in vitro. Infect Immun. 1982 Jan;35(1):79–85. doi: 10.1128/iai.35.1.79-85.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R. T., Garner R. E., Hudson J. A. Arg-Gly-Asp (RGD) peptides alter hepatic killing of Candida albicans in the isolated perfused mouse liver model. Infect Immun. 1992 Jan;60(1):213–218. doi: 10.1128/iai.60.1.213-218.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E., Lehrer N., Ofek I. Adherence of Candida albicans to human vaginal epithelial cells: inhibition by amino sugars. Exp Cell Biol. 1982;50(1):13–17. doi: 10.1159/000163121. [DOI] [PubMed] [Google Scholar]
- Skerl K. G., Calderone R. A., Segal E., Sreevalsan T., Scheld W. M. In vitro binding of Candida albicans yeast cells to human fibronectin. Can J Microbiol. 1984 Feb;30(2):221–227. doi: 10.1139/m84-033. [DOI] [PubMed] [Google Scholar]
- Sobel J. D., Myers P. G., Kaye D., Levison M. E. Adherence of Candida albicans to human vaginal and buccal epithelial cells. J Infect Dis. 1981 Jan;143(1):76–82. doi: 10.1093/infdis/143.1.76. [DOI] [PubMed] [Google Scholar]
- Strunk R. C., Eidlen D. M., Mason R. J. Pulmonary alveolar type II epithelial cells synthesize and secrete proteins of the classical and alternative complement pathways. J Clin Invest. 1988 May;81(5):1419–1426. doi: 10.1172/JCI113472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellotti G. M., Platt J. L., Bach F. H., Dalmasso A. P. Neutrophil adhesion to xenogeneic endothelium via iC3b. J Immunol. 1991 Jan 15;146(2):730–734. [PubMed] [Google Scholar]
- Wright S. D., Reddy P. A., Jong M. T., Erickson B. W. C3bi receptor (complement receptor type 3) recognizes a region of complement protein C3 containing the sequence Arg-Gly-Asp. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1965–1968. doi: 10.1073/pnas.84.7.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Weitz J. I., Huang A. J., Levin S. M., Silverstein S. C., Loike J. D. Complement receptor type three (CD11b/CD18) of human polymorphonuclear leukocytes recognizes fibrinogen. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7734–7738. doi: 10.1073/pnas.85.20.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zach T. L., Hill L. D., Herrman V. A., Leuschen M. P., Hostetter M. K. Effect of glucocorticoids on C3 gene expression by the A549 human pulmonary epithelial cell line. J Immunol. 1992 Jun 15;148(12):3964–3969. [PubMed] [Google Scholar]