Abstract
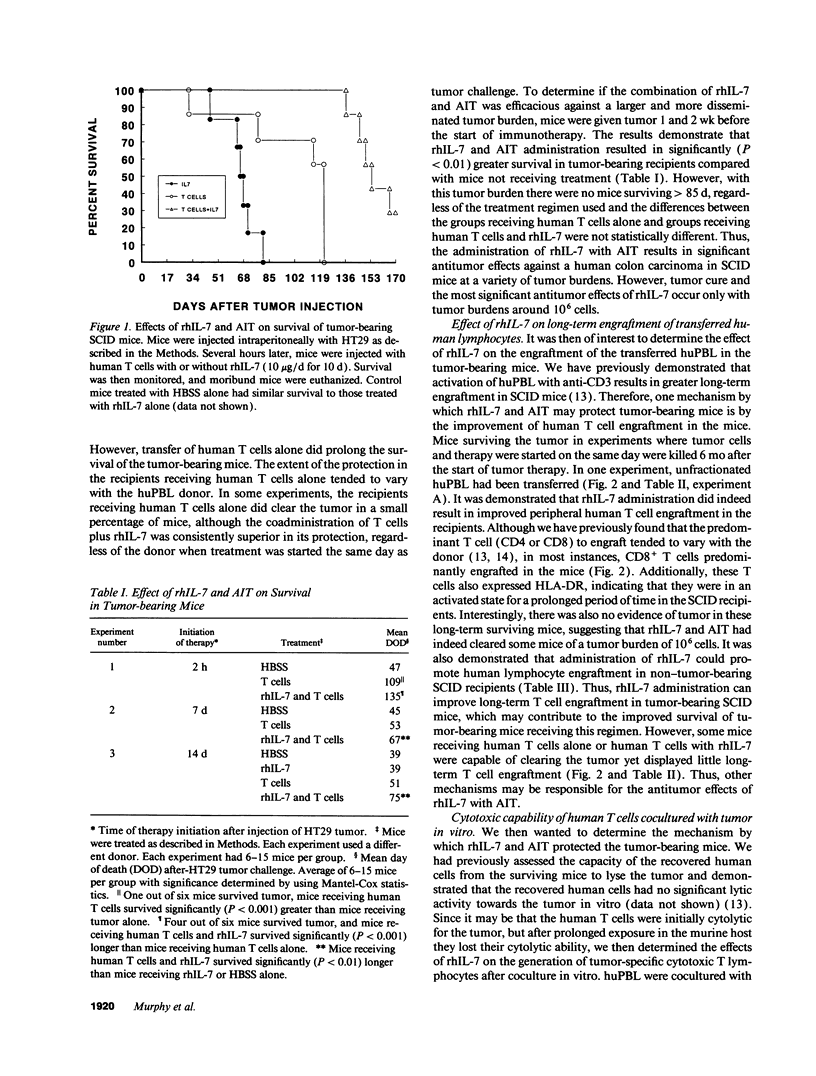
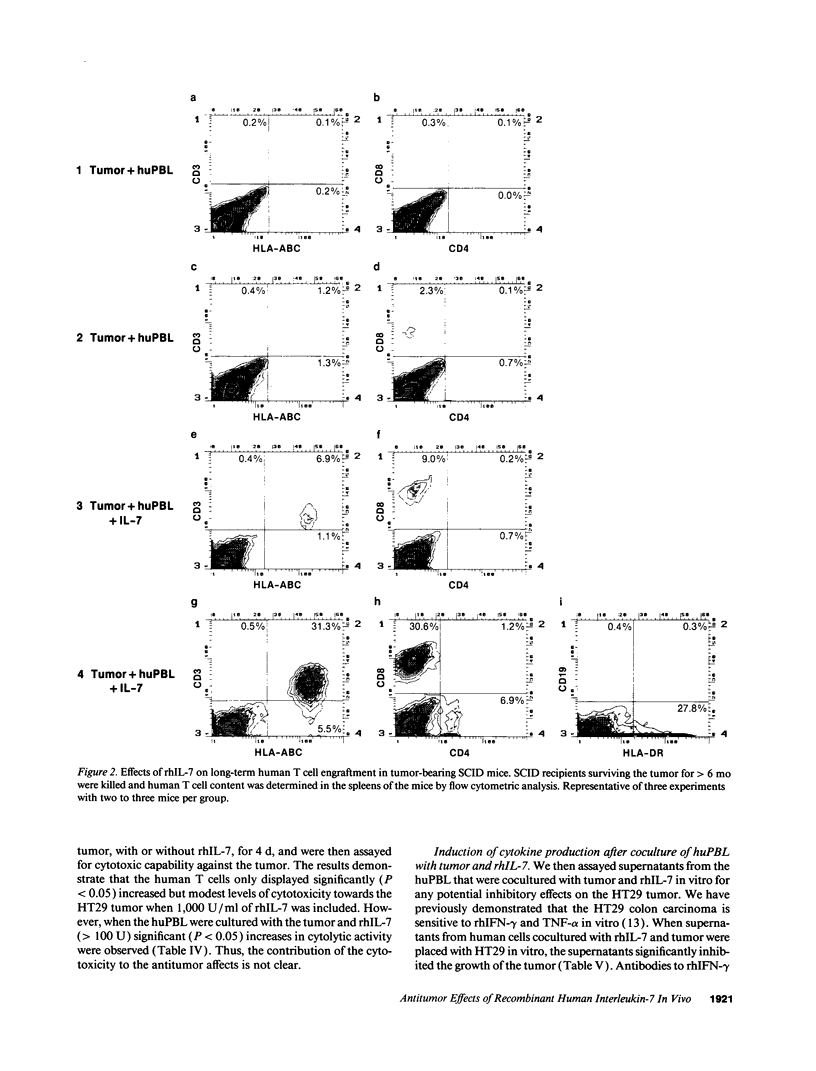
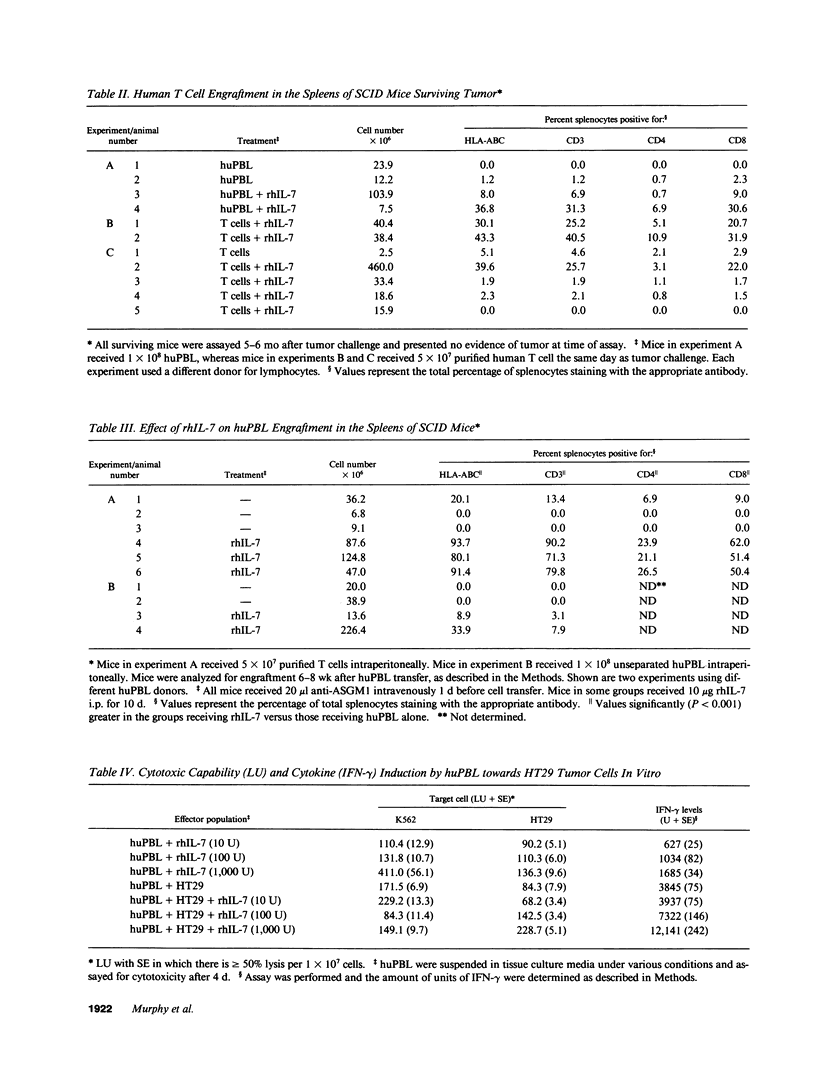
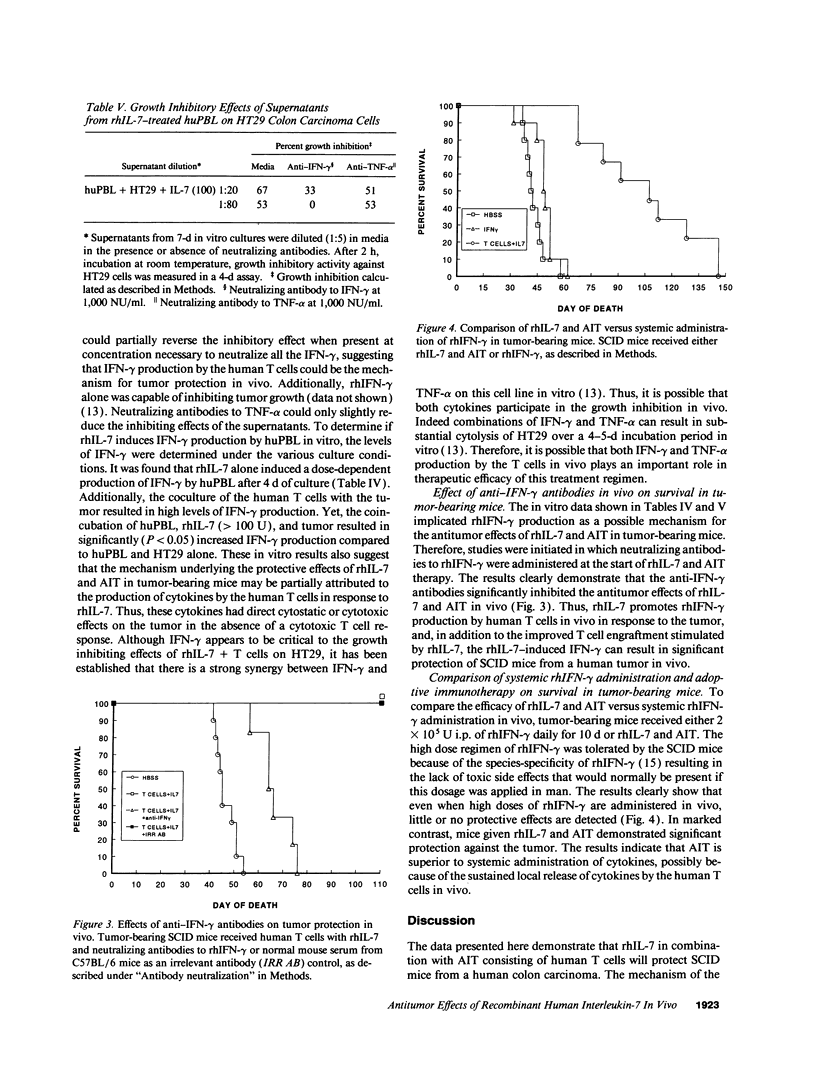
The antitumor properties of recombinant human IL-7 (rhIL-7) on a human tumor was evaluated by engrafting a human colon carcinoma into immunodeficient mice and then treating the mice with rhIL-7 and adoptively transferred human peripheral blood T cells. It was found that rhIL-7 alone had no effect on the survival of the tumor-bearing recipients. However, the combination of rhIL-7 and human T cells significantly promoted the survival of the recipients compared with mice receiving either treatment by itself. When the surviving mice were analyzed 6 mo later for the degree of human cell engraftment, the recipients receiving both rhIL-7 and human T cells had greater numbers of human CD8+ T cells in the spleens. However, the human T cells recovered from the surviving mice showed low lytic activity against the tumor in vitro. Supernatants from human T cells cultured with the tumor and rhIL-7 in vitro were found to inhibit tumor growth and were demonstrated to contain high levels of IFN-gamma. Antibodies to IFN-gamma neutralized the growth inhibition of the tumor both in vitro and in vivo demonstrating that the in vivo mechanism underlying the antitumor effects of this regimen was partly dependent on the production of IFN-gamma by the T cells and not their cytolytic capability. Interestingly, systemic administration of rhIFN-gamma to tumor-bearing mice yielded little antitumor effect suggesting that adoptive immunotherapy with rhIL-7 was superior possibly because of the continuous local release of the cytokines. Therefore, rhIL-7 may be of clinical use as an antineoplastic agent and the human/mouse model is a potentially important preclinical model for in vivo evaluation of the efficacy of this and other immunotherapies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderson M. R., Sassenfeld H. M., Widmer M. B. Interleukin 7 enhances cytolytic T lymphocyte generation and induces lymphokine-activated killer cells from human peripheral blood. J Exp Med. 1990 Aug 1;172(2):577–587. doi: 10.1084/jem.172.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth R. J., Jr, Mulé J. J., Spiess P. J., Rosenberg S. A. Interferon gamma and tumor necrosis factor have a role in tumor regressions mediated by murine CD8+ tumor-infiltrating lymphocytes. J Exp Med. 1991 Mar 1;173(3):647–658. doi: 10.1084/jem.173.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon P. J., Morrissey P. J., Nordan R. P., Grabstein K. H., Prickett K. S., Reed S. G., Goodwin R., Cosman D., Namen A. E. Murine thymocytes proliferate in direct response to interleukin-7. Blood. 1989 Sep;74(4):1368–1373. [PubMed] [Google Scholar]
- Goodwin R. G., Lupton S., Schmierer A., Hjerrild K. J., Jerzy R., Clevenger W., Gillis S., Cosman D., Namen A. E. Human interleukin 7: molecular cloning and growth factor activity on human and murine B-lineage cells. Proc Natl Acad Sci U S A. 1989 Jan;86(1):302–306. doi: 10.1073/pnas.86.1.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabstein K. H., Namen A. E., Shanebeck K., Voice R. F., Reed S. G., Widmer M. B. Regulation of T cell proliferation by IL-7. J Immunol. 1990 Apr 15;144(8):3015–3020. [PubMed] [Google Scholar]
- Hock H., Dorsch M., Diamantstein T., Blankenstein T. Interleukin 7 induces CD4+ T cell-dependent tumor rejection. J Exp Med. 1991 Dec 1;174(6):1291–1298. doi: 10.1084/jem.174.6.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jicha D. L., Mulé J. J., Rosenberg S. A. Interleukin 7 generates antitumor cytotoxic T lymphocytes against murine sarcomas with efficacy in cellular adoptive immunotherapy. J Exp Med. 1991 Dec 1;174(6):1511–1515. doi: 10.1084/jem.174.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D. H., Namen A. E., Miller R. E. In vivo evaluation of the effects of interleukins 2, 4 and 7 on enhancing the immunotherapeutic efficacy of anti-tumor cytotoxic T lymphocytes. Eur J Immunol. 1991 Dec;21(12):2977–2985. doi: 10.1002/eji.1830211212. [DOI] [PubMed] [Google Scholar]
- McBride W. H., Thacker J. D., Comora S., Economou J. S., Kelley D., Hogge D., Dubinett S. M., Dougherty G. J. Genetic modification of a murine fibrosarcoma to produce interleukin 7 stimulates host cell infiltration and tumor immunity. Cancer Res. 1992 Jul 15;52(14):3931–3937. [PubMed] [Google Scholar]
- McCune J. M., Namikawa R., Kaneshima H., Shultz L. D., Lieberman M., Weissman I. L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988 Sep 23;241(4873):1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- Morrissey P. J., Goodwin R. G., Nordan R. P., Anderson D., Grabstein K. H., Cosman D., Sims J., Lupton S., Acres B., Reed S. G. Recombinant interleukin 7, pre-B cell growth factor, has costimulatory activity on purified mature T cells. J Exp Med. 1989 Mar 1;169(3):707–716. doi: 10.1084/jem.169.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., Baird S. M., Wilson D. B. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988 Sep 15;335(6187):256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- Murphy W. J., Bennett M., Anver M. R., Baseler M., Longo D. L. Human-mouse lymphoid chimeras: host-vs.-graft and graft-vs.-host reactions. Eur J Immunol. 1992 Jun;22(6):1421–1427. doi: 10.1002/eji.1830220614. [DOI] [PubMed] [Google Scholar]
- Murphy W. J., Conlon K. C., Sayers T. J., Wiltrout R. H., Back T. C., Ortaldo J. R., Longo D. L. Engraftment and activity of anti-CD3-activated human peripheral blood lymphocytes transferred into mice with severe combined immune deficiency. J Immunol. 1993 Apr 15;150(8 Pt 1):3634–3642. [PubMed] [Google Scholar]
- Namen A. E., Lupton S., Hjerrild K., Wignall J., Mochizuki D. Y., Schmierer A., Mosley B., March C. J., Urdal D., Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988 Jun 9;333(6173):571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- Rabinowich H., Vitolo D., Altarac S., Herberman R. B., Whiteside T. L. Role of cytokines in the adoptive immunotherapy of an experimental model of human head and neck cancer by human IL-2-activated natural killer cells. J Immunol. 1992 Jul 1;149(1):340–349. [PubMed] [Google Scholar]
- Welch P. A., Namen A. E., Goodwin R. G., Armitage R., Cooper M. D. Human IL-7: a novel T cell growth factor. J Immunol. 1989 Dec 1;143(11):3562–3567. [PubMed] [Google Scholar]



