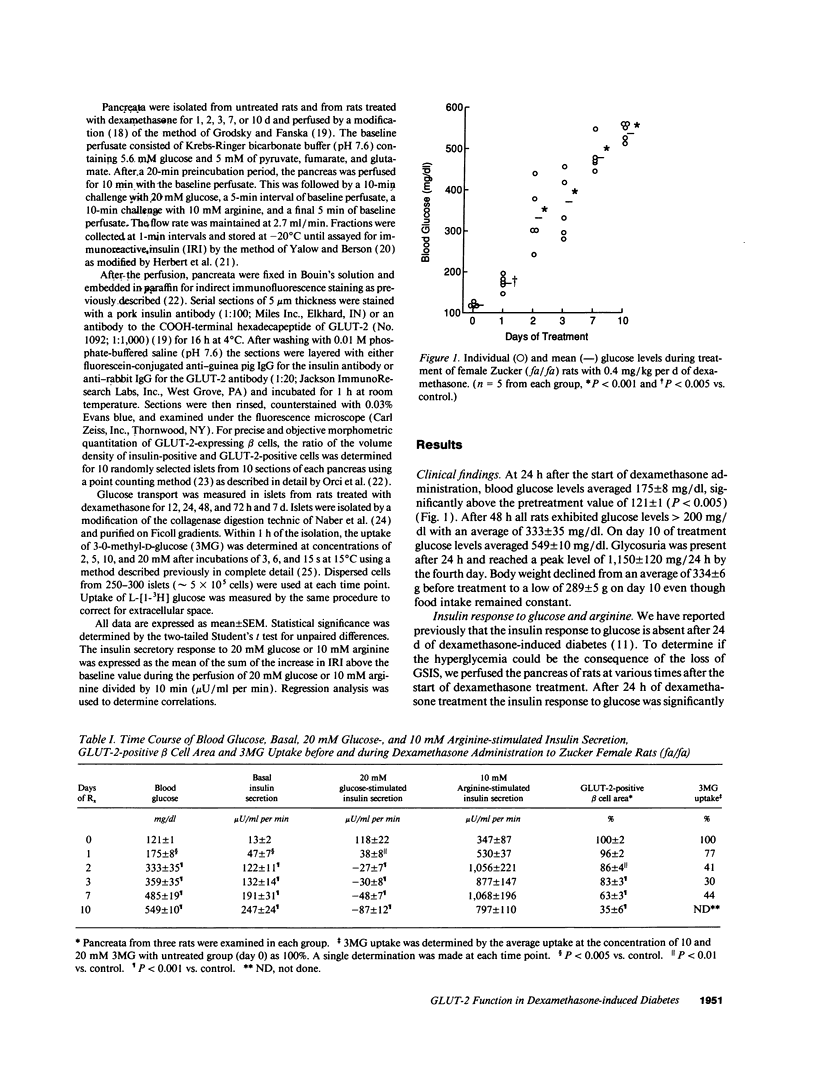
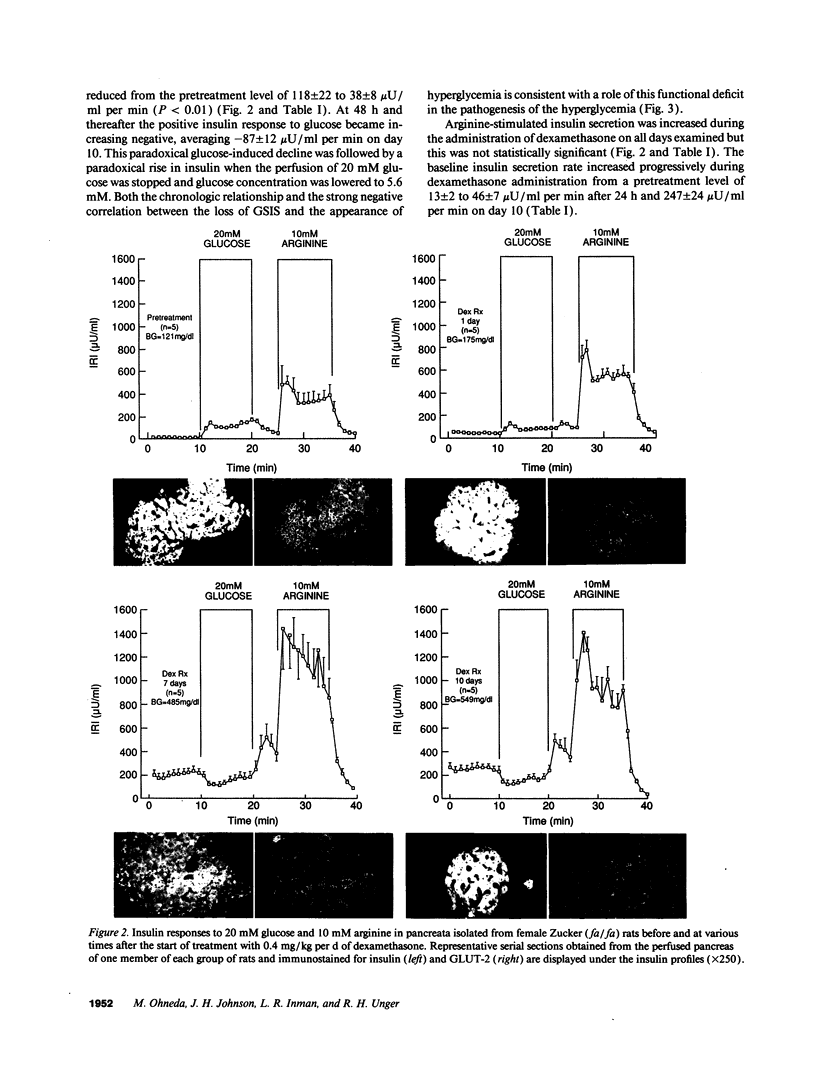
Abstract
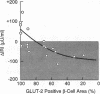
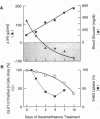
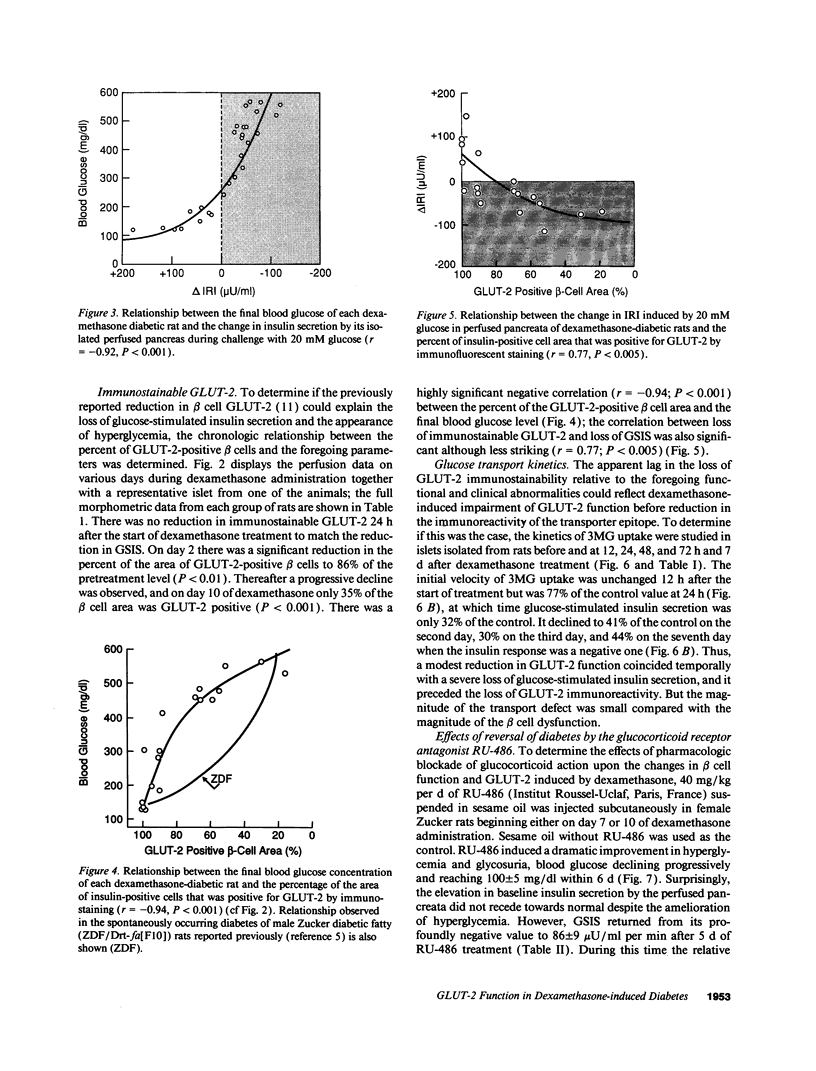
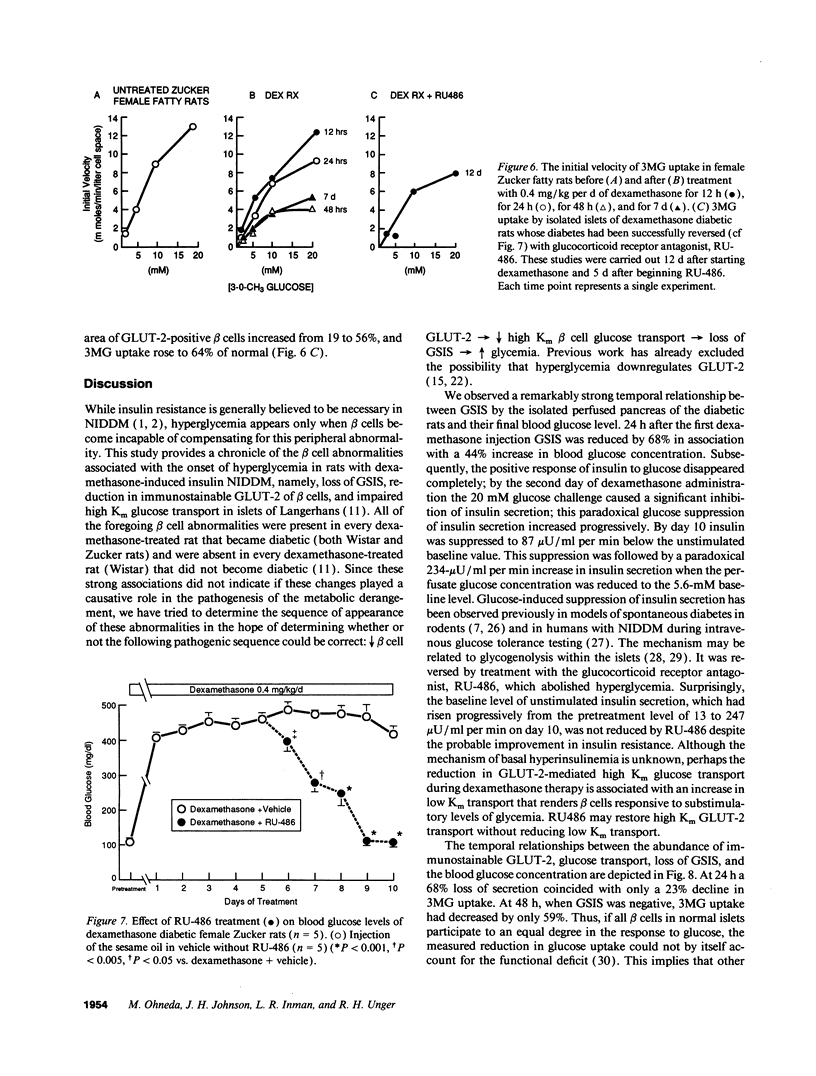
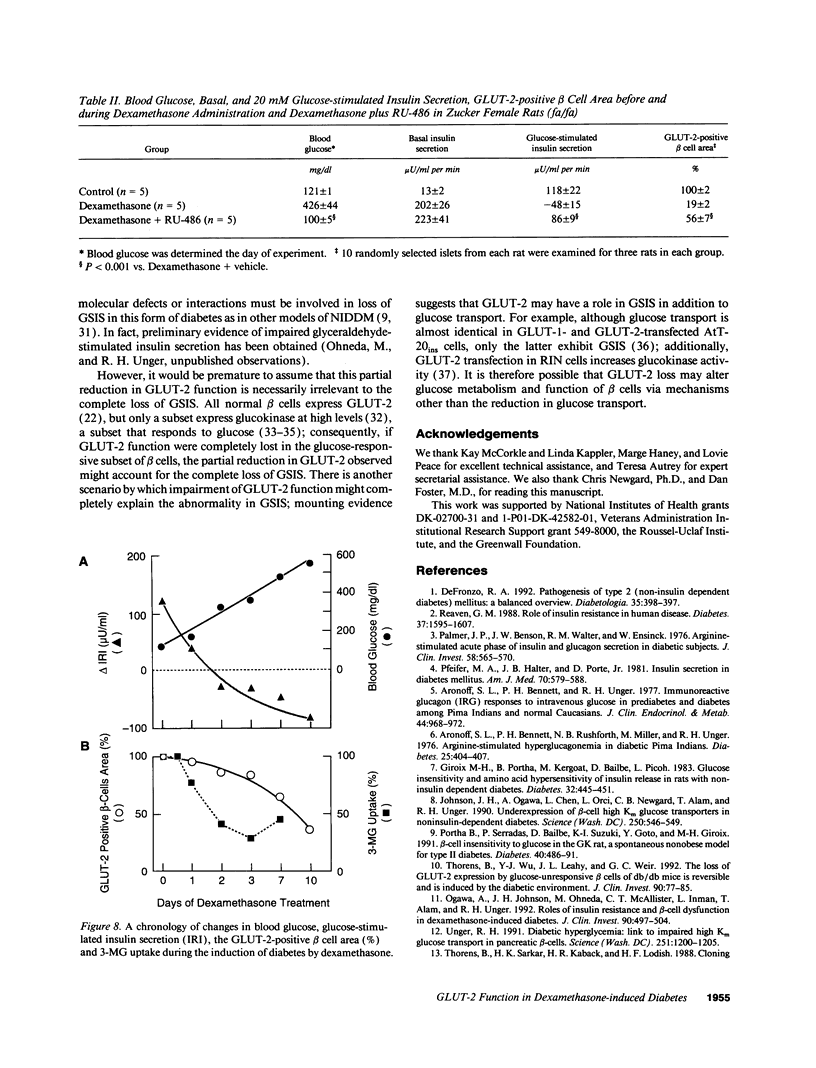
Spontaneous and dexamethasone-induced noninsulin-dependent diabetes mellitus (NIDDM) in rats is associated with loss of glucose-stimulated insulin secretion (GSIS) and a reduction in both GLUT-2-positive beta cells and high Km glucose transport. To determine if the chronology and correlation of these abnormalities is consistent with a causal relationship, Zucker (fa/fa) rats were studied longitudinally before and during 10 d of dexamethasone-induced (0.4 mg/kg per d i.p.) NIDDM. Within 24 h of dexamethasone treatment blood glucose rose and GSIS declined, becoming paradoxically negative (-87 +/- 12 microU/ml per min) on day 10. Blood glucose was negatively correlated with GSIS (r = -0.92; P < 0.001). 3-0-methyl-D-glucose (3MG) transport was unchanged at 12 h, 23% below normal on day 1, and declined further to a nadir 59% below normal. The GLUT-2-positive beta cell area did not decline until 48 h, reaching a nadir of 35% of normal at 10 d. The area of GLUT-2-positive beta cells was correlated with GSIS (r = 0.77; P < 0.005). We conclude that the chronology and correlation between GSIS loss and hyperglycemia is consistent with a cause-effect relationship, but that the subtotal impairment in glucose transport by itself cannot explain the total loss of GSIS if one assumes that normal beta cells are functionally homogenous.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronoff S. L., Bennett P. H., Rushforth N. B., Miller M., Unger R. H. Arginine-stimulated hyperglucagonemia in diabetic Pima Indians. Diabetes. 1976 May;25(5):404–407. doi: 10.2337/diab.25.5.404. [DOI] [PubMed] [Google Scholar]
- Aronoff S. L., Bennett P. H., Unger R. H. Immunoreactive glucagon (IRG) responses to intravenous glucose in prediabetes and diabetes among Pima Indians and normal Caucasians. J Clin Endocrinol Metab. 1977 May;44(5):968–972. doi: 10.1210/jcem-44-5-968. [DOI] [PubMed] [Google Scholar]
- Chen L., Alam T., Johnson J. H., Hughes S., Newgard C. B., Unger R. H. Regulation of beta-cell glucose transporter gene expression. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4088–4092. doi: 10.1073/pnas.87.11.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A. Pathogenesis of type 2 (non-insulin dependent) diabetes mellitus: a balanced overview. Diabetologia. 1992 Apr;35(4):389–397. doi: 10.1007/BF00401208. [DOI] [PubMed] [Google Scholar]
- Giordano E., Bosco D., Cirulli V., Meda P. Repeated glucose stimulation reveals distinct and lasting secretion patterns of individual rat pancreatic B cells. J Clin Invest. 1991 Jun;87(6):2178–2185. doi: 10.1172/JCI115251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroix M. H., Baetens D., Rasschaert J., Leclercq-Meyer V., Sener A., Portha B., Malaisse W. J. Enzymic and metabolic anomalies in islets of diabetic rats: relationship to B cell mass. Endocrinology. 1992 May;130(5):2634–2640. doi: 10.1210/endo.130.5.1315252. [DOI] [PubMed] [Google Scholar]
- Giroix M. H., Portha B., Kergoat M., Bailbe D., Picon L. Glucose insensitivity and amino-acid hypersensitivity of insulin release in rats with non-insulin-dependent diabetes. A study with the perfused pancreas. Diabetes. 1983 May;32(5):445–451. doi: 10.2337/diab.32.5.445. [DOI] [PubMed] [Google Scholar]
- Grodsky G. M., Fanska R. E. The in vitro perfused pancreas. Methods Enzymol. 1975;39:364–372. doi: 10.1016/s0076-6879(75)39033-2. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Hughes S. D., Quaade C., Johnson J. H., Ferber S., Newgard C. B. Transfection of AtT-20ins cells with GLUT-2 but not GLUT-1 confers glucose-stimulated insulin secretion. Relationship to glucose metabolism. J Biol Chem. 1993 Jul 15;268(20):15205–15212. [PubMed] [Google Scholar]
- Jetton T. L., Magnuson M. A. Heterogeneous expression of glucokinase among pancreatic beta cells. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2619–2623. doi: 10.1073/pnas.89.7.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. H., Crider B. P., McCorkle K., Alford M., Unger R. H. Inhibition of glucose transport into rat islet cells by immunoglobulins from patients with new-onset insulin-dependent diabetes mellitus. N Engl J Med. 1990 Mar 8;322(10):653–659. doi: 10.1056/NEJM199003083221003. [DOI] [PubMed] [Google Scholar]
- Johnson J. H., Ogawa A., Chen L., Orci L., Newgard C. B., Alam T., Unger R. H. Underexpression of beta cell high Km glucose transporters in noninsulin-dependent diabetes. Science. 1990 Oct 26;250(4980):546–549. doi: 10.1126/science.2237405. [DOI] [PubMed] [Google Scholar]
- Kiekens R., In 't Veld P., Mahler T., Schuit F., Van De Winkel M., Pipeleers D. Differences in glucose recognition by individual rat pancreatic B cells are associated with intercellular differences in glucose-induced biosynthetic activity. J Clin Invest. 1992 Jan;89(1):117–125. doi: 10.1172/JCI115551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisse W. J., Maggetto C., Leclercq-Meyer V., Sener A. Interference of glycogenolysis with glycolysis in pancreatic islets from glucose-infused rats. J Clin Invest. 1993 Feb;91(2):432–436. doi: 10.1172/JCI116219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisse W. J., Marynissen G., Sener A. Possible role of glycogen accumulation in B-cell glucotoxicity. Metabolism. 1992 Aug;41(8):814–819. doi: 10.1016/0026-0495(92)90160-c. [DOI] [PubMed] [Google Scholar]
- Marynissen G., Leclercq-Meyer V., Sener A., Malaisse W. J. Perturbation of pancreatic islet function in glucose-infused rats. Metabolism. 1990 Jan;39(1):87–95. doi: 10.1016/0026-0495(90)90153-4. [DOI] [PubMed] [Google Scholar]
- Metz S. A., Halter J. B., Robertson R. P. Paradoxical inhibition of insulin secretion by glucose in human diabetes mellitus. J Clin Endocrinol Metab. 1979 May;48(5):827–835. doi: 10.1210/jcem-48-5-827. [DOI] [PubMed] [Google Scholar]
- Naber S. P., McDonald J. M., Jarett L., McDaniel M. L., Ludvigsen C. W., Lacy P. E. Preliminary characterization of calcium binding in islet-cell plasma membranes. Diabetologia. 1980 Nov;19(5):439–444. doi: 10.1007/BF00281823. [DOI] [PubMed] [Google Scholar]
- Ogawa A., Johnson J. H., Ohneda M., McAllister C. T., Inman L., Alam T., Unger R. H. Roles of insulin resistance and beta-cell dysfunction in dexamethasone-induced diabetes. J Clin Invest. 1992 Aug;90(2):497–504. doi: 10.1172/JCI115886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohneda M., Johnson J. H., Inman L. R., Chen L., Suzuki K., Goto Y., Alam T., Ravazzola M., Orci L., Unger R. H. GLUT2 expression and function in beta-cells of GK rats with NIDDM. Dissociation between reductions in glucose transport and glucose-stimulated insulin secretion. Diabetes. 1993 Jul;42(7):1065–1072. doi: 10.2337/diab.42.7.1065. [DOI] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Baetens D., Inman L., Amherdt M., Peterson R. G., Newgard C. B., Johnson J. H., Unger R. H. Evidence that down-regulation of beta-cell glucose transporters in non-insulin-dependent diabetes may be the cause of diabetic hyperglycemia. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9953–9957. doi: 10.1073/pnas.87.24.9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Thorens B., Ravazzola M., Lodish H. F. Localization of the pancreatic beta cell glucose transporter to specific plasma membrane domains. Science. 1989 Jul 21;245(4915):295–297. doi: 10.1126/science.2665080. [DOI] [PubMed] [Google Scholar]
- Palmer J. P., Benson J. W., Walter R. M., Ensinck J. W. Arginine-stimulated acute phase of insulin and glucagon secretion in diabetic subjects. J Clin Invest. 1976 Sep;58(3):565–570. doi: 10.1172/JCI108502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer M. A., Halter J. B., Porte D., Jr Insulin secretion in diabetes mellitus. Am J Med. 1981 Mar;70(3):579–588. doi: 10.1016/0002-9343(81)90579-9. [DOI] [PubMed] [Google Scholar]
- Portha B., Serradas P., Bailbé D., Suzuki K., Goto Y., Giroix M. H. Beta-cell insensitivity to glucose in the GK rat, a spontaneous nonobese model for type II diabetes. Diabetes. 1991 Apr;40(4):486–491. doi: 10.2337/diab.40.4.486. [DOI] [PubMed] [Google Scholar]
- Reaven G. M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988 Dec;37(12):1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Schuit F. C., In't Veld P. A., Pipeleers D. G. Glucose stimulates proinsulin biosynthesis by a dose-dependent recruitment of pancreatic beta cells. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3865–3869. doi: 10.1073/pnas.85.11.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal M., Liang Y., Najafi H., Lodish H. F., Matschinsky F. M. Expression and function of GLUT-1 and GLUT-2 glucose transporter isoforms in cells of cultured rat pancreatic islets. J Biol Chem. 1992 Aug 25;267(24):17241–17247. [PubMed] [Google Scholar]
- Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Thorens B., Weir G. C., Leahy J. L., Lodish H. F., Bonner-Weir S. Reduced expression of the liver/beta-cell glucose transporter isoform in glucose-insensitive pancreatic beta cells of diabetic rats. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6492–6496. doi: 10.1073/pnas.87.17.6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B., Wu Y. J., Leahy J. L., Weir G. C. The loss of GLUT2 expression by glucose-unresponsive beta cells of db/db mice is reversible and is induced by the diabetic environment. J Clin Invest. 1992 Jul;90(1):77–85. doi: 10.1172/JCI115858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M., Komiya I., Johnson J. H., Inman L., Alam T., Moltz J., Crider B., Stefan Y., Baetens D., McCorkle K. Loss of insulin response to glucose but not arginine during the development of autoimmune diabetes in BB/W rats: relationships to islet volume and glucose transport rate. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9749–9753. doi: 10.1073/pnas.83.24.9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H. Diabetic hyperglycemia: link to impaired glucose transport in pancreatic beta cells. Science. 1991 Mar 8;251(4998):1200–1205. doi: 10.1126/science.2006409. [DOI] [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960 Jul;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]