Abstract
Activating mutations in the tyrosine kinase domain of HER2 (ErbB2) have been identified in human cancers. Compared to wild-type HER2, mutant HER2 shows constitutively activate kinase activity and increased oncogenicity. Cells transformed by mutant HER2 are resistant to EGFR tyrosine kinase inhibitors and exhibit an attenuated response to the HER2 antibody trastuzumab. We investigated herein pathways through which mutant HER2 alters the extracellular environment, potentially leading to drug resistance and the effect of simultaneously targeting HER2 and the tumor cell microenvironment with a therapeutic intent. Expression of mutant HER2 in mammary epithelial cells activated autocrine transforming growth factor (TGF) β1 signaling through a mechanism involving Rac1 and JNK-AP1-dependent transcription. Cells transformed by an activating mutant of H-Ras (G12V) also expressed higher TGF-β1 level through Rac1 activation. In addition, mutant HER2 induced the EGFR ligands TGF-α and amphiregulin at the mRNA and protein levels. Vascular endothelial growth factor (VEGF), a target of the TGF-β-Smad transcriptional regulation, was also induced as a result of expression of mutant HER2. Inhibition of TGF-β signaling with the Alk5 small molecule inhibitor LY2109761 reduced growth and invasiveness of cells expressing mutant HER2. Combined inhibition of intracellular and paracrine effects of mutant HER2 by trastuzumab and the EGFR antibody cetuximab was more efficient than single-agent therapies. These data suggest that mutations in oncogenes such as HER2 and Ras not only alter intracellular signaling and also influence on other components of the tumor microenvironment by inducing several pro-invasive growth factors. In turn, these serve as extracellular targets of novel therapeutic strategies directed at both cancer-driving oncogenes and the modified tumor microenvironment.
Keywords: HER2/ErbB2, Ras, TGF-β, VEGF, EGFR ligands, tumor microenvironment
Introduction
The majority of cancer occurs as a result of alterations in oncogenes. Among these, gene mutation, amplification or overexpression of HER2/Neu (ErbB2) and epidermal growth factor receptor (EGFR, ErbB1) have been found in various human cancers (Sharma et al., 2007; Yarden and Sliwkowski, 2001). Ligands of EGFR bind to the ectodomain of the receptor, leading to the formation of catalytically active receptor homo- and heterodimers in which the EGFR associates with other ErbB receptors. Although HER2 cannot bind any of the ErbB ligands directly, its catalytic activity can potently amplify signaling by ErbB-containing heterodimers (Graus-Porta et al., 1997; Pinkas-Kramarski et al., 1996; Wang et al., 1998; Worthylake et al., 1999; Yarden and Sliwkowski, 2001). A number of studies have shown mutations in the kinase domain of EGFR and HER2 resulting in ligand-independent kinase activity and activation of cytoplasmic signal transducers that regulate proliferation, differentiation, motility, adhesion, protection from apoptosis, and transformation (Sharma et al., 2007; Wang et al., 2006a).
Intragenic somatic mutations in the HER2 gene were reported in 5% of non-small-cell lung cancers (NSCLC), 5% of gastric carcinomas, 3% of colorectal carcinomas, and <5% of breast carcinomas (Lee et al., 2006; Shigematsu et al., 2005; Stephens et al., 2004). These involve in-frame duplications/insertions in a small stretch within exon 20 that correspond to the identical nine-codon region in exon 20 of the EGFR gene, where duplications/insertions have also been reported (Shigematsu et al., 2005; Stephens et al., 2004). We have previously shown that mutant HER2 with an in-frame YVMA insertion at residue 776 (HER2YVMA), the most common abnormality detected in NSCLC specimens (Shigematsu et al., 2005), results in a gain of function compared to wild-type HER2 (HER2WT) thus transforming normal epithelial cells and inducing tumors in vivo (Wang et al., 2006a). HER2YVMA is potently autophosphorylated and induces transphosphorylation of kinase-dead EGFR. Cells expressing HER2YVMA are resistant to the EGFR tyrosine kinase inhibitors (TKIs) such as erlotinib and gefitinib, and exhibit an attenuated response to the HER2 inhibitors lapatinib and trastuzumab (Wang et al., 2006a), suggesting that tumors with HER2 mutations may have a suboptimal response to these therapies when given single agents. In this study, we demonstrate that mutant HER2 significantly up-regulates expression of multiple growth factors including TGF-β, TGF-α, amphiregulin and VEGF. These ligands, upon entering the surrounding extracellular matrix, initiate not only autocrine but also paracrine signaling that enhance the growth and survival of co-cultured cells. Induction of TGF-β is also detected in cells expressing mutant H-Ras, and is dependent on the JNK-AP1 pathway which is activated in cells expressing mutant HER2 or H-Ras. These results suggest a mechanism through which gain of function oncogene mutations amplify their transforming potential by modifying the tumor microenvironment and, second, therapeutic strategies that simultaneously target both cancer-driving oncogenes and the tumor microenvironment.
Results
Autocrine TGF-β signaling is upregulated in cells expressing mutant HER2
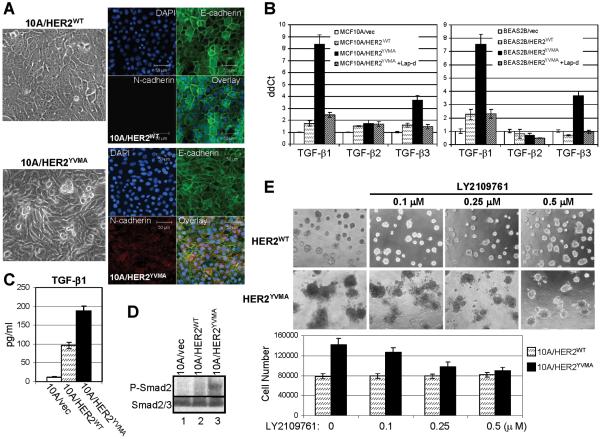
MCF10A human mammary epithelial cells stably transfected by HER2WT maintain an epithelial morphology. However, stable expression of HER2YVMA induces a morphological change consistent with an epithelial-to-mesenchymal transition (EMT), including disruption of E-cadherin localization at the cell junctions and expression of the mesenchymal N-cadherin (Fig. 1A). Because TGF-β is a potent inducer of EMT (Willis and Borok, 2007), we examined the mRNA levels for all 3 isoforms of human TGF-βs. Quantitative RT-PCR indicated that both TGF-β1 and TGF-β3 but not TGF-β2 were markedly upregulated in cells expressing HER2YVMA. An 8.3-fold and 3.8-fold increase were observed for TGF-β1 and TGF-β3, respectively, in MCF10A/HER2YVMA cells compared to cells expressing the empty vector (MCF10A/vec), whereas the increase of both ligands was less than 2-fold in MCF10A/HER2WT cells (Fig. 1B). These changes were also observed in BEAS2B human bronchial epithelial cells. The upregulation of TGF-β1 and TGF-β3 RNAs was abrogated by treating the HER2YVMA-expressing cells with the HER2 tyrosine kinase inhibitor lapatinib (Rusnak et al., 2001) (Fig. 1B). Using the conditioned medium (CM) harvested from MCF10A cells expressing HER2WT, HER2YVMA or vector, we detected a 4-fold and 7.5-fold increase in TGF-β1 protein resulting from the expression of HER2WT and HER2YVMA, respectively, when compared to cells transfected with vector alone (Fig. 1C).
Fig. 1.
Autocrine TGF-β signaling is increased in cells expressing mutant HER2. A. Phase contrast (left) and immunofluorescence (right) images of MCF10A/HER2WT and MCF10A/HER2YVMA cells cultured to reach confluence. For immunofluorescence staining, cells growing on glass coverslips were fixed and stained using antibodies against E-cadherin (green) and N-cadherin (red). DAPI (blue), nuclear staining. Bar equals 50 μm. B. MCF10A (left) or BEAS2B (right) cells stably expressing HER2WT, HER2YVMA or empty vector were grown in complete medium and treated with lapatinib for 16 h or left untreated. Total RNA was extracted and subjected to reverse transcription followed by quantitative PCR for TGF-β1, -β2 and -β3 as described in Materials and Methods. Data were normalized to the MCF10A/vec or BEAS2B/vec control cells. Each data represents the mean ± S.D. of 3 experiments. C. Cells grown on 100-mm dishes (1×106 cells/dish) were incubated for 24 h in serum-free medium. Conditioned medium (CM) was collected and analyzed for total amount of TGF-β1 by ELISA as indicated in Materials and Methods. Data are normalized to pg/ml/106 cells/24 h. Each data represents the mean ± S.D. of 3 experiments. D. Cells grown on 6-well plate were serum-starved for 16 h before lysed. Cell lysates were subjected to immunoblot using indicated antibodies. E. Top: MCF10A/HER2WT and MCF10A/HER2YVMA cells were plated in Matrigel in 8-well chambers and allowed to grow in the absence or presence of LY2109761 at the indicated concentrations. The inhibitor was added to the top medium 12 h after cell seeding and replenished every 3 days. Phase contrast images shown were recorded 9 days after the initial seeding of cells. Bottom: 9-day acini were trypsinized and total cell number was determined in a Coulter counter. Each bar graph represents the mean ± S.D. of 4 wells.
Phosphorylation of Smad2 which serves as an indicator of TGF-β signaling was only detectable in MCF10A/HER2YVMA cells but not in control or HER2WT-expressing cells in the absence of added TGF-β (Fig. 1D), suggesting that autocrine TGF-β pathway is activated in the former. When cultured in Matrigel, MCF10A/HER2YVMA cells but not MCF10A/HER2WT cells formed multiacinar structures that invaded the surrounding matrix. Inhibition of TGF-β signaling by LY2109761, a small molecule kinase inhibitor of type I and II TGF-β receptor kinases (Peng et al., 2005; Sawyer et al., 2004), reduced the size, invasiveness and cell number of colonies expressing mutant HER2 but had no effect on wild type HER2 expressing colonies (Fig. 1E).
Mutant HER2 induces TGF-β through activating Rac1 and JNK-AP1 pathway
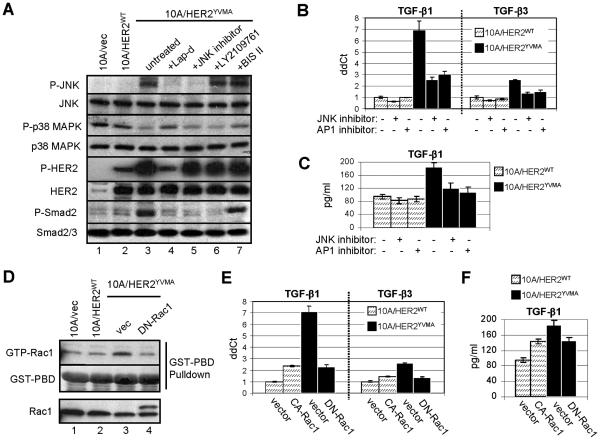
Previous study have indicated that the activating protein 1 (AP1) mediates activation of the TGF-β1 promoter in both human and rat cells. Unlike this promoter, the human TGF-β2 promoter does not contain AP1 binding sites and is therefore under different transcriptional regulation (Kim et al., 1990; Malipiero et al., 1990; Weigert et al., 2000). As the expression of HER2YVMA induced TGF-β1 production but lacked an effect on TGF-β2 (Fig. 1B), we speculated that HER2YVMA may upregulate TGF-β1 transcription via activation of the c-Jun N-terminal kinase (JNK)-AP1 pathway. Indeed, the level of phosphorylated (active) JNK was significantly elevated in MCF10A/HER2YVMA cells compared to vector control or HER2WT-expressing cells (Fig. 2A). Both lapatinib and a small molecule JNK inhibitor suppressed P-JNK as well as P-Smad2 in these cells, whereas the protein kinase C (PKC) inhibitor bisindolylmaleimide II (BIS II) and TGF-β receptor kinase inhibitor (LY2109761) showed no effect on P-JNK (Fig. 2A). This suggests that JNK activation by HER2YVMA is independent of PKC and upstream of TGF-β signal transduction. It has been reported that p38 MAPK also upregulates TGF-β1 (Weigert et al., 2000). However, MCF10A/HER2YVMA exhibited a lower level of P-p38 compared to control cells arguing against p38 signaling as a mechanism for increased TGF-β1 expression. Both the JNK inhibitor and the AP1 inhibitor curcumin reduced TGF-β1 and TGF-β3 mRNA levels in MCF10A/HER2YVMA cells (Fig. 2B) and the level of TGF-β1 in the CM (Fig. 2C), further supporting a role for the JNK-AP1 pathway in TGF-β overproduction in these cells.
Fig. 2.
HER2 mutant induces TGF-β through activating Rac1 and JNK-AP1 pathway. A. MCF10A/vec, MCF10A/HER2WT and MCF10A/HER2YVMA cells were serum-starved for 16 h before treated with lapatinib (5 μM), JNK inhibitor (30 μM), LY2109761 (0.5 μM) or BIS II (0.5 μM) for 4 h, and lysed for immunoblot. B. MCF10A/HER2WT and MCF10A/HER2YVMA cells were grown in complete medium and treated with JNK inhibitor (30 μM) or AP1 inhibitor (5 μM) for 16 h or left untreated. Total RNA was extracted for RT-PCR. C. Cells were treated as in (B) before incubated for 24 h in serum-free medium. CM was collected and analyzed for total amount of TGF-β1 by ELISA. D. MCF10A/vec, MCF10A/HER2WT and MCF10A/HER2YVMA cells grown on 6-well plates were transiently transfected with a dominant negative (DN)-Rac1 construct or vector. At 40 h post-transfection, cells were serum-starved for 8 h and lysed prior to incubation with GST-PBD. GST-PBD-bound proteins were eluted, separated by SDS-PAGE and subjected to immunoblot using Rac1 antibody. Ponceau staining of the GST-PBD fusion was used as loading control. E. MCF10A/HER2WT and MCF10A/HER2YVMA cells were transiently transfected with plasmid DNA encoding DN-Rac1, constitutively active (CA)-Rac1 or vector. At 48 h post-transfection, total RNA was extracted for RT-PCR. F. Cells were transfected as in (E) before incubated for 24 h in serum-free medium. CM was collected and analyzed for total amount of TGF-β1 by ELISA.
A body of previous work has elucidated a signaling cascade to activate JNK during oncogenesis. This includes activation of several MAP3Ks, usually initiated by growth factors and mediated by the Rho-family GTPases, and the subsequent activation of MKK4/7, the kinase that activates JNK [reviewed in(Heasley and Han, 2006)]. Activity of the Rho-family GTPases was examined by GST-PBD (for Rac1 and Cdc42) or GST-rhotekin (for RhoA) pulldown assay. Elevated Rac1 activity was observed in MCF10A/HER2YVMA cells (Fig. 2D), whereas the Cdc42 and RhoA activities were comparable to those detected in control cells (data not shown). Expression of a dominant negative (DN) Rac1 markedly decreased TGF-β1 and TGF-β3 mRNA levels in MCF10A/HER2YVMA cells and the TGF-β1 level in the CM, whereas a constitutively active (CA) Rac1 was sufficient to increase TGF-β1 transcript by 2.5-fold (Fig. 2E) and TGF-β1 level in the CM by 1.5-fold (Fig. 2F) in MCF10A control cells.
Mutant H-Ras also induces autocrine TGF-β autocrine through Rac1 and JNK-AP1
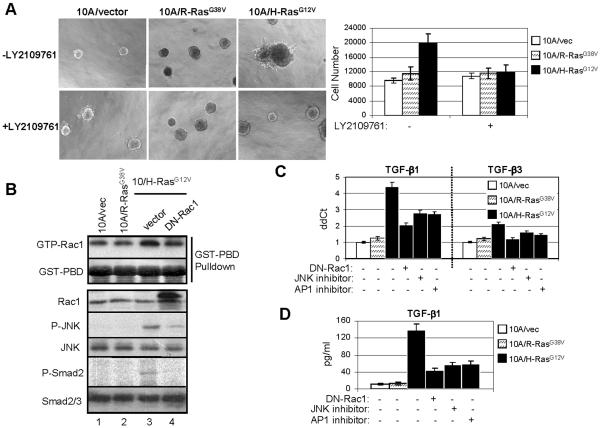
TGF-β is also engaged in Ras-mediated oncogenesis (Janda et al., 2002; Kim et al., 2005). H-Ras induces autocrine TGF-β production and TGF-β-dependent EMT in cells transformed by this oncogene (Janda et al., 2002). In keratinocytes containing mutant H-Ras, TGF-β1 production is enhanced while TGF-β2 is undetectable (Fahey et al., 1996). Further, both H-Ras and elevated levels of activated nuclear Smad2 are required for acquisition of EMT and metastatic progression in vivo (Oft et al., 2002). To determine whether Rac1 and JNK-AP1 mediated transcription were also involved in TGF-β overproduction in cells transformed by mutant Ras, we stably expressed the active mutants of R-Ras (R-RasG38V) and H-Ras (H-RasG12V) in MCF10A cells. When cultured in Matrigel, H-Ras-transformed cells but not R-Ras- or vector-expressing cells formed multiacinar invasive structures, whose growth was inhibited by the Alk5 kinase inhibitor LY2109761 (Fig. 3A). H-Ras-transformed cells contained higher levels of active Rac1, phosphorylated JNK and phosphorylated Smad2 compared to cells expressing the vector alone or R-Ras. P-JNK and P-Smad2 were decreased by the expression of DN-Rac1 (Fig. 3B), suggesting that the Rac1-JNK-AP1 axis induces autocrine TGF-β in H-Ras-transformed cells. Further, quantitative RT-PCR and ELISA indicated that H-Ras-transformed cells expressed significantly higher level of TGF-β1 than the other two lines and expression of DN-Rac1 or treatment with JNK or AP1 inhibitors markedly impaired TGF-β1 production (Fig. 3C&D).
Fig. 3.
H-Ras mutant also induces TGF-β autocrine through Rac1 and JNK-AP1 pathway. A. Left: MCF10A/vec, MCF10A/R-RasG38V and MCF10A/H-RasG12V cells were plated in Matrigel in 8-well chambers and allowed to grow in the absence or presence of LY2109761 (0.5 μM). The inhibitor was added to the top medium 12 h after cell seeding and replenished every 3 days. Phase contrast images shown were recorded 9 days after the initial seeding of cells. Right: 9-day acini were trypsinized and total cell number was determined in a Coulter counter. Each bar graph represents the mean ± S.D. of 4 wells. B. Cells grown on 6-well plates were transiently transfected with DN-Rac1 construct or vector. At 40 h post-transfection, cells were serum-starved for 8 h and lysed for GST-PBD pull-down assay and immunoblot. C. Cells were transiently transfected with plasmid DNA encoding DN-Rac1, or treated with JNK or AP1 inhibitors as indicated. At 48 h post-transfection or 16 h after treatment, total RNA was extracted for RT-PCR. D. Cells were treated as in (C) before incubated for 24 h in serum-free medium. CM was collected and analyzed for total amount of TGF-β1 by ELISA.
Mutant HER2 induces expression of EGFR ligands
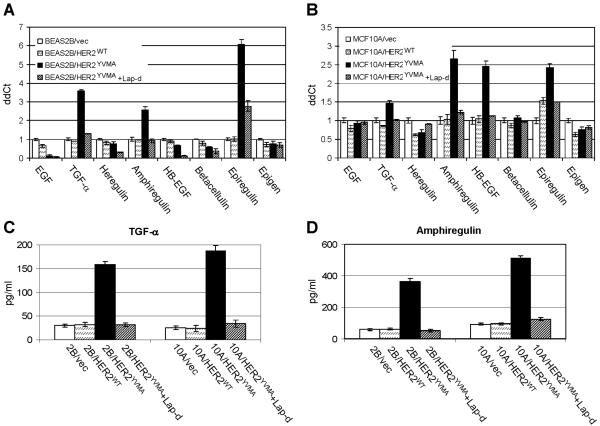
We have previously shown that cells expressing HER2YVMA exihibit higher level of EGFR phosphorylation as a result of the constitutive association of EGFR and mutant HER2 and the transphosphorylation of EGFR by the latter (Wang et al., 2006a). It is known that EGFR ligands auto-induce their expression and can also cross-induce other EGFR ligands. For example, TGF-α, amphiregulin, HB-EGF and betacellulin can induce the mRNA expression of the remaining family members (Barnard et al., 1994). Treatment with epiregulin induces the mRNA levels of TGF-α, amphiregulin, HB-EGF and epiregulin within 1 h. In turn, EGF, TGF-α, amphiregulin and HB-EGF can all induce epiregulin expression (Shirakata et al., 2000). Therefore, we speculated that the ligand-independent activation of EGFR in cells expressing HER2YVMA can also induce expression of multiple EGFR ligands. This was examined in MCF10A and BEAS2B cells expressing HER2WT, HER2YVMA or vector alone by quantitative RT-PCR using ligand-specific primers specific. In BEAS2B cells, expression of HER2YVMA but not HER2WT induced the mRNA levels of TGF-α, amphiregulin and epiregulin by 2.7 to 6-fold (Fig. 4A). Significant increase of mRNAs encoding TGF-α, amphiregulin, HB-EGF and epiregulin was also observed in MCF10A cells expressing HER2YVMA compared to cells expressing HER2WT or vector alone (Fig. 4B). In both cell lines, treatment with lapatinib inhibited the induction of EGFR ligands (Fig. 4A&B). We next tested the levels of TGF-α and amphiregulin in the conditioned medium prepared from these cells using specific immunoassays. HER2YVMA-expressing cells produced 4- to 7.5-fold higher levels of TGF-α (Fig. 4C) and amphiregulin protein (Fig. 4D) compared to the other two cell lines.
Fig. 4.
HER2 mutant induces expression of various EGFR ligands. A&B: BEAS2B cells (A) or MCF10A cells (B) stably expressing HER2WT, HER2YVMA or empty vector were grown in complete medium and treated with lapatinib (5 μM) for 16 h or left untreated. Total RNA was extracted and subjected to RT-PCR. Data were normalized to the BEAS2B/vec (A) or MCF10A/vec (B) control cells. Each data represents the mean ± S.D. of 3 experiments. C&D: Cells grown on 100-mm dishes (1×106 cells/dish) were incubated for 24 h in serum-free medium containing cetuximab (10 μg/ml) in the presence or absence of lapatinib (5 μM). Conditioned medium was collected and analyzed for total amount of TGF-α (C) or amphiregulin (D) by immunoassay as indicated in Materials and Methods. Data are normalized to pg/ml/106 cells/24 h. Each data represents the mean ± S.D. of 3 experiments.
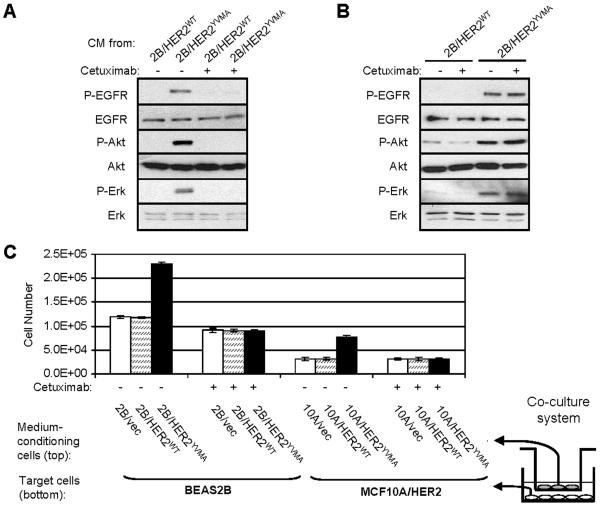
EGFR ligands produced by cells expressing mutant HER2 stimulate paracrine signaling
Cells expressing mutant HER2 exhibit ligand-independent constitutive EGFR and HER2 signaling. We speculated that their increased production of EGFR ligands will stimulate adjacent cells where activation of the EGFR is ligand-dependent. This was first tested in the wild-type BEAS2B cells treated with serum-free conditioned medium (CM) harvested from cells expressing HER2YVMA or HER2WT. CM of HER2YVMA-expressing cells induced phosphorylation of EGFR and activation of the downstream effectors Akt and Erk in wild-type BEAS2B cells (Fig. 5A). These responses were inhibited by preincubating BEAS2B cells with the EGFR antibody cetuximab which blocks ligand binding (Fig. 5A). As expected, cetuximab had no effect on the ligand-independent EGFR and HER2 signaling in cells expressing HER2YVMA (Fig. 5B). We then used a co-culture system in which wild-type target cells (either BEAS2B or MCF10A/HER2) growing in plates were co-incubated with but separated by a 0.4-μm pore-size filter from oncogene-expressing cells to determine the effect of CM from these cells on wild-type cell growth after 72 h. Co-incubation of BEAS2B or MCF10A/HER2 cells with cells expressing HER2YVMA but not with cells expressing HER2WT or vector alone resulted in a significant increase in BEAS2B or MCF10A/HER2 target cell number (Fig. 5C). This increase in target cell number as a result of coincubation with cells expressing mutant HER2 was abrogated by treatment with cetuximab (Fig. 5C), implying it was mediated by paracrine effects of EGFR ligands.
Fig. 5.
EGFR ligands produced by cells expressing mutant HER2 stimulate paracrine signaling. A: Conditioned medium (CM) was prepared by incubating BEAS2B/HER2WT or BEAS2B/HER2YVMA cells grown on 100-mm dishes (1×106 cells/dish) in serum-free medium for 24 h followed by filtration of harvested medium through a 0.22-μm sterile filter. Wild-type BEAS2B cells grown on 6-well plate were serum-starved for 16 h before treated with different CM for 1 h. In the indicated lanes, cetuximab (10 μg/ml) was added to the target cells 2 h prior the addition of the CM. Cell lysates were subjected to immunoblot with indicated antibodies. B: BEAS2B/HER2WT or BEAS2B/HER2YVMA cells were serum-starved for 16 h and treated with cetuximab (10 μg/ml) for 16 h or left untreated. Cell lysates were subjected to immunoblot with indicated antibodies. C: Co-culture assay was performed as described in Materials and Methods using transwell tissue-culture inserts. Co-cultures were carried out as indicated in serum-free medium in the presence or absence of cetuximab (10 μg/ml) for 72 h. Target cells were harvested by trypsinization and cell number was determined in a Coulter counter. Each data represents the mean ± S.D. of 3 wells. Right: A schematic representation of the two-story co-culture experiments.
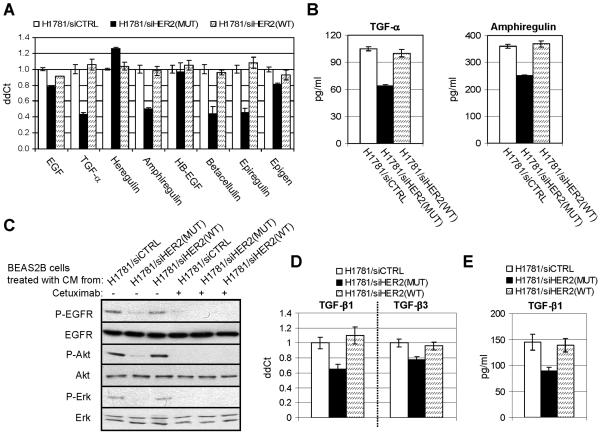
Mutant HER2 in H1781 cells is required for production of EGFR and TGF-β ligands
To validate our findings in cells naturally carrying HER2 mutation, we performed HER2 RNA interference in NCI-H1781 lung cancer cells, which contain a VC insertion at G776 in exon 20 of the HER2 gene (Shigematsu et al., 2005). We and others have previously shown that H1781 cells are homozygous and do not express wild type HER2 (Shigematsu et al., 2005; Wang et al., 2006a). Small-interfering RNA oligonucleotides targeting HER2WT, HER2MUT, or a control sequence were transfected into H1781 cells as described (Wang et al., 2006a). Three days post-transfection, the mRNA levels of TGF-α, amphiregulin, betacellulin and epiregulin were decreased by 50-60% only in cells transfected with siHER2(MUT) (Fig. 6A). Consistent with these results, levels of TGF-α and amphiregulin in the CM of transfected cells were also decreased by siHER2(MUT) but not siHER2(WT) (Fig. 6B). When the CM were used to treated BEAS2B cells, addition of medium harvested from H1781 cells transfected with control siRNA or siHER2(WT) induced P-EGFR, P-Akt, and P-Erk in BEAS2B target cells; this was not observed with the CM from siHER2(MUT)-transfected H1781 cells (Fig. 6C). In addition, knock-down of HER2MUT by siRNA also decreased TGF-β1 mRNA levels (Fig. 6D) and protein levels in CM (Fig. 6E), suggesting that mutant HER2 is required for the production of EGFR ligands and TGF-β1 in H1781 cells.
Fig. 6.
RNA interference of mutant HER2 inhibits growth factor production of H1781 lung cancer cells. A&D. H1781 cells were transfected by siRNA oligonucleotides targeting HER2WT, HER2MUT, or a control sequence. At day 3 after transfection, total RNA was extracted and subjected to RT-PCR. Data were normalized to the H1781 cells transfected by control siRNA. Each data represents the mean ± S.D. of 3 experiments. B&E. At day 3 after siRNA transfection, H1781 cells grown on 100-mm dishes (1×106 cells/dish) were incubated for 24 h in serum-free medium. CM was collected and analyzed for total amount of TGF-β amphiregulin (B) or TGF-β1 (E) by immunoassays. Data are normalized to pg/ml/106 cells/24 h. Each data represents the mean ± S.D. of 3 experiments. C. CM collected from H1781 cells transfected by siRNA was added to serum-starved BEAS2B cells. In the indicated lanes, cetuximab (10 μg/ml) was added to the target cells 2 h prior the addition of the CM. BEAS2B cell lysates were prepared after treatment with CM for 1 h and subjected to immunoblot with indicated antibodies.
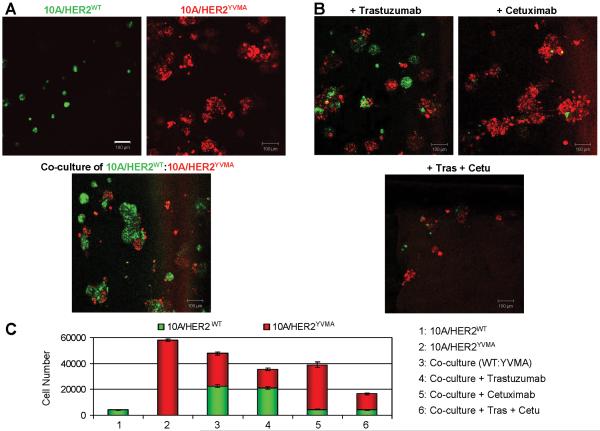
Combined inhibition of intracellular and paracrine effects of mutant HER2
We have previously shown that in 3D Matrigel supplemented with EGF, MCF10A/HER2WT cells form round-shaped acinus-like structures with a hollow lumen, whereas MCF10A/HER2YVMA cells form multiacinar structures with invading protrusions into the surrounding matrix (Wang et al., 2006a)(Fig. 1E). However, when no EGF was added into the top medium, MCF10A/HER2WT cells showed highly restricted growth (Fig. 7A) thus providing a model to test the biological effect of EGFR ligands secreted by adjacent HER2YVMA-expressing cells. The MCF10A/HER2WT cells and MCF10A/HER2YVMA cells were differentially labeled with fluorescent linkers and cultured on Matrigel in the absence of EGF either alone or in a 1:1 combination. On day 6, the MCF10A/HER2YVMA cells grew into multiacinar structures in an EGF-independent manner, whereas the MCF10A/HER2WT cells did not when cultured alone. However, the MCF10A/HER2WT cells regained the ability to grow into regular-sized acini when co-cultured with the MCF10A/HER2YVMA cells (Fig. 7A). Treatment with trastuzumab modestly inhibited growth and invasiveness of HER2YVMA but not EGF-stimulated HER2WT acini in 3D co-culture (Fig. 7B&C). This lack of effect of trastuzumab on MCF10A/HER2WT cells is consistent with the reported inability of the antibody to interfere with ErbB ligand-induced growth and/or EGFR/HER2 heterodimerization (Agus et al., 2002; Moulder, 2001; Ye et al., 1999). In contrast, cetuximab completely inhibited growth of MCF10A/HER2WT cells but had no effect on the ligand-independent growth of MCF10A/HER2YVMA cells in the co-culture system (Fig. 7B&C). When the two antibodies were added to the co-culture, growth of both cell types was inhibited (Fig. 7B&C).
Fig. 7.
Combined inhibition of intracellular and paracrine effects of mutant HER2. A: MCF10A/HER2WT cells were labeled with PKH67 green fluorescent cell linker amd MCF10A/HER2YVMA cells were labeled with PKH26 red fluorescent cell linker. Labeled cells were immediately seeded in Matrigel for 3D culture in the absence of EGF in the top medium. Top: For single cell type culture, 6×103 cells were seeded on day 0. Bottom: For co-culture of mixed cell types, 3×103 cells of each cell type (a total of 6×103 cells) were seeded on day 0. The fluorescent images were captured on day 6. Bars equal 100 μm. B: Co-culture of differently labeled 10A/HER2WT and 10A/HER2YVMA cells were set up as described in A. At 12 h after cell seeding, trastuzumab (10 μg/ml) and/or cetuximab (10 μg/ml) was added into the top medium as indicated. Antibodies were replenished every 3 days. The fluorescent images were photographed on day 6. C: The 6-day acini in A&B were trypsinized and total cell number of each labeled cell type was determined. Each bar graph represents the mean ± S.D. of 3 wells.
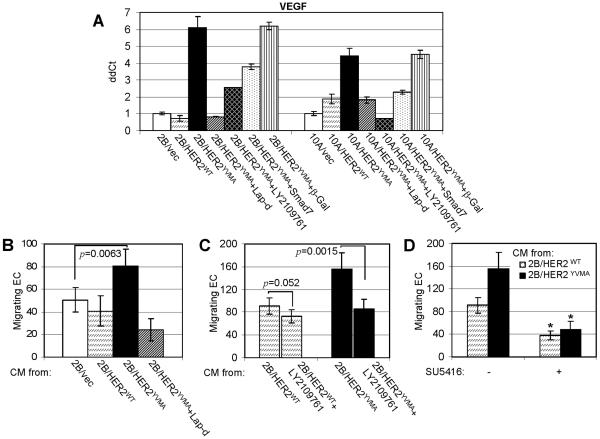
Mutant HER2 upregulates VEGF expression
Vascular endothelial growth factor (VEGF) is an important cancer-promoting angiogenic factor and thus a therapeutic target in the tumor microenvironment. Transcription of VEGF gene is upregulated by numerous transcription factors and signaling pathways, including the hypoxia-inducible factor (HIF)-1α and TGF-β (Josko and Mazurek, 2004). Hypoxia and TGF-β cooperate to induce VEGF gene expression through a region on the VEGF promoter which contains functional DNA-binding sequences for HIF-1α and Smads (Sanchez-Elsner et al., 2001). Therefore, we investigated if activated autocrine TGF-β in cells expressing HER2YVMA upregulates VEGF expression using quantitative RT-PCR. In both BEAS2B and MCF10A cells, expression of HER2YVMA significantly increased VEGF transcription by 6- and 4.5-fold, respectively (Fig. 8A). This induction was inhibited by lapatinib, LY2109761, or transduction with a Smad7 adenovirus (Fig. 8A). Finally, we examined the effect of conditioned medium (CM) collected from BEAS2B cells expressing HER2WT, HER2YVMA or vector on endothelial cell (EC) migration. CM from BEAS2B/HER2YVMA cells exhibited a higher potential to induce EC migration through the transwell filters than CM from the other two lines (Fig. 8B). This paracrine effect required HER2, TGF-β receptor, and VEGF receptor kinase activities as lapatinib, LY2109761, and SU5416, a small molecule inhibitor of VEGFR tyrosine kinases, inhibited CM-stimulated endothelial cell migration (Fig. 8B,C,D).
Fig. 8.
Mutant HER2 upregulates VEGF expression in TGF-β dependent fashion. A. Cells were either treated with lapatinib or LY2109761 or infected with an adenovirus encoding Smad7 or β-Gal (MOI 1:5) as described (Wang et al., 2005) 16 h before total RNA was extracted for RT-PCR using primers for VEGF. B. Conditioned medium (CM) was prepared from cells treated with lapatinib or from untreated cells as described in Fig. 1C. After concentrated by 10-fold as described in Materials and Methods, CM was added to endothelial cells (EC) in EC migration assay. Each data represents the mean ± S.D. of 3 experiments. C. CM was prepared from cells treated with LY2109761 or from untreated cells, concentrated, and examined in EC migration assay. D. CM from 2B/HER2WT and 2B/HER2YVMA cells were examined in EC migration assay as described in Materials and Methods. SU5416 (5 μM) was added to EC cells at the beginning of migration assay when indicated. * p<0.005.
Discussion
Solid tumors are heterogeneous tissues composed of tumor, stromal and immune cells, extracellular matrix, connective tissue and blood vessels. In the tumor niche, cells harboring oncogenes such as HER2 and Ras mutations coexist with genetically wild-type host cells. As a result of these gain-of-function gene mutations, cells expressing oncogenes exhibit advantageous growth and survival over their wild-type countertypes, leading to clonal selection in the tumor microenvironment. Meanwhile, these oncogene-expressing cells may also influence adjacent wild-type cells by modifying this microenvironment. Herein we showed that an activating mutant of HER2 upregulates expression of multiple growth factors including TGF-β, VEGF and a variety of EGFR ligands including TGF-α and amphiregulin, both of which have shown special relevance to tumor growth among other EGFR ligands (Normanno et al., 2001). These growth factors overproduced by the cells carrying oncogenes initiate not only autocrine but also paracrine signaling that favors tumor growth. Our data also suggest that the efficacy of current oncogene-targeted therapies may be potentiated by rationally co-targeting both the intracellular and the environmental effects of the oncogene. In the mixed culture of cells each expressing mutant HER2 and wild-type HER2, the HER2 and EGFR antibodies trastuzumab or cetuximab, respectively, inhibited only one cell population. Simultaneous treatment with both antibodies was required for inhibition of both cell populations (Fig. 7B&C).
Among the growth factors induced by HER2 and Ras oncogenes, TGF-β ligands are known to foster cancer progression by mechanisms that include an increase in tumor neoangiogenesis and extracellular matrix production, upregulation of peri-tumor proteases, and inhibition of mechanisms of immune surveillance in the cancer host, among others (Dumont and Arteaga, 2003). Many of the cancer-promoting functions of TGF-β are exerted via the cooperation between TGF-β and transforming oncogenes, such as ErbB2/HER2/Neu, polyomavirus middle T antigen (PyVmT) and Ras (Janda et al., 2002; Muraoka-Cook et al., 2004; Muraoka et al., 2003; Seton-Rogers et al., 2004; Siegel et al., 2003; Ueda et al., 2004). Among the three TGF-β homologous forms in human, TGF-β1 is most frequently overexpressed in tumor cells (Derynck et al., 2001). Interference of TGF-β signaling with a small molecule inhibitor of the type I receptor serine threonine kinase blocked invasiveness of tumor cells expressing mutant HER2 or mutant Ras (Figs. 1E & 3A), suggesting tumor cell autonomous mechanisms of dependence on TGF-β signaling in oncogene transformed cells.
A link between EGFR, Ras and TGF-β signaling has been established. For example, Ras-MEK downstream of EGFR signaling induces phosphorylation of TGIF, a Smad co-repressor that competes with the co-activator p300 for Smad2 association. This results in TGIF stabilization, formation of Smad2-TGIF co-repressor complexes, and abrogation of TGF-β-induced inhibition of cell proliferation mediated by Smad2 target genes (Lo et al., 2001). Our study herein suggests that mutants of HER2 and H-Ras also upregulate autocrine TGF-β signaling by increasing the production of TGF-β ligands, such that other cells in the vicinity will be affected by cells carrying these mutations. This upregulation was mediated by the Rac1-JNK-AP1 axis which is activated by HER2 and H-Ras. Notably, transient expression of constitutively active Rac1 was sufficient to induce 2.5-fold increase of TGF-β1 expression (Fig. 2E). This mechanism may also apply to cells harboring other oncogenes that activate Rac1 or JNK-AP1. Overexpression of Rac1 occurs in many tumor types including cancers of the breast, lung, and colon (Fritz et al., 1999; Schnelzer et al., 2000). Both JNK and AP1 are known to be activated during tumor development and are potential therapeutic targets in cancer (Wagner and Nebreda, 2009). Our report suggests that elevated level/activity of Rac1, JNK or AP1 are causal to the high levels of TGF-β expression observed in many human cancers.
The Rac1 GTPase has been shown to contribute to TGF-β-mediated cellular and transcriptional responses (Atfi et al., 1997; Mucsi et al., 1996). In addition, TGF-β can rapidly activate RhoA and Rac1, contributing to EMT and enhanced cell motility (Bakin et al., 2002; Bhowmick et al., 2001). Rac1 activity in situ is higher in mouse mammary cancers expressing Neu (ErbB2) and active TGF-β1 transgenes compared with transgenic tumors expressing the Neu oncogene alone (Muraoka et al., 2003). Rac1 is one of the immediately early targets of growth factors and oncogenes such as HER2 and Ras signaling (Adam et al., 1998; Bourguignon et al., 2001). Therefore, these data suggest that oncogenic signaling that results in Rac1 activation contributes to high levels of TGF-β1 at tumor sites which, in turn, facilitate the cooperation between TGF-β1 and oncogenes to promote cancer progression and further stimulates Rac1 activity in a positive feedback fashion. Moreover, blockade of Rac1-induced TGF-β overproduction by JNK or AP1 inhibitors may serve as another strategy to target the high TGF-β levels in oncogene-driven tumors, in addition to the direct tumor cell autonomous effect of these inhibitors.
Mutant HER2 also upregulated VEGF expression and inhibition of the HER2 tyrosine kinase by lapatinib blocked this effect (Fig. 8). Along those lines, inhibition of EGFR, Ras or PI3K has been shown to decrease VEGF promoter activity and mRNA level in glioblastoma cell line through a mechanism distinct from signals induced by hypoxia (Maity et al., 2000). Herein we also showed that inhibition of TGF-β (by LY2109761) or Smad2/3 (by Smad7) also suppressed the induction of VEGF by mutant HER2. Considering that blockade of TGF-β signaling by LY2109761 inhibited the malignant phenotype of oncogene transformed cells (Figs. 1E&3A), the capacity of TGF-β-targeted therapy to inhibit both cancer cell growth and tumor-induced angiogenesis makes it a promising therapeutic strategy that aims at multiple compartments in the cancer niche. Future clinical investigation of TGF-β pathway inhibitors in combination with anti-oncogene therapies in selected types of cancers will shed light on this possibility.
Materials and Methods
Cell lines and reagents
BEAS2B human bronchial epithelial cells and MCF10A human mammary epithelial cells stably expressing wild-type HER2 (HER2WT), mutant HER2 with a G776YVMA insertion in exon 20 (HER2YVMA) or vector alone, as well as NCI-H1781 lung cancer cells have been described elsewhere (Shigematsu et al., 2005; Wang et al., 2006a). MCF10A cells stably expressing R-RasG38V or H-RasG12V were generated using LZRS-GFP retroviral vector encoding the Ras mutants (Erdogan et al., 2007) and selection for GFP-positivity. Human endothelial cells (ECs) were cultured as described (DeBusk et al., 2004). Cetuximab and trastuzumab were purchased at the Vanderbilt University Medical Center Pharmacy. Lapatinib ditosylate was purchased from LC Laboratories. The small molecule TβRI and TβRII inhibitor LY2109761 was kindly provided by Dr. Jonathan Yingling (Eli Lilly Research Laboratories). JNK inhibitor II was purchased from Calbiochem. AP1 inhibitor curcumin and protein kinase C (PKC) inhibitor bisindolylmaleimide II (BIS II) were purchased from Sigma. The VEGFR tyrosine kinase inhibitor SU5416 was provided by SUGEN Inc. (Fong et al., 1999). Constructs encoding dominant negative (DN)-Rac1(T17N) and constitutively active (CA)-Rac1(Q61L) are described elsewhere (Wang et al., 2006b).
Immunoblot analysis and Rac1 activity assay
Immunoblot analyses were performed as described previously (Wang et al., 2006a). Primary antibodies included P-p38, p38, P-ERK1/2, ERK1/2, P-AktS473, Akt, P-EGFRY1068, P-HER2Y1248, P-JNKT183/Y185, JNK (Cell Signaling), Rac1 (BD Transduction Laboratories), HER2, and EGFR (NeoMarkers). To pull-down GTP-bound (active) Rac, a glutathione S-transferase (GST) fusion protein of Pak binding domain (PBD) pre-coupled to agarose-glutathione beads (Cytoskeleton Inc., Denver, CO), was utilized as described previously (Ueda et al., 2004). Eluted Rac1 was detected by immunoblot.
Transfection of DNA and siRNA, RNA extraction, reverse transcription and real time quantitative PCR (qPCR) analysis
Cell transfection and the sequences of HER2WT and HER2MUT siRNA oligonucleotides were described previously (Wang et al., 2006a). RNA was isolated using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s procedures. After treatment with RQ1 RNase-free DNase (Promega), RNA samples were subjected to first-strand cDNA synthesis using Superscript™ II reverse transcriptase (Invitrogen) following the manufacturer’s protocols. Gene expression was quantified by qPCR using iQ™ SYBR green supermix (BioRad) and 50 ng of cDNA per reaction. The sequences of the primer sets used for this analysis are as follows: VEGF-A: F 5′-TTGCCTTGCTGCTCTACCTC-3′, R 5′-AAATGCTTTCTCCGCTCTGA-3′; TGF-β1: F 5′-GCACGTGGAGCTGTACCA-3′, R 5′-CAGCCGGTTGCTGAGGTA-3′; TGF-β2: F 5′-CAAAGGGTACAATGCCAACTT-3′, R 5′-CAGATGCTTCTGGATTTATGGTATT-3′; TGF-β3: F 5′-AAGAAGCGGGCTTTGGAC-3′, R 5′-CACACAGCAGTTCTCCTCCA-3′, where F and R are the forward and reverse primers, respectively. Primers for the ErbB ligands are described elsewhere (Wang et al., 2008). Human cDNA FLJ22101 fis (GenBank accession number AK025754) was used as a housekeeping gene for normalization. Primer sets for FLJ22101 are as follows: F 5′-TTCCCTGTGGCACTTGACATT-3′, R 5′-CTTTTGCCTCTGGCAGTACTCA-3′. An annealing temperature of 57 °C was used for all the primers. PCR reactions were performed in a standard 96-well plate format with BioRad iQ™5 multicolor real time PCR detection system. For data analysis, raw Ct was first normalized to the housekeeping gene for each sample to obtain dCt. The normalized dCt was then calibrated to control cell samples to obtain ddCt.
TGF-β1, TGF-α and amphiregulin immunoassays
Cells grown on 100-mm dishes (1×106 cells/dish) were incubated for 24 h in serum-free medium. Conditioned medium was collected, pre-cleared by centrifugation, and analyzed for total amount of TGF-β1, TGF-α and amphiregulin using Quantikine™ human TGF-β1 or TGF-α immunoassay kit (R&D Systems) and DuoSet™ human amphiregulin kit (R&D Systems), respectively. Plate preparation and assay procedures were performed according to the manufacturer’s protocols. Each value was normalized by total protein concentration of each sample. For immunoassays of TGF-α and amphiregulin, cetuximab (10 μg/ml) was added at the beginning of the incubation to prevent ligand binding to the EGFR and their subsequent internalization.
Co-culture cell growth assays
Co-culture assays were performed using transwell tissue-culture inserts with a 0.4-μm microporous membrane (Becton Dickinson Labware). Target cells were plated (2×104) onto the bottom of 12-well plates. Medium-conditioning cells (5×103) were plated on the permeable membrane of tissue culture inserts which were then introduced into target cell-containing wells. Co-cultures were carried out in serum-free medium in the presence or absence of cetuximab (10 μg/ml). After 72 h, the test cells on the bottom of the wells were harvested by trypsinization and cell number was determined in a Coulter counter. A schematic representation of the co-culture experiments is shown in Fig. 5C.
Three-dimensional morphogenesis and indirect immunofluorescence
Cells were seeded on Growth Factor Reduced Matrigel (BD Biosciences) in 8-well chamber slides following the protocol described by Debnath et al. (Debnath et al., 2003). In the case of co-culture, cells were labeled with PKH67 green (for MCF10A/HER2WT cells) or PKH26 red (for MCF10A/HER2YVMA cells) fluorescent cell linkers (Sigma) according to manufacturer’s protocol. Labeled cells were immediately seeded on Matrigel following the protocol described by Debnath et al. (Debnath et al., 2003) except that EGF was omitted from the top medium. For single-cell cultures, 6×103 cells were seeded on day 0, whereas for co-culture of mixed cell types, 3×103 cells of each cell type (a total of 6×103 cells) were seeded. Inhibitors were added into the medium 12 h after cell seeding. The fluorescent images were captured on day 6 using Zeiss LSM510 confocal microscopy system. Acini were trypsinized and total cell number of each labeled cell type was determined under an upright fluorescent microscope. Indirect immunofluorescence assay (IFA) was performed as described previously (Wang et al., 2005). Fluorescent images were captured using a Princeton Instruments cooled CCD digital camera from a Zeiss Axiophot upright microscope. Primary antibodies include E-cadherin and N-cadherin. The fluorescent antibodies are Oregon Green-α-mouse IgG and Texas Red-α-rabbit IgG (Molecular Probes).
Endothelial cell migration assay
Polyvinylpyrrolidone-free polycarbonate transwells with 8-μm pores (Costar) were pre-coated with a mixture of collagen I (20 μg/ml) and collagen IV (10 μg/ml) overnight at 4°C. After blocking the filters with 3% BSA in PBS to inhibit nonspecific migration, the lower wells of the chamber were filled with 0.4 ml of concentrated conditioned medium harvested from BEAS2B/vec, BEAS2B/HER2WT or BEAS2B/HER2YVMA cells. Added CM had been concentrated 10-fold using 5K Centrifugal Filters (Amicon). Human endothelial cells (ECs) were collected from subconfluent cultures and resuspended in the same concentrated conditioned medium. A total of 5×104 cells/100 μl were added to the upper chamber and then incubated for 4 h at 37°C. At the end of the incubation, cells remaining on the top of the filter were removed by wiping. Filters were fixed in 3% formaldehyde in PBS and cells that had migrated to the underside of the transwells were with 1% crystal violet and counted under microscopy.
Acknowledgements
This work was supported by NCI K99/R00 CA125892 (SEW), NCI R01 CA62212 (CLA), R01 CA80195 (CLA), ACS Clinical Research Professorship Grant CRP-07-234 (CLA), Breast Cancer Specialized Program of Research Excellence (SPORE) P50 CA98131, and Vanderbilt-Ingram Comprehensive Cancer Center Support Grant P30 CA68485.
Footnotes
Conflict of Interest
The authors hereby declare that there are no competing financial interests in relation to the work described.
References
- Adam L, Vadlamudi R, Kondapaka SB, Chernoff J, Mendelsohn J, Kumar R. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J Biol Chem. 1998;273:28238–46. doi: 10.1074/jbc.273.43.28238. [DOI] [PubMed] [Google Scholar]
- Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–37. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- Atfi A, Djelloul S, Chastre E, Davis R, Gespach C. Evidence for a role of Rho-like GTPases and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) in transforming growth factor beta-mediated signaling. J Biol Chem. 1997;272:1429–32. doi: 10.1074/jbc.272.3.1429. [DOI] [PubMed] [Google Scholar]
- Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci. 2002;115:3193–206. doi: 10.1242/jcs.115.15.3193. [DOI] [PubMed] [Google Scholar]
- Barnard JA, Graves-Deal R, Pittelkow MR, DuBois R, Cook P, Ramsey GW, et al. Auto- and cross-induction within the mammalian epidermal growth factor-related peptide family. J Biol Chem. 1994;269:22817–22. [PubMed] [Google Scholar]
- Bhowmick NA, Ghiassi M, Bakin A, Aakre M, Lundquist CA, Engel ME, et al. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12:27–36. doi: 10.1091/mbc.12.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon LY, Zhu H, Zhou B, Diedrich F, Singleton PA, Hung MC. Hyaluronan promotes CD44v3-Vav2 interaction with Grb2-p185(HER2) and induces Rac1 and Ras signaling during ovarian tumor cell migration and growth. J Biol Chem. 2001;276:48679–92. doi: 10.1074/jbc.M106759200. [DOI] [PubMed] [Google Scholar]
- Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–68. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- DeBusk LM, Hallahan DE, Lin PC. Akt is a major angiogenic mediator downstream of the Ang1/Tie2 signaling pathway. Exp Cell Res. 2004;298:167–77. doi: 10.1016/j.yexcr.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–29. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- Dumont N, Arteaga CL. Targeting the TGF beta signaling network in human neoplasia. Cancer Cell. 2003;3:531–6. doi: 10.1016/s1535-6108(03)00135-1. [DOI] [PubMed] [Google Scholar]
- Erdogan M, Pozzi A, Bhowmick N, Moses HL, Zent R. Signaling pathways regulating TC21-induced tumorigenesis. J Biol Chem. 2007;282:27713–20. doi: 10.1074/jbc.M703037200. [DOI] [PubMed] [Google Scholar]
- Fahey MS, Paterson IC, Stone A, Collier AJ, Heung YL, Davies M, et al. Dysregulation of autocrine TGF-beta isoform production and ligand responses in human tumour-derived and Ha-ras-transfected keratinocytes and fibroblasts. Br J Cancer. 1996;74:1074–80. doi: 10.1038/bjc.1996.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong TA, Shawver LK, Sun L, Tang C, App H, Powell TJ, et al. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999;59:99–106. [PubMed] [Google Scholar]
- Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int J Cancer. 1999;81:682–7. doi: 10.1002/(sici)1097-0215(19990531)81:5<682::aid-ijc2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. Embo J. 1997;16:1647–55. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasley LE, Han SY. JNK regulation of oncogenesis. Mol Cells. 2006;21:167–73. [PubMed] [Google Scholar]
- Janda E, Lehmann K, Killisch I, Jechlinger M, Herzig M, Downward J, et al. Ras and TGF[beta] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J Cell Biol. 2002;156:299–313. doi: 10.1083/jcb.200109037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josko J, Mazurek M. Transcription factors having impact on vascular endothelial growth factor (VEGF) gene expression in angiogenesis. Med Sci Monit. 2004;10:RA89–98. [PubMed] [Google Scholar]
- Kim ES, Kim MS, Moon A. Transforming growth factor (TGF)-beta in conjunction with H-ras activation promotes malignant progression of MCF10A breast epithelial cells. Cytokine. 2005;29:84–91. doi: 10.1016/j.cyto.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Angel P, Lafyatis R, Hattori K, Kim KY, Sporn MB, et al. Autoinduction of transforming growth factor beta 1 is mediated by the AP-1 complex. Mol Cell Biol. 1990;10:1492–7. doi: 10.1128/mcb.10.4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Soung YH, Seo SH, Kim SY, Park CH, Wang YP, et al. Somatic mutations of ERBB2 kinase domain in gastric, colorectal, and breast carcinomas. Clin Cancer Res. 2006;12:57–61. doi: 10.1158/1078-0432.CCR-05-0976. [DOI] [PubMed] [Google Scholar]
- Lo RS, Wotton D, Massague J. Epidermal growth factor signaling via Ras controls the Smad transcriptional co-repressor TGIF. EMBO J. 2001;20:128–36. doi: 10.1093/emboj/20.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity A, Pore N, Lee J, Solomon D, O’Rourke DM. Epidermal growth factor receptor transcriptionally up-regulates vascular endothelial growth factor expression in human glioblastoma cells via a pathway involving phosphatidylinositol 3′-kinase and distinct from that induced by hypoxia. Cancer Res. 2000;60:5879–86. [PubMed] [Google Scholar]
- Malipiero U, Holler M, Werner U, Fontana A. Sequence analysis of the promoter region of the glioblastoma derived T cell suppressor factor/transforming growth factor (TGF)-beta 2 gene reveals striking differences to the TGF-beta 1 and -beta 3 genes. Biochem Biophys Res Commun. 1990;171:1145–51. doi: 10.1016/0006-291x(90)90804-v. [DOI] [PubMed] [Google Scholar]
- Moulder SL, Yakes FM, Muthuswamy SK, Bianco R, Simpson JF, Arteaga CL. Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Research. 2001;61:8887–8895. [PubMed] [Google Scholar]
- Mucsi I, Skorecki KL, Goldberg HJ. Extracellular signal-regulated kinase and the small GTP-binding protein, Rac, contribute to the effects of transforming growth factor- beta1 on gene expression. J Biol Chem. 1996;271:16567–72. doi: 10.1074/jbc.271.28.16567. [DOI] [PubMed] [Google Scholar]
- Muraoka-Cook RS, Kurokawa H, Koh Y, Forbes JT, Roebuck LR, Barcellos-Hoff MH, et al. Conditional overexpression of active transforming growth factor beta1 in vivo accelerates metastases of transgenic mammary tumors. Cancer Res. 2004;64:9002–11. doi: 10.1158/0008-5472.CAN-04-2111. [DOI] [PubMed] [Google Scholar]
- Muraoka RS, Koh Y, Roebuck LR, Sanders ME, Brantley-Sieders D, Gorska AE, et al. Increased malignancy of Neu-induced mammary tumors overexpressing active transforming growth factor beta1. Mol Cell Biol. 2003;23:8691–703. doi: 10.1128/MCB.23.23.8691-8703.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanno N, Bianco C, De Luca A, Salomon DS. The role of EGF-related peptides in tumor growth. Front Biosci. 2001;6:D685–707. doi: 10.2741/normano. [DOI] [PubMed] [Google Scholar]
- Oft M, Akhurst RJ, Balmain A. Metastasis is driven by sequential elevation of H-ras and Smad2 levels. Nat Cell Biol. 2002;4:487–94. doi: 10.1038/ncb807. [DOI] [PubMed] [Google Scholar]
- Peng SB, Yan L, Xia X, Watkins SA, Brooks HB, Beight D, et al. Kinetic characterization of novel pyrazole TGF-beta receptor I kinase inhibitors and their blockade of the epithelial-mesenchymal transition. Biochemistry. 2005;44:2293–304. doi: 10.1021/bi048851x. [DOI] [PubMed] [Google Scholar]
- Pinkas-Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L, et al. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. Embo J. 1996;15:2452–67. [PMC free article] [PubMed] [Google Scholar]
- Rusnak DW, Lackey K, Affleck K, Wood ER, Alligood KJ, Rhodes N, et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther. 2001;1:85–94. [PubMed] [Google Scholar]
- Sanchez-Elsner T, Botella LM, Velasco B, Corbi A, Attisano L, Bernabeu C. Synergistic cooperation between hypoxia and transforming growth factor-beta pathways on human vascular endothelial growth factor gene expression. J Biol Chem. 2001;276:38527–35. doi: 10.1074/jbc.M104536200. [DOI] [PubMed] [Google Scholar]
- Sawyer JS, Beight DW, Britt KS, Anderson BD, Campbell RM, Goodson T, Jr., et al. Synthesis and activity of new aryl- and heteroaryl-substituted 5,6-dihydro-4H-pyrrolo[1,2-b]pyrazole inhibitors of the transforming growth factor-beta type I receptor kinase domain. Bioorg Med Chem Lett. 2004;14:3581–4. doi: 10.1016/j.bmcl.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Schnelzer A, Prechtel D, Knaus U, Dehne K, Gerhard M, Graeff H, et al. Rac1 in human breast cancer: overexpression, mutation analysis, and characterization of a new isoform, Rac1b. Oncogene. 2000;19:3013–20. doi: 10.1038/sj.onc.1203621. [DOI] [PubMed] [Google Scholar]
- Seton-Rogers SE, Lu Y, Hines LM, Koundinya M, LaBaer J, Muthuswamy SK, et al. Cooperation of the ErbB2 receptor and transforming growth factor beta in induction of migration and invasion in mammary epithelial cells. Proc Natl Acad Sci U S A. 2004;101:1257–62. doi: 10.1073/pnas.0308090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–81. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- Shigematsu H, Takahashi T, Nomura M, Majmudar K, Suzuki M, Lee H, et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res. 2005;65:1642–6. doi: 10.1158/0008-5472.CAN-04-4235. [DOI] [PubMed] [Google Scholar]
- Shirakata Y, Komurasaki T, Toyoda H, Hanakawa Y, Yamasaki K, Tokumaru S, et al. Epiregulin, a novel member of the epidermal growth factor family, is an autocrine growth factor in normal human keratinocytes. J Biol Chem. 2000;275:5748–53. doi: 10.1074/jbc.275.8.5748. [DOI] [PubMed] [Google Scholar]
- Siegel PM, Shu W, Cardiff RD, Muller WJ, Massague J. Transforming growth factor beta signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis. Proc Natl Acad Sci U S A. 2003;100:8430–5. doi: 10.1073/pnas.0932636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens P, Hunter C, Bignell G, Edkins S, Davies H, Teague J, et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature. 2004;431:525–6. doi: 10.1038/431525b. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Wang S, Dumont N, Yi JY, Koh Y, Arteaga CL. Overexpression of HER2 (erbB2) in human breast epithelial cells unmasks transforming growth factor beta-induced cell motility. J Biol Chem. 2004;279:24505–13. doi: 10.1074/jbc.M400081200. [DOI] [PubMed] [Google Scholar]
- Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–49. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- Wang LM, Kuo A, Alimandi M, Veri MC, Lee CC, Kapoor V, et al. ErbB2 expression increases the spectrum and potency of ligand-mediated signal transduction through ErbB4. Proc Natl Acad Sci U S A. 1998;95:6809–14. doi: 10.1073/pnas.95.12.6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SE, Narasanna A, Perez-Torres M, Xiang B, Wu FY, Yang S, et al. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell. 2006a;10:25–38. doi: 10.1016/j.ccr.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Wang SE, Shin I, Wu FY, Friedman DB, Arteaga CL. HER2/Neu (ErbB2) Signaling to Rac1-Pak1 Is Temporally and Spatially Modulated by Transforming Growth Factor {beta} Cancer Res. 2006b;66:9591–600. doi: 10.1158/0008-5472.CAN-06-2071. [DOI] [PubMed] [Google Scholar]
- Wang SE, Wu FY, Shin I, Qu S, Arteaga CL. Transforming growth factor {beta} (TGF-{beta})-Smad target gene protein tyrosine phosphatase receptor type kappa is required for TGF-{beta} function. Mol Cell Biol. 2005;25:4703–15. doi: 10.1128/MCB.25.11.4703-4715.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SE, Xiang B, Guix M, Olivares MG, Parker J, Chung CH, et al. Transforming growth factor beta engages TACE and ErbB3 to activate phosphatidylinositol-3 kinase/Akt in ErbB2-overexpressing breast cancer and desensitizes cells to trastuzumab. Mol Cell Biol. 2008;28:5605–20. doi: 10.1128/MCB.00787-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert C, Sauer U, Brodbeck K, Pfeiffer A, Haring HU, Schleicher ED. AP-1 proteins mediate hyperglycemia-induced activation of the human TGF-beta1 promoter in mesangial cells. J Am Soc Nephrol. 2000;11:2007–16. doi: 10.1681/ASN.V11112007. [DOI] [PubMed] [Google Scholar]
- Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–34. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- Worthylake R, Opresko LK, Wiley HS. ErbB-2 amplification inhibits down-regulation and induces constitutive activation of both ErbB-2 and epidermal growth factor receptors. J Biol Chem. 1999;274:8865–74. doi: 10.1074/jbc.274.13.8865. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Ye D, Mendelsohn J, Fan Z. Augmentation of a humanized anti-HER2 mAb 4D5 induced growth inhibition by a human-mouse chimeric anti-EGF receptor mAb C225. Oncogene. 1999;18:731–8. doi: 10.1038/sj.onc.1202319. [DOI] [PubMed] [Google Scholar]