Abstract
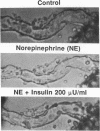
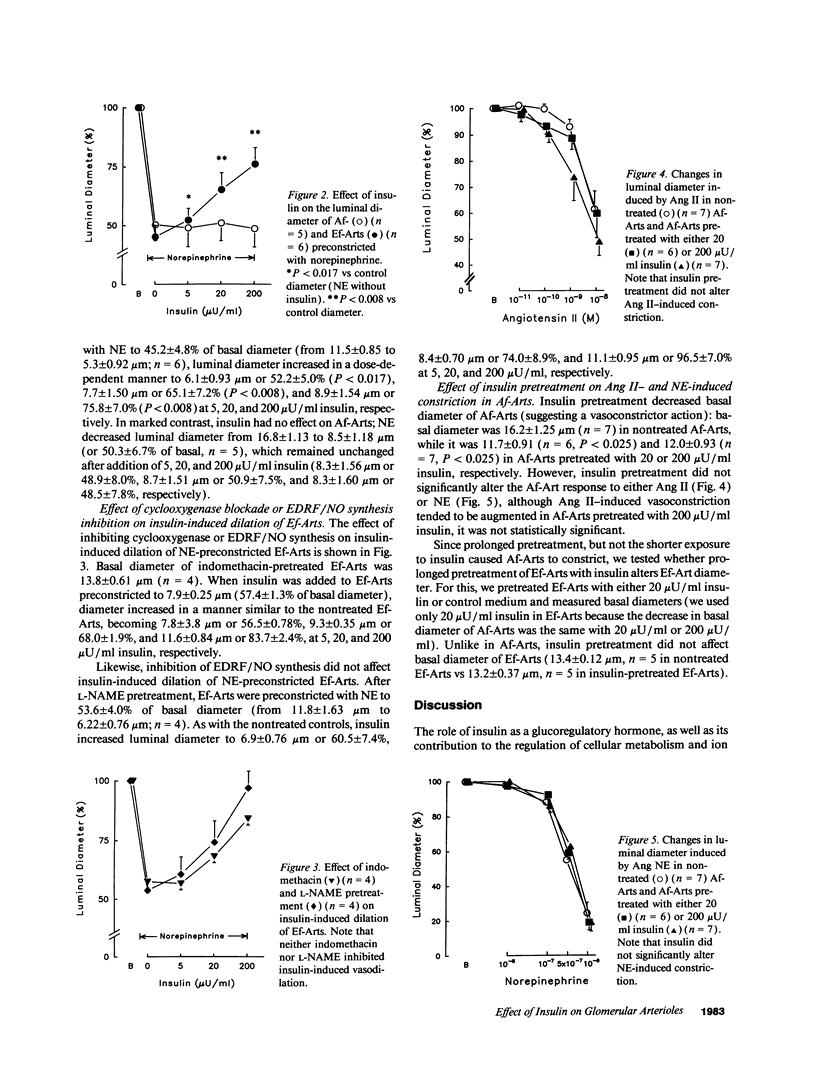
Despite evidence that insulin per se may be an important regulator of glomerular hemodynamics, little is known about its direct action on the glomerular afferent arterioles (Af-Art) and efferent arterioles (Ef-Art), the crucial vascular segments that control glomerular hemodynamics. In the present study, we examined the direct effect of physiological concentrations of insulin on isolated microperfused rabbit Af- and Ef-Arts. After cannulation, vessels were equilibrated in insulin-free medium for 30 min. To determine whether insulin causes vasodilation or constriction, increasing doses (5, 20, and 200 microU/ml) were added to the bath and lumen of arterioles that were either preconstricted to 50% of control diameter with norepinephrine or left nonpreconstricted. Insulin caused no vasoconstriction in either Af- or Ef-Arts, but it reversed norepinephrine-induced constriction in Ef-Arts but not Af-Arts (suggesting a vasodilator action selective to the Ef-Art): at 200 microU/ml, insulin increased Ef-Art luminal diameter by 75.8 +/- 7.0% from the preconstricted level (n = 6; P < 0.008). The vasorelaxant effect of insulin on Ef-Arts was not affected by blockade of either endothelium-derived relaxing factor/nitric oxide or prostaglandin synthesis. Despite the lack of effect of insulin on Af-Art when added after the equilibration period, when Af-Arts were equilibrated in the presence of either 20 or 200 microU/ml insulin, their basal diameter was significantly reduced (11.7 +/- 0.9 microns; P < 0.025, n = 6, and 12.0 +/- 0.9 microns; P < 0.025, n = 7, respectively) compared with nontreated Af-Arts (16.2 +/- 1.3 microns; n = 7). In conclusion, this study demonstrates that at physiological concentrations, insulin dilates NE-constricted Ef-Arts, while insulin pretreatment enhances Af-Art tone. The disparate actions of insulin on the Af- vs the Ef-Art may contribute to its beneficial effect on glomerular hypertension.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrass C. K., Raugi G. J., Gabourel L. S., Lovett D. H. Insulin and insulin-like growth factor I binding to cultured rat glomerular mesangial cells. Endocrinology. 1988 Nov;123(5):2432–2439. doi: 10.1210/endo-123-5-2432. [DOI] [PubMed] [Google Scholar]
- Bank N., Klose R., Aynedjian H. S., Nguyen D., Sablay L. B. Evidence against increased glomerular pressure initiating diabetic nephropathy. Kidney Int. 1987 Apr;31(4):898–905. doi: 10.1038/ki.1987.83. [DOI] [PubMed] [Google Scholar]
- Butlen D., Vadrot S., Roseau S., Morel F. Insulin receptors along the rat nephron: [125I] insulin binding in microdissected glomeruli and tubules. Pflugers Arch. 1988 Oct;412(6):604–612. doi: 10.1007/BF00583761. [DOI] [PubMed] [Google Scholar]
- Cavaliere T. A., Taylor D. G. The effects of insulin on vasoconstrictor responses in pithed rats. J Clin Pharmacol. 1981 Jul;21(7):275–279. doi: 10.1002/j.1552-4604.1981.tb01766.x. [DOI] [PubMed] [Google Scholar]
- Christiansen J. S., Gammelgaard J., Frandsen M., Parving H. H. Increased kidney size, glomerular filtration rate and renal plasma flow in short-term insulin-dependent diabetics. Diabetologia. 1981 Apr;20(4):451–456. doi: 10.1007/BF00253406. [DOI] [PubMed] [Google Scholar]
- Cohen A. J., McCarthy D. M., Stoff J. S. Direct hemodynamic effect of insulin in the isolated perfused kidney. Am J Physiol. 1989 Oct;257(4 Pt 2):F580–F585. doi: 10.1152/ajprenal.1989.257.4.F580. [DOI] [PubMed] [Google Scholar]
- Creager M. A., Liang C. S., Coffman J. D. Beta adrenergic-mediated vasodilator response to insulin in the human forearm. J Pharmacol Exp Ther. 1985 Dec;235(3):709–714. [PubMed] [Google Scholar]
- DeFronzo R. A., Cooke C. R., Andres R., Faloona G. R., Davis P. J. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest. 1975 Apr;55(4):845–855. doi: 10.1172/JCI107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Goldberg M., Agus Z. S. The effects of glucose and insulin on renal electrolyte transport. J Clin Invest. 1976 Jul;58(1):83–90. doi: 10.1172/JCI108463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot S. J., Conti F. G., Striker L. J., Striker G. E. Mouse glomerular endothelial cells have an insulin receptor. Horm Metab Res. 1990 Nov;22(11):557–560. doi: 10.1055/s-2007-1004972. [DOI] [PubMed] [Google Scholar]
- Hall J. E., Brands M. W., Kivlighn S. D., Mizelle H. L., Hildebrandt D. A., Gaillard C. A. Chronic hyperinsulinemia and blood pressure. Interaction with catecholamines? Hypertension. 1990 May;15(5):519–527. doi: 10.1161/01.hyp.15.5.519. [DOI] [PubMed] [Google Scholar]
- Hall J. E., Brands M. W., Mizelle H. L., Gaillard C. A., Hildebrandt D. A. Chronic intrarenal hyperinsulinemia does not cause hypertension. Am J Physiol. 1991 May;260(5 Pt 2):F663–F669. doi: 10.1152/ajprenal.1991.260.5.F663. [DOI] [PubMed] [Google Scholar]
- Hostetter T. H., Rennke H. G., Brenner B. M. The case for intrarenal hypertension in the initiation and progression of diabetic and other glomerulopathies. Am J Med. 1982 Mar;72(3):375–380. doi: 10.1016/0002-9343(82)90490-9. [DOI] [PubMed] [Google Scholar]
- Hostetter T. H., Troy J. L., Brenner B. M. Glomerular hemodynamics in experimental diabetes mellitus. Kidney Int. 1981 Mar;19(3):410–415. doi: 10.1038/ki.1981.33. [DOI] [PubMed] [Google Scholar]
- Ito S., Arima S., Ren Y. L., Juncos L. A., Carretero O. A. Endothelium-derived relaxing factor/nitric oxide modulates angiotensin II action in the isolated microperfused rabbit afferent but not efferent arteriole. J Clin Invest. 1993 May;91(5):2012–2019. doi: 10.1172/JCI116423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Carretero O. A. An in vitro approach to the study of macula densa-mediated glomerular hemodynamics. Kidney Int. 1990 Dec;38(6):1206–1210. doi: 10.1038/ki.1990.335. [DOI] [PubMed] [Google Scholar]
- Ito S., Johnson C. S., Carretero O. A. Modulation of angiotensin II-induced vasoconstriction by endothelium-derived relaxing factor in the isolated microperfused rabbit afferent arteriole. J Clin Invest. 1991 May;87(5):1656–1663. doi: 10.1172/JCI115181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P. K., Christiansen J. S., Steven K., Parving H. H. Renal function in streptozotocin-diabetic rats. Diabetologia. 1981 Oct;21(4):409–414. [PubMed] [Google Scholar]
- Jensen P. K., Steven K., Blaehr H., Christiansen J. S., Parving H. H. Effects of indomethacin on glomerular hemodynamics in experimental diabetes. Kidney Int. 1986 Feb;29(2):490–495. doi: 10.1038/ki.1986.26. [DOI] [PubMed] [Google Scholar]
- Kirchner K. A. Insulin increases loop segment chloride reabsorption in the euglycemic rat. Am J Physiol. 1988 Dec;255(6 Pt 2):F1206–F1213. doi: 10.1152/ajprenal.1988.255.6.F1206. [DOI] [PubMed] [Google Scholar]
- Kurokawa K., Silverblatt F. J., Klein K. L., Wang M. S., Lerner R. L. Binding of 125I-insulin to the isolated glomeruli of rat kidney. J Clin Invest. 1979 Nov;64(5):1357–1364. doi: 10.1172/JCI109592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels L. D., Davidman M., Keane W. F. Determinants of glomerular filtration and plasma flow in experimental diabetic rats. J Lab Clin Med. 1981 Dec;98(6):869–885. [PubMed] [Google Scholar]
- Moore R. D., Rabovsky J. L. Mechanism of insulin action on resting membrane potential of frog skeletal muscle. Am J Physiol. 1979 May;236(5):C249–C254. doi: 10.1152/ajpcell.1979.236.5.C249. [DOI] [PubMed] [Google Scholar]
- Osgood R. W., Patton M., Hanley M. J., Venkatachalam M., Reineck H. J., Stein J. H. In vitro perfusion of the isolated dog glomerulus. Am J Physiol. 1983 Mar;244(3):F349–F354. doi: 10.1152/ajprenal.1983.244.3.F349. [DOI] [PubMed] [Google Scholar]
- Scholey J. W., Meyer T. W. Control of glomerular hypertension by insulin administration in diabetic rats. J Clin Invest. 1989 Apr;83(4):1384–1389. doi: 10.1172/JCI114026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standley P. R., Zhang F., Ram J. L., Zemel M. B., Sowers J. R. Insulin attenuates vasopressin-induced calcium transients and a voltage-dependent calcium response in rat vascular smooth muscle cells. J Clin Invest. 1991 Oct;88(4):1230–1236. doi: 10.1172/JCI115426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker B. J., Anderson C. M., Thies R. S., Collins R. C., Blantz R. C. Glomerular hemodynamic alterations during acute hyperinsulinemia in normal and diabetic rats. Kidney Int. 1992 Nov;42(5):1160–1168. doi: 10.1038/ki.1992.400. [DOI] [PubMed] [Google Scholar]
- Yagi S., Takata S., Kiyokawa H., Yamamoto M., Noto Y., Ikeda T., Hattori N. Effects of insulin on vasoconstrictive responses to norepinephrine and angiotensin II in rabbit femoral artery and vein. Diabetes. 1988 Aug;37(8):1064–1067. doi: 10.2337/diab.37.8.1064. [DOI] [PubMed] [Google Scholar]
- Yanagisawa-Miwa A., Ito H., Sugimoto T. Effects of insulin on vasoconstriction induced by thromboxane A2 in porcine coronary artery. Circulation. 1990 May;81(5):1654–1659. doi: 10.1161/01.cir.81.5.1654. [DOI] [PubMed] [Google Scholar]
- ZIERLER K. L. Effect of insulin on membrane potential and potassium content of rat muscle. Am J Physiol. 1959 Sep;197:515–523. doi: 10.1152/ajplegacy.1959.197.3.515. [DOI] [PubMed] [Google Scholar]
- ZIERLER K. L. Increase in resting membrane potential of skeletal muscle produced by insulin. Science. 1957 Nov 22;126(3282):1067–1068. doi: 10.1126/science.126.3282.1067. [DOI] [PubMed] [Google Scholar]
- Zatz R., Dunn B. R., Meyer T. W., Anderson S., Rennke H. G., Brenner B. M. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J Clin Invest. 1986 Jun;77(6):1925–1930. doi: 10.1172/JCI112521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierler K., Rogus E. M. Effects of peptide hormones and adrenergic agents on membrane potentials of target cells. Fed Proc. 1981 Feb;40(2):121–124. [PubMed] [Google Scholar]