Abstract
Exposure of rabbit peritoneal exudate macrophages (PEM) or whole blood to picomolar concentrations of LPS induces adaptation or hyporesponsiveness to LPS. Because of the importance of plasma LPS-binding protein (LBP) and the macrophage cell membrane protein CD14 in recognition of LPS, we examined the effect of LBP on LPS-induced adaptation in PEM. PEM exposed to LPS in the presence of LBP for 8 h were markedly less responsive to subsequent stimulation by LPS than monocytes/macrophages (M phi) adapted in the absence of LBP. LPS-induced expression of TNF was sharply reduced in LBP-LPS-adapted PEM, but in contrast these cells remained fully responsive to Staphylococcus aureus peptidoglycan. We considered that specific hyporesponsiveness in LPS-adapted M phi or in blood monocytes could be due to decreased expression of CD14 or diminished binding of LBP-LPS complexes to CD14. However, flow cytometry analysis revealed only minimal reduction of CD14 expression or CD14-dependent binding of a fluorescent LPS derivative when normo- and hyporesponsive cells were compared. These results show that complexes of LPS and LBP are more effective than LPS alone in inducing adaptation to LPS, and LPS-induced hyporesponsiveness probably results from changes in cellular elements distinct from CD14 that are involved in either LPS recognition or LPS-specific signal transduction.
Full text
PDF

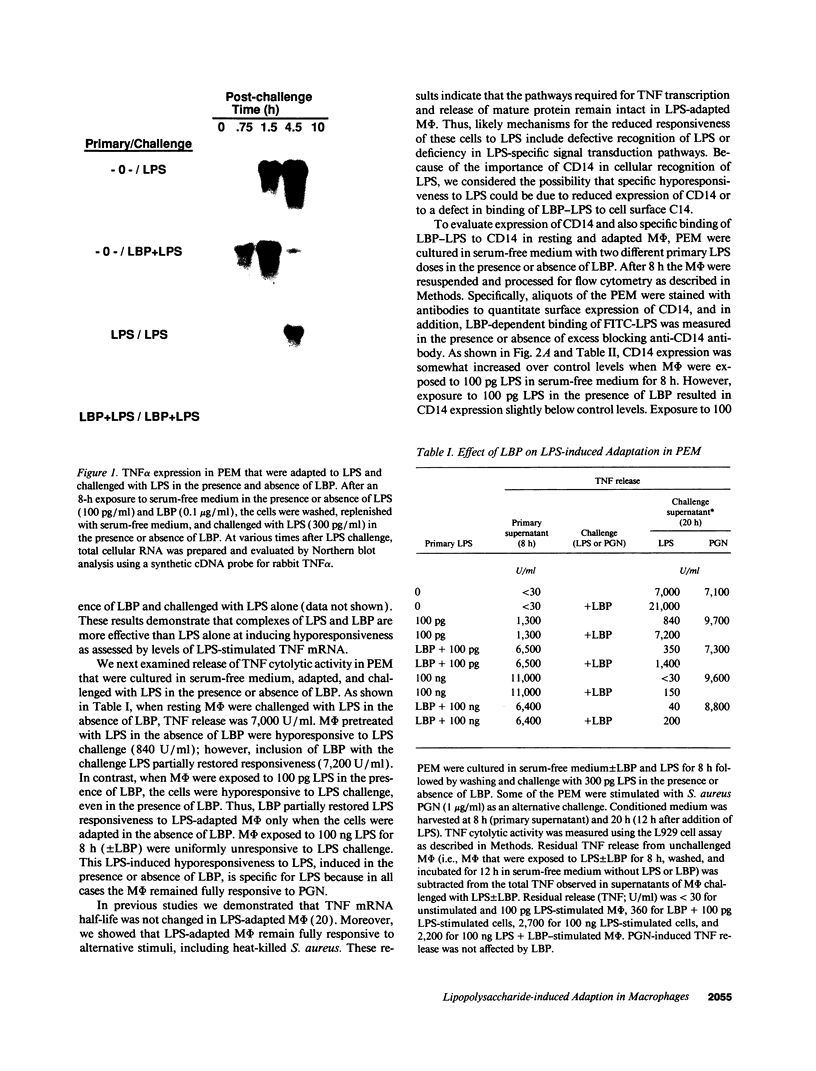




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annenkov A. Y., Baranova F. S. Lipopolysaccharide-dependent and lipopolysaccharide-independent pathways of monocyte desensitisation to lipopolysaccharides. J Leukoc Biol. 1991 Sep;50(3):215–222. doi: 10.1002/jlb.50.3.215. [DOI] [PubMed] [Google Scholar]
- Beutler B. A., Milsark I. W., Cerami A. Cachectin/tumor necrosis factor: production, distribution, and metabolic fate in vivo. J Immunol. 1985 Dec;135(6):3972–3977. [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987 Feb 12;316(7):379–385. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Blanchard D. K., Djeu J. Y., Klein T. W., Friedman H., Stewart W. E., 2nd Protective effects of tumor necrosis factor in experimental Legionella pneumophila infections of mice via activation of PMN function. J Leukoc Biol. 1988 May;43(5):429–435. doi: 10.1002/jlb.43.5.429. [DOI] [PubMed] [Google Scholar]
- Corradin S. B., Mauël J., Gallay P., Heumann D., Ulevitch R. J., Tobias P. S. Enhancement of murine macrophage binding of and response to bacterial lipopolysaccharide (LPS) by LPS-binding protein. J Leukoc Biol. 1992 Oct;52(4):363–368. doi: 10.1002/jlb.52.4.363. [DOI] [PubMed] [Google Scholar]
- Danner R. L., Elin R. J., Hosseini J. M., Wesley R. A., Reilly J. M., Parillo J. E. Endotoxemia in human septic shock. Chest. 1991 Jan;99(1):169–175. doi: 10.1378/chest.99.1.169. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F. Trace levels of bacterial lipopolysaccharide prevent interferon-gamma or tumor necrosis factor-alpha from enhancing mouse peritoneal macrophage respiratory burst capacity. J Immunol. 1987 Sep 15;139(6):1971–1977. [PubMed] [Google Scholar]
- Ding A. H., Sanchez E., Srimal S., Nathan C. F. Macrophages rapidly internalize their tumor necrosis factor receptors in response to bacterial lipopolysaccharide. J Biol Chem. 1989 Mar 5;264(7):3924–3929. [PubMed] [Google Scholar]
- Dziarski R. Demonstration of peptidoglycan-binding sites on lymphocytes and macrophages by photoaffinity cross-linking. J Biol Chem. 1991 Mar 15;266(8):4713–4718. [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Glode L. M., Mergenhagen S. E., Rosenstreich D. L. Significant contribution of spleen cells in mediating the lethal effects of endotoxin in vivo. Infect Immun. 1976 Sep;14(3):626–630. doi: 10.1128/iai.14.3.626-630.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas J. G., Thiel C., Blömer K., Weiss E. H., Riethmüller G., Ziegler-Heitbrock H. W. Downregulation of tumor necrosis factor expression in the human Mono-Mac-6 cell line by lipopolysaccharide. J Leukoc Biol. 1989 Jul;46(1):11–14. doi: 10.1002/jlb.46.1.11. [DOI] [PubMed] [Google Scholar]
- Heumann D., Gallay P., Barras C., Zaech P., Ulevitch R. J., Tobias P. S., Glauser M. P., Baumgartner J. D. Control of lipopolysaccharide (LPS) binding and LPS-induced tumor necrosis factor secretion in human peripheral blood monocytes. J Immunol. 1992 Jun 1;148(11):3505–3512. [PubMed] [Google Scholar]
- Kitchens R. L., Ulevitch R. J., Munford R. S. Lipopolysaccharide (LPS) partial structures inhibit responses to LPS in a human macrophage cell line without inhibiting LPS uptake by a CD14-mediated pathway. J Exp Med. 1992 Aug 1;176(2):485–494. doi: 10.1084/jem.176.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. D., Kato K., Tobias P. S., Kirkland T. N., Ulevitch R. J. Transfection of CD14 into 70Z/3 cells dramatically enhances the sensitivity to complexes of lipopolysaccharide (LPS) and LPS binding protein. J Exp Med. 1992 Jun 1;175(6):1697–1705. doi: 10.1084/jem.175.6.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. R., Mathison J. C., Tobias P. S., Letúrcq D. J., Moriarty A. M., Maunder R. J., Ulevitch R. J. Lipopolysaccharide binding protein enhances the responsiveness of alveolar macrophages to bacterial lipopolysaccharide. Implications for cytokine production in normal and injured lungs. J Clin Invest. 1992 Dec;90(6):2209–2219. doi: 10.1172/JCI116106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathison J. C., Tobias P. S., Wolfson E., Ulevitch R. J. Plasma lipopolysaccharide (LPS)-binding protein. A key component in macrophage recognition of gram-negative LPS. J Immunol. 1992 Jul 1;149(1):200–206. [PubMed] [Google Scholar]
- Mathison J. C., Virca G. D., Wolfson E., Tobias P. S., Glaser K., Ulevitch R. J. Adaptation to bacterial lipopolysaccharide controls lipopolysaccharide-induced tumor necrosis factor production in rabbit macrophages. J Clin Invest. 1990 Apr;85(4):1108–1118. doi: 10.1172/JCI114542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathison J. C., Wolfson E., Ulevitch R. J. Participation of tumor necrosis factor in the mediation of gram negative bacterial lipopolysaccharide-induced injury in rabbits. J Clin Invest. 1988 Jun;81(6):1925–1937. doi: 10.1172/JCI113540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek S. M., Moore R. N., McGhee J. R., Rosenstreich D. L., Mergenhagen S. E. The primary role of lymphoreticular cells in the mediation of host responses to bacterial endotoxim. J Infect Dis. 1980 Jan;141(1):55–63. doi: 10.1093/infdis/141.1.55. [DOI] [PubMed] [Google Scholar]
- Michie H. R., Manogue K. R., Spriggs D. R., Revhaug A., O'Dwyer S., Dinarello C. A., Cerami A., Wolff S. M., Wilmore D. W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988 Jun 9;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Munoz C., Carlet J., Fitting C., Misset B., Blériot J. P., Cavaillon J. M. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Invest. 1991 Nov;88(5):1747–1754. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane A., Minagawa T., Kato K. Endogenous tumor necrosis factor (cachectin) is essential to host resistance against Listeria monocytogenes infection. Infect Immun. 1988 Oct;56(10):2563–2569. doi: 10.1128/iai.56.10.2563-2569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y., Onozuka K., Terada Y., Shinomiya H., Nakano M. Protective effect of recombinant tumor necrosis factor-alpha in murine salmonellosis. J Immunol. 1990 Mar 1;144(5):1935–1941. [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst M. J., Hedegaard H. B., Johnston R. B., Jr Cultured human monocytes require exposure to bacterial products to maintain an optimal oxygen radical response. J Immunol. 1982 Jan;128(1):123–128. [PubMed] [Google Scholar]
- Schumann R. R., Leong S. R., Flaggs G. W., Gray P. W., Wright S. D., Mathison J. C., Tobias P. S., Ulevitch R. J. Structure and function of lipopolysaccharide binding protein. Science. 1990 Sep 21;249(4975):1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- Sherry B., Cerami A. Cachectin/tumor necrosis factor exerts endocrine, paracrine, and autocrine control of inflammatory responses. J Cell Biol. 1988 Oct;107(4):1269–1277. doi: 10.1083/jcb.107.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly R. R., Munkenbeck P., Morrison D. C. Stimulation of T-independent antibody responses by hapten-lipopolysaccharides without repeating polymeric structure. Infect Immun. 1979 Feb;23(2):287–293. doi: 10.1128/iai.23.2.287-293.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias P. S., Soldau K., Ulevitch R. J. Isolation of a lipopolysaccharide-binding acute phase reactant from rabbit serum. J Exp Med. 1986 Sep 1;164(3):777–793. doi: 10.1084/jem.164.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Virca G. D., Kim S. Y., Glaser K. B., Ulevitch R. J. Lipopolysaccharide induces hyporesponsiveness to its own action in RAW 264.7 cells. J Biol Chem. 1989 Dec 25;264(36):21951–21956. [PubMed] [Google Scholar]
- Vosbeck K., Tobias P., Mueller H., Allen R. A., Arfors K. E., Ulevitch R. J., Sklar L. A. Priming of polymorphonuclear granulocytes by lipopolysaccharides and its complexes with lipopolysaccharide binding protein and high density lipoprotein. J Leukoc Biol. 1990 Feb;47(2):97–104. doi: 10.1002/jlb.47.2.97. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Tobias P. S., Ulevitch R. J., Ramos R. A. Lipopolysaccharide (LPS) binding protein opsonizes LPS-bearing particles for recognition by a novel receptor on macrophages. J Exp Med. 1989 Oct 1;170(4):1231–1241. doi: 10.1084/jem.170.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman S. H., Evans G. F. Endotoxin tolerance: in vivo regulation of tumor necrosis factor and interleukin-1 synthesis is at the transcriptional level. Cell Immunol. 1992 Apr;140(2):513–519. doi: 10.1016/0008-8749(92)90216-c. [DOI] [PubMed] [Google Scholar]






