Abstract
Overexpression of cyclooxygenase-2 (COX-2) and elevated levels of its enzymatic product prostaglandin E2 (PGE2) occur in the majority of colorectal cancers and play important roles in colorectal tumorigenesis. However, despite the established prosurvival role of PGE2 in cancer, the underlying mechanisms are not fully understood. Here, we have shown that PGE2 suppresses apoptosis via repression of the proapoptotic BH3-only protein Bim in human colorectal adenoma cells. Repression of Bim expression was dependent upon PGE2-mediated activation of the Raf-MEK-ERK1/2 pathway which promoted Bim phosphorylation and proteasomal degradation. Reduction of Bim expression using RNA interference reduced spontaneous apoptosis in adenoma cells and abrogated PGE2-dependent apoptosis suppression. Treatment of COX-2-expressing colorectal carcinoma cells with COX-2-selective NSAIDs induced Bim expression, suggesting that Bim repression via PGE2 signalling may be opposed by COX-2 inhibition. Examination of Bim expression in two established in vitro models of the adenoma-carcinoma sequence revealed that downregulation of Bim expression was associated with tumour progression towards an anchorage-independent phenotype. Finally, immunohistochemical analyses revealed that Bim expression is markedly reduced in approximately 40% of human colorectal carcinomas in vivo. These observations highlight the COX-2/PGE2 pathway as an important negative regulator of Bim expression in colorectal tumours and suggest that Bim repression may be an important step during colorectal cancer tumorigenesis.
Introduction
Evidence from clinical trials and population-based studies has demonstrated that the cyclooxygenases (COX-1 and COX-2), the enzymes inhibited by non-steroidal anti-inflammatory drugs (NSAIDs), are bona fide targets for colorectal cancer prevention and therapy (Brown & DuBois, 2005). Of particular interest is COX-2 (the inducible form of cyclooxygenase), the key enzyme in prostaglandin E2 (PGE2) biosynthesis, which is overexpressed in the majority of colorectal cancers and thought to influence most – if not all – of the hallmarks of cancer (reviewed in Greenhough, et al., 2009). Understanding the molecular mechanisms of PGE2 signalling and NSAID action is important, as this may lead to the identification of novel targets for cancer prevention or therapy.
Impaired apoptosis is a hallmark of cancer which underpins both tumorigenesis and resistance to cytotoxic cancer therapies (Hall, et al., 1994, Hanahan & Weinberg, 2000, Johnstone, et al., 2002, Yang, et al., 2009). Indeed, both traditional and COX-2-selective NSAIDs are thought to exert their chemopreventive effects, at least in part, by promoting apoptosis (Chan, et al., 1998, Elder, et al., 1996, Grosch, et al., 2001, Hanif, et al., 1996, Loveridge, et al., 2008). The major pathway of apoptosis in vertebrate cells is the intrinsic pathway; however, while COX-2/PGE2 has previously been reported to activate prosurvival pathways including Raf-MEK-ERK1/2 (Pozzi, et al., 2004, Wang, et al., 2005), PI3K/Akt (Sheng, et al., 2001, Wang, et al., 2004), cAMP/protein kinase A (Hawcroft, et al., 2007, Nishihara, et al., 2003) and Wnt/β-catenin (Castellone, et al., 2005, Goessling, et al., 2009), it is still unclear how PGE2 signalling couples to the intrinsic cell death machinery, and clinically relevant downstream mediators of PGE2-mediated apoptosis suppression and NSAID-induced apoptosis remain elusive.
The intrinsic pathway of apoptosis is governed by the balance between opposing factions of the Bcl-2 protein family, wherein the proapoptotic activities of Bax and Bak are held in check by the inhibitory binding of prosurvival Bcl-2 family members such as Bcl-xL and Mcl-1 (Youle & Strasser, 2008). The life-death switch is controlled by the damage-sensing BH3-only members of the Bcl-2 family, which reside upstream of Bax and Bak (Cheng, et al., 2001) and promote apoptosis by antagonising the antiapoptotic activity of prosurvival Bcl-2 family members (Chen, et al., 2005, Willis, et al., 2007), or in some cases by directly activating Bax or Bak (Gallenne, et al., 2009, Gavathiotis, et al., 2008, Letai, et al., 2002, Lovell, et al., 2008). Bim is one of the most powerful BH3-only proteins, able to engage all prosurvival proteins potently (Certo, et al., 2006, Chen, et al., 2005) and activate Bax directly (Gavathiotis, et al., 2008). Evidence suggests that Bim is a tumour suppressor both in haematopoietic malignancies (Egle, et al., 2004) and in solid tumours of epithelial origin (Tan, et al., 2005). Bim expression and activity is controlled by both transcriptional and posttranslational mechanisms; for example, Bim is transcriptionally upregulated by FoxO3a in response to cytokine deprivation or PI3K-Akt pathway inhibition (Dijkers, et al., 2000) and by CHOP-C/EBPα under conditions of ER stress (Puthalakath, et al., 2007). Furthermore, phosphorylation of BimEL by ERK1/2 (Ley, et al., 2003, Luciano, et al., 2003) precludes its binding to prosurvival members of the Bcl-2 family (Ewings, et al., 2007) and primes it for additional phosphorylation events (Dehan, et al., 2009) resulting in its ubiquitination and proteasomal turnover (Ley, et al., 2004).
Recent investigations have revealed that Bim is a key mediator of apoptosis in response to several cancer therapeutics, including targeted therapies in ‘oncogene addicted’ cancer cell lines (for a review see Balmanno & Cook, 2009, and references therein). For example, repression of BimEL by ERK1/2 signalling is an important mechanism by which mutant-BRAFV600E (occurring in 10-20% of colorectal tumours) promotes cell survival (Wickenden, et al., 2008), and Bim mediates MEK inhibitor-induced apoptosis in BRAFV600E-mutant cancer cell lines (Cragg, et al., 2008, Wickenden, et al., 2008). Although the COX-2/PGE2 pathway is a known activator of Raf-MEK-ERK1/2 signalling, whether Bim expression is regulated by PGE2 or NSAIDs has not been reported. Furthermore, whether Bim is an important regulator of apoptosis during the premalignant adenoma stage of colorectal tumorigenesis is unknown, and the mechanisms underlying PGE2-mediated apoptosis suppression in human colorectal adenoma cells have not previously been investigated. We present evidence showing that PGE2 signalling leads to Bim repression and apoptosis suppression in human colorectal adenoma cells. Furthermore, we found that COX-2-selective NSAIDs induce Bim expression in colorectal carcinoma cell lines. Finally, we show that tumour progression is associated with decreased Bim expression in two in vitro models of the colorectal adenoma-carcinoma sequence, and while Bim is invariably expressed in the normal colonic epithelium, its expression is markedly reduced in ~40% of human colorectal carcinomas in vivo. These data highlight Bim as an important target of COX-2/PGE2 signalling in colorectal tumour cells and suggest that repression of Bim may be an important step during colorectal tumorigenesis.
Materials and methods
Cells and cell culture reagents
Human colorectal adenoma- and carcinoma-derived cell lines were maintained as described previously (Chell, et al., 2006, Kaidi, et al., 2007, Williams, et al., 2007). The RG/C2, RG/GV, AA/C1 and AA/C1/SB10C cell lines were derived in this laboratory and are described in detail elsewhere (Chell, et al., 2006, Paraskeva, et al., 1989, Williams, et al., 1990). Briefly, the AA/C1/SB10C cell line is an in vitro transformed anchorage-independent and tumorigenic variant of the anchorage-dependent and non-tumorigenic adenoma cell line AA/C1 (Williams, et al., 1990); the RG/GV cell line is an in vitro transformed anchorage-independent variant of the anchorage-dependent RG/C2 adenoma cell line (Chell, et al., 2006). HT29 cells were from the ATCC (Rockville, MD, USA); HCA7 cells were a kind gift from Susan Kirkland (Imperial College London, UK). Bim+/+ and Bim−/− immortalised mouse embryonic fibroblasts (iMEFs) (Bouillet, et al., 1999) were kindly provided by David Huang (WEHI, Melbourne, Australia). PGE2 was from Sigma (Poole, UK). The Akt inhibitor (Akt Inhibitor VIII, Isozyme-Selective, Akti-1/2), caspase inhibitor QVD-OPh, proteasome inhibitor MG132, and EGF-receptor inhibitor CL-387,785 were all from Calbiochem (EMD Biosciences, La Jolla, CA). The MEK inhibitor U0126 and lambda protein phosphatase (λ-PPase) were from Cell Signaling Technology (Danvers, MA, USA). The COX-2 selective NSAID NS-398 was from Cayman Chemical (Ann Arbor, MI, USA); Rofecoxib was kindly provided by Merck. All experiments were carried out in DMEM containing 10% FBS.
Western blot analysis
Cells were washed with ice-cold PBS prior to disruption on ice for ten minutes with Triton-X100-containing lysis buffer supplemented with protease inhibitors (Roche Diagnostics, East Sussex, UK). Equal amounts of protein were separated by SDS-PAGE, transferred to nitrocellulose membranes, probed with primary/secondary antibodies, and visualised using a chemiluminescence detection kit (KPL, Gaithersburg, MD). The following antibodies were used for immunoblotting: Bim (AB17003) was from Chemicon (Temecula, CA, USA); COX-2 (SC-19999), Bax N-20 (SC-493), Bcl-2 (SC-509), Bcl-xL (SC-1041), Mcl-1 (SC-819) were from Santa Cruz (CA, USA); cleaved (Asp175) caspase-3 (9664), phospho-Ser112-Bad (9291), Bad (9292), Puma (4976), Bmf (4692), ERK1/2 (9102), phospho-ERK1/2 (4377), Akt (9272), phospho-Ser473-Akt (4058), phospho-Thr24-FoxO1/phospho-Thr32-FoxO3a (9464), FoxO1 (9454), FoxO3a (9467) were from Cell Signaling Technology; PARP (C2-10) was from Alexis (San Diego, CA, USA); α-tubulin (T9206) was from Sigma; Bak (556382) was from BD Pharmingen (San Diego, CA, USA).
RNAi
Small interfering RNAs (siRNAs) were from Ambion (Huntingdon, Cambridgeshire, UK). Cells were reverse transfected with siRNA sequences targeted to human Bim, Bad, or a validated non-targeting negative control siRNA using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) as described previously (Kaidi, et al., 2007). The following Ambion siRNA sequences were used: Bad siRNA1, ID 120388; Bad siRNA2, ID 120807; Bim siRNA1, ID s195012; Bim siRNA2, ID s194474.
Immunohistochemistry
Samples of formalin-fixed, paraffin-embedded human colonic adenocarcinoma and normal colon tissue were obtained from the Department of Histopathology, Bristol Royal Infirmary. This was approved by the local research ethics committee. Tissue sections (4μM) were stained with a rabbit polyclonal antibody to Bim (AB17003) at 1:4000 dilution and visualized using the Vectastain ABC Elite Kit (Vector Laboratories, Burlingame, CA) as described previously (Clemo, et al., 2008). Sections were graded as follows: low/undetectable (–), expressed (+), highly expressed (++) as performed previously (Chell, et al., 2006, Elder, et al., 2002). The slides were graded by three observers (M.M., A.C.W. and A.G.) independently. In the few cases where there was a discrepancy, these were reconsidered and a consensus was reached.
Immunohistochemistry antibody validation
Wild-type (Bim+/+) and Bim knockout (Bim−/−) iMEF cell lines were grown to confluence and serum-starved in the presence of 10μM U0126 for 8 hours to induce Bim expression (Ewings, et al., 2007). RG/C2 cells were treated with control or Bim siRNA and grown in 10% FBS containing media for 72 hours. Following incubation, cells were washed in PBS and fixed in 10% neutral-buffered formalin for 1 hour at room temperature. After fixing, cells were washed, scraped, and pelleted in high-melting temperature agar (3.3%) to facilitate embedding in paraffin as described previously (Clemo, et al., 2008).
PGE2 quantification
A competitive enzyme immunoassay for PGE2 (Cayman) was performed according to the manufacturer's instructions as described previously (Kaidi, et al., 2006).
Apoptosis assays
Flow cytometry was carried out as described previously (Kaidi, et al., 2007). Briefly, the attached (those remaining adhered to the tissue culture flask) and floating (those having detached from the tissue culture flask) cells were collected, pooled, fixed in 70% ethanol and stained with propidium iodide in the presence of RNase A. Flow cytometry was performed on a LSR-II flow cytometer (BD Biosciences, San Jose, CA, USA) and the proportion of cells exhibiting sub-G1 DNA was determined using ModFit LT (Verity Software House, Topsham, ME, USA). For cell counts, the percentage apoptosis was determined by measuring the proportion of apoptotic floating cells as a percentage of the total (attached plus floating) cell population, as described previously (Elder, et al., 1996, Greenhough, et al., 2007).
Statistical analyses
Statistical analyses were performed using Student's t-test and expressed as: *p < 0.05, *p < 0.01, ***p <0.001, NS: not significant.
Results
PGE2 suppresses apoptosis in RG/C2 colorectal adenoma cells
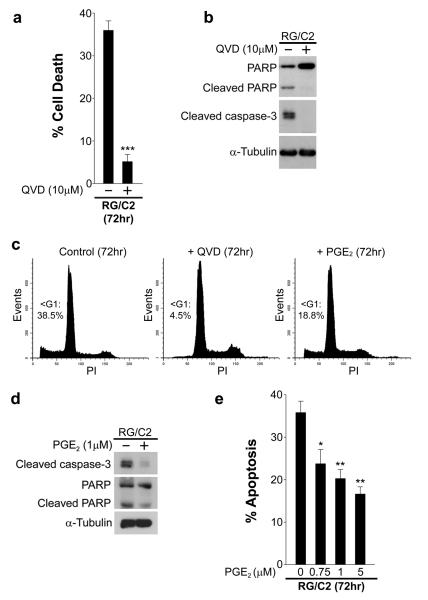
The majority of colorectal carcinomas are thought to arise from benign adenomas, with progression to malignancy via the adenoma-carcinoma sequence. Colorectal adenoma development and malignant transformation are associated with the progressive suppression of apoptosis (Bedi, et al., 1995). To study the apoptosis-suppressive effects of PGE2, we exploited an anchorage-dependent and non-tumorigenic premalignant colorectal adenoma cell line, RG/C2, derived from a single sporadic colonic tubular adenoma (Paraskeva, et al., 1989). RG/C2 cells exhibit relatively high basal levels of cell death and therefore represent a good model system to study apoptosis-modulating factors (N.B., RG/C2 cells express wild-type KRAS, BRAF, PTEN, PIK3CA, and Akt1/2; unpublished data). We sought to determine the mechanisms underlying PGE2-mediated apoptosis suppression during the early stages of human colorectal tumorigenesis, which have not previously been investigated. Initially, we confirmed that the spontaneous cell death observed in RG/C2 cells was classical apoptosis by examining cells for evidence of apoptosis in the presence or absence of the potent broad-spectrum caspase inhibitor QVD-OPh. As shown in Fig. 1a, 10μM QVD markedly reduced cell death in RG/C2 cells. Furthermore, QVD completely abrogated the basal levels of cleaved (active) caspase-3 and prevented cleavage of its substrate PARP (Fig. 1b). In addition, flow cytometry revealed that the sub-G1 population of RG/C2 cells was dramatically reduced upon addition of QVD (Fig. 1c; compare left and middle panels). Having confirmed that the spontaneous cell death displayed by RG/C2 cells was indeed classical apoptosis, we investigated whether PGE2 was able to suppress apoptosis in these cells. Treatment of RG/C2 cells with PGE2 (1μM) decreased the fraction of sub-G1 containing cells (Fig. 1c; right panel) and reduced levels of cleaved caspase-3 and PARP (Fig. 1d). Increasing concentrations (750nM – 5μM) of PGE2 led to a dose-dependent decrease in the proportion of apoptotic cells (Fig. 1e). These data indicate that PGE2 suppresses apoptosis in the human colorectal adenoma cell line RG/C2.
Figure 1. PGE2 suppresses apoptosis in RG/C2 cells.
(a) RG/C2 cells exhibit relatively high levels of spontaneous cell death that can be abolished by caspase inhibition. RG/C2 cells were cultured in the presence and absence of the broad spectrum caspase inhibitor QVD for 72 hours; the proportion of apoptotic cells was significantly reduced by QVD. Columns show the mean of three independent experiments performed in triplicate; ***p<0.001. (b) QVD prevents spontaneous caspase-3 and PARP cleavage in RG/C2 cells. Western blotting was performed on whole cell lysates from the total population of cells (attached plus floating). QVD increased intact PARP (116kDa) levels and inhibited PARP cleavage (89kDa). (c) QVD and PGE2 reduced the proportion of sub-G1 DNA-containing RG/C2 cells. Cells were treated with vehicle control, QVD, or PGE2, for 72 hours, after which the total population of cells was subjected to propidium iodide staining and flow cytometric analysis. Representative histograms and percentage sub-G1 DNA containing cells are shown. (d) PGE2 reduces spontaneous caspase-3 and PARP cleavage in RG/C2 cells. RG/C2 cells were cultured for 72 hours in the presence or absence of PGE2 and western blotting was performed as described in (b). (e) Increasing concentrations of PGE2 suppress apoptosis in RG/C2 cells. RG/C2 cells were treated with PGE2 for 72 hours and the proportion of apoptotic cells was determined as described in materials and methods. Columns show the mean of three independent experiments performed in triplicate; *p<0.05; p**<0.01.
PGE2-mediated apoptosis-suppression is associated with Bim repression
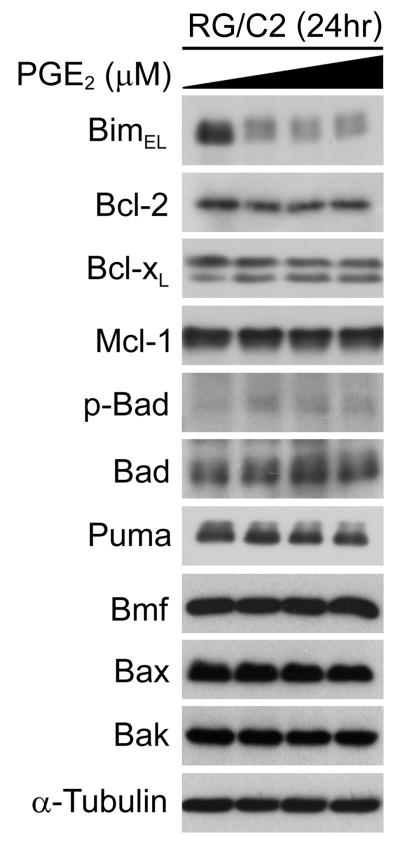
Given that the balance of prosurvival and proapoptotic members of the Bcl-2 protein family is a major determinant of apoptosis in mammalian cells, we examined the expression levels of Bcl-2 family members in RG/C2 cells prior to- and post-PGE2 treatment. Under control conditions, RG/C2 cells expressed readily detectable levels of several prosurvival, BH3-only, and multidomain proapoptotic members of the Bcl-2 family (Fig. 2). Treatment of RG/C2 cells with PGE2 for 24 hours did not significantly alter the expression of any of the prosurvival Bcl-2 family members examined (e.g., Bcl-2, Bcl-xL, Mcl-1) or of the multidomain proapoptotic proteins Bax and Bak (Fig. 2). However, we observed a striking downregulation of the proapoptotic BH3-only protein BimEL (Fig. 2), which was by far the most abundant Bim isoform in these cells (BimL and BimS were detectable upon longer film exposures but did not change significantly upon PGE2 treatment; data not shown). In addition, we noted a small increase in the levels of phosphorylated Bad at the ERK1/2-RSK dependent site, Ser112. In comparison to the regulation of Bim expression, no significant changes were observed to the expression levels of BH3-only proteins Bad, Puma and Bmf (Fig. 2). These data indicate that PGE2 promotes BimEL downregulation and Bad phosphorylation in colorectal adenoma cells.
Figure 2. PGE2 promotes Bim repression and Bad (Ser112) phosphorylation in RG/C2 cells.
RG/C2 cells were treated with increasing concentrations of PGE2 (0.75 – 5μM) for 24 hours. Whole cell lysates were probed with antibodies to prosurvival, BH3-only and multidomain proapoptotic members of the Bcl-2 protein family as indicated. PGE2 treatment of RG/C2 cells led to a striking repression of BimEL and also caused a small increase in Bad (Ser112) phosphorylation. Levels of other Bcl-2 family members did not change significantly. Equal loading was confirmed by re-probing immunoblots with an antibody to α-tubulin.
PGE2 promotes ERK1/2-dependent but Akt-independent Bim repression
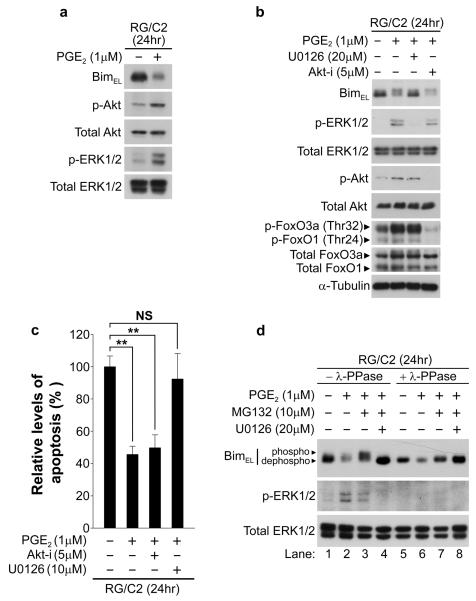
Following our screen of Bcl-2 family members, we focused on the novel observation that PGE2 treatment decreased Bim expression. Bim expression can be induced transcriptionally by FoxOs (e.g., FoxO3a; Dijkers, et al., 2000), which are negatively regulated by Akt-mediated phosphorylation, leading to their nuclear export and sequestration in the cytoplasm (Brunet, et al., 1999). Conversely, BimEL is directly phosphorylated by ERK1/2, an event that promotes its degradation via the proteasome (reviewed in Ley, et al., 2005). Western blot analysis of phosphorylated ERK1/2 and Akt levels after exposure to PGE2 revealed that PGE2 promoted the activation of both ERK1/2 and Akt signalling in RG/C2 cells; this correlated with decreased expression of BimEL (Fig. 3a). To determine whether activation of either or both pathways contributed to BimEL downregulation, we treated RG/C2 cells with PGE2 in conjunction with the selective MEK inhibitor U0126 (to block Raf-MEK-ERK1/2 signalling) or an Akt inhibitor (Akt-i, to block Akt-mediated FoxO phosphorylation). Pharmacological blockade of MEK or Akt completely prevented PGE2-mediated activation of ERK1/2 and Akt, respectively, confirming that these inhibitors selectively blocked their target pathways (Fig. 3b). Surprisingly, PGE2-mediated BimEL downregulation was unaffected by the Akt inhibitor – despite Akt inhibition preventing PGE2-mediated phosphorylation of FoxO3a (Fig. 3b). However, PGE2-mediated BimEL repression was prevented by inhibition of the MEK-ERK1/2 pathway with U0126 (Fig. 3b). Furthermore, treatment of RG/C2 cells with PGE2 in the presence of U0126 also attenuated its apoptosis-suppressive properties, whereas Akt inhibition had little effect (Fig. 3c). These results suggest that in colorectal adenoma cells, PGE2 promotes repression of BimEL expression principally via a MEK-ERK1/2-dependent mechanism.
Figure 3. PGE2 promotes ERK1/2-dependent Bim phosphorylation and proteasomal degradation.
(a) Treatment of RG/C2 cells with PGE2 for 24 hours leads to activation of ERK1/2 and Akt (b) PGE2 represses Bim in RG/C2 cells via an MEK-ERK1/2-dependent but Akt/FoxO-independent mechanism. RG/C2 cells were pre-treated with a MEK inhibitor (U0126) or an Akt inhibitor (Akt-i) for three hours prior to exposure to PGE2 for 24 hours; western blot analysis was performed on whole cell lysates with the indicated antibodies. PGE2-mediated Bim repression was reversed by U0126 but not Akt-i. Akt inhibition abolished FoxO1 and FoxO3a phosphorylation, but did not alter Bim expression. Probing for total ERK1/2, Akt, FoxO1/3a, and α-tubulin confirmed equal loading. (c) Inhibition of MEK-ERK1/2 signalling but not Akt signalling reverses PGE2-mediated apoptosis suppression. RG/C2 cells were treated as described in (b) and the proportion of apoptotic cells was determined as described in materials and methods; p**<0.01; NS, not significant. (d) PGE2 promotes ERK1/2-mediated phosphorylation (mobility shift) and proteasomal degradation of BimEL. RG/C2 cells were pre-treated with U0126, the proteasome inhibitor MG132, or both for three hours prior to exposure to PGE2 for 24 hours. Whole cell lysates were incubated for two hours at 30°C in the absence (lanes 1-4) or presence (lanes 5-8) of 400 units of λ-phosphatase (λ-PPase). MG132 reversed PGE2-mediated Bim repression and caused its accumulation in a hyperphosphorylated state (mobility shift); this was prevented by U0126. λ-PPase treatment established that the slower migrating forms of BimEL were as a result of phosphorylation. The absence of phosphorylated ERK1/2 in the λ-PPase-treated lysates confirmed PPase activity.
PGE2 promotes ERK1/2-dependent Bim phosphorylation and proteasomal degradation
The reduction in BimEL protein expression mediated by PGE2 was also associated with an apparent increase in the molecular weight (mobility shift) of BimEL (see Fig. 3b), suggesting that PGE2 induces BimEL phosphorylation (which would lead to its proteasomal degradation). To determine whether PGE2 promotes degradation of BimEL via the proteasome, we treated RG/C2 cells with the proteasome inhibitor MG132 prior to and during PGE2 treatment. While PGE2 reduced the mobility of BimEL in the presence of MG132, its expression levels were restored to control levels (Fig. 3d, compare lanes 1-3); this suggests that PGE2 promotes degradation of BimEL via the proteasome. Both the repression and mobility shift of BimEL were prevented by co-administration of U0126 with MG132 (Fig. 3d, lane 4), indicating that these events were dependent upon the MEK-ERK1/2 pathway. To confirm that the slower migrating forms of BimEL resulted from PGE2-induced BimEL hyperphosphorylation, the same whole cell lysates were incubated with lambda phosphatase (λ-PPase) for 1 hour. Phosphorylated ERK1/2 was undetectable in lysates following λ-PPase treatment, which served as a useful control to confirm phosphatase activity (Fig. 3d, lanes 5-8). λ-PPase treatment converted BimEL to hypophosphorylated forms with a faster migration on SDS-PAGE (Fig. 3d, lanes 5-8); again, BimEL expression was reduced by PGE2 (lane 6) and restored by MG132 (lane 7). These data show that PGE2 promotes MEK-ERK1/2-dependent BimEL phosphorylation and proteasomal degradation.
Reduction of Bim, but not Bad, protein expression mimics PGE2-mediated apoptosis-suppression and abrogates the antiapoptotic effects of PGE2
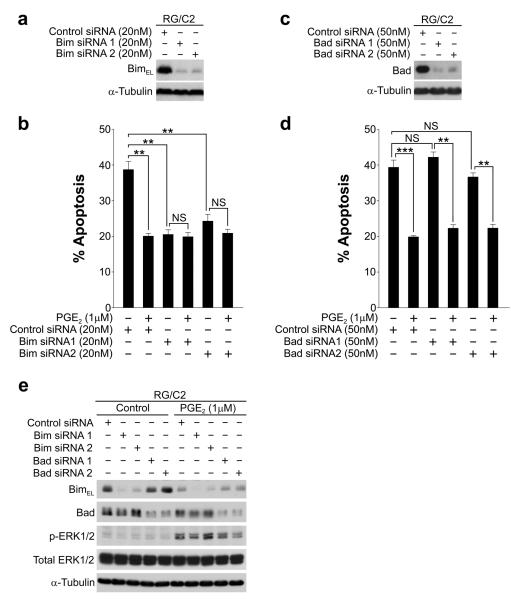
The ability of PGE2 to suppress apoptosis concomitant with BimEL repression suggests that the reduction in BimEL protein expression may be involved in the antiapoptotic effects of PGE2. To examine whether Bim downregulation was sufficient for the suppression of apoptosis in RG/C2 cells, we used RNAi to reduce Bim protein levels. Transfection of RG/C2 cells with two independent siRNA sequences targeted to Bim resulted in an almost complete knockdown of BimEL (Fig. 4a). Bim knockdown considerably reduced the basal apoptotic rate of RG/C2 cells (Fig. 4b), suggesting an important role for Bim in colorectal adenoma cell apoptosis. By comparison, knockdown of Bad (Fig. 4c) — another proapoptotic BH3-only protein — failed to reduce this basal apoptotic rate (Fig. 4d). Combined knockdown of Bim and Bad did not reduce basal apoptosis further than Bim knockdown alone (data not shown). In order to determine whether PGE2-mediated apoptosis suppression requires Bim repression, we treated Bim knockdown cells with PGE2. PGE2 failed to significantly further suppress apoptosis in Bim knockdown cells, (Fig. 4b), suggesting that PGE2 suppresses apoptosis via Bim downregulation. However, PGE2 was still able to suppress apoptosis in Bad knockdown cells (Fig. 4d), and PGE2-mediated apoptosis suppression correlated with ERK1/2 activation and BimEL repression (Fig. 4e). This suggests that the posttranslational inhibition of Bad (by phosphorylation on Ser112) mediated by PGE2 (shown earlier in Fig. 2) may be insufficient to suppress apoptosis. These findings specifically implicate Bim repression as a key mediator of PGE2-mediated apoptosis suppression in RG/C2 colorectal adenoma cells.
Figure 4. Reduction of Bim protein expression reduces spontaneous apoptosis in RG/C2 cells and abrogates PGE2-mediated apoptosis suppression.
(a) Knockdown of Bim expression in RG/C2 cells. RG/C2 cells were reverse transfected with two independent sequences to human Bim or a non-targeting control siRNA; reduction of BimEL protein expression was confirmed by western blotting. (b) Bim knockdown significantly reduced spontaneous apoptosis in RG/C2 cells. PGE2 suppressed apoptosis in control siRNA treated cells, but could not further suppress apoptosis in Bim siRNA treated cells. (c) Knockdown of Bad expression in RG/C2 cells was carried out as in (a) with two independent sequences targeted to human Bad. (d) Bad knockdown did not affect spontaneous apoptosis in RG/C2 cells or the ability of PGE2 to suppress apoptosis. PGE2 significantly suppressed apoptosis in Bad knockdown cells, which correlated with Bim repression shown in (e). (e) Western blotting confirmed Bim and Bad knockdown in control and PGE2 treated RG/C2 cells. PGE2 activated ERK1/2 and promoted Bim repression but did not affect Bad expression. Data is representative of three independent experiments, **p<0.01; p***<0.001; NS, not significant.
COX-2-selective inhibitors induce BimEL expression in COX-2 expressing colorectal carcinoma cell lines
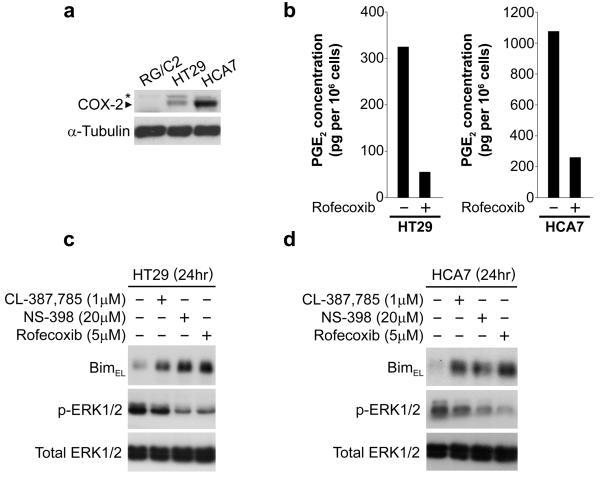
COX-2-selective NSAIDs are chemopreventive for colorectal cancer, with their principal modes of action thought to be their ability to reduce prostaglandin levels and to induce apoptosis (Elder & Paraskeva, 1998). We and others have previously shown that NSAIDs can reduce the activity of the Raf-MEK-ERK1/2 pathway in colorectal carcinoma cell lines (Kaidi, et al., 2006, Rice, et al., 2004). Having shown that addition of exogenous PGE2 to colorectal tumour cells leads to repression of BimEL, we sought to determine whether inhibition of COX-2 (and hence endogenous PGE2 production) increased BimEL expression. Since RG/C2 cells expressed low/undetectable levels of COX-2 protein (Fig. 5a), to address this question we treated the COX-2-expressing colorectal cancer cell lines HT29 and HCA7 (Fig. 5a) with the COX-2-selective NSAIDs NS398 and rofecoxib at concentrations which greatly reduced PGE2 levels (as shown previously, Crew, et al., 2000; and Fig. 5b). Alongside the COX-2-selective NSAIDs, we also treated the cells with the EGFR inhibitor CL-387,785 as a positive control for BimEL induction (Costa, et al., 2007). Treatment of HT29 cells with either NS398 or rofecoxib led to a strong induction of BimEL expression (Fig. 5c); this correlated with reduced expression of phosphorylated ERK1/2. Similar results were observed in a second COX-2-expressing cell line, HCA7 (Fig. 5d). These data suggest that inhibition of PGE2 production with COX-2-selective NSAIDs leads to increased expression of BimEL in colorectal carcinoma cell lines.
Figure 5. Inhibition of COX-2 and PGE2 secretion in COX-2 expressing colorectal carcinoma cell lines induces Bim expression.
(a) COX-2 expression in RG/C2, HT29 and HCA7 cell lines. RG/C2 cells express low/undetectable levels of COX-2; HT29 and HCA7 cells express moderate and high levels of COX-2, respectively. *Non-specific band. (b) PGE2 assay: Rofecoxib (5μM) inhibits PGE2 production in HT29 and HCA7 cell lines. (c & d) Induction of Bim by NSAIDs in HT29 (c) and HCA7 (d) cells. HT29 and HCA7 cells were pre-treated with the indicated drugs for three hours, the media removed (to remove PGE2), and fresh treatment applied for 24 hours. COX-2-selective NSAIDs NS398 and rofecoxib strongly induced BimEL expression. BimEL induction correlated with reduced phosphorylated ERK1/2 levels.
Bim expression is reduced during the adenoma-carcinoma sequence in two in vitro models of colorectal tumour progression
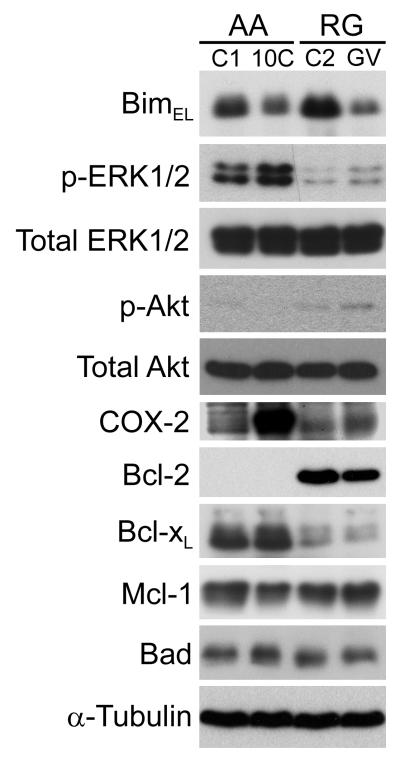
Taken together with a previous report implicating Bim repression as an important cell survival mechanism in BRAFV600E-mutant colorectal carcinoma cell lines (Wickenden, et al., 2008), our study suggests that reductions in Bim expression (or activity) may be associated with colorectal tumorigenesis. In addition, Bim has previously been shown to be a critical mediator of anoikis in mammary epithelial cells (Reginato, et al., 2003), suggesting that negative regulation of Bim expression may contribute to anchorage independence in epithelial cells (Schmelzle, et al., 2007). Initially, we examined Bim expression levels in two established in vitro models of colorectal tumour progression (Chell, et al., 2006, Williams, et al., 1990) to determine whether alterations to Bim expression occur during the adenoma-carcinoma sequence. Comparison of the non-tumorigenic anchorage-dependent colorectal adenoma cell line, AA/C1, with its in vitro transformed anchorage-independent and tumorigenic counterpart, AA/C1/SB10C, revealed that BimEL expression was reduced in the more progressed cell line (Fig. 6). This correlated with increased COX-2 and p-ERK1/2 (but not p-Akt) levels (Fig. 6). A similar correlation was observed during progression of RG cell line series (RG/C2 to RG/GV), but in this case decreased Bim expression also correlated with increased p-Akt levels (Fig. 6). Expression levels of other Bcl-2 family members did not change significantly between the non-transformed and transformed cell line pairs. These results suggest that progression of colorectal adenoma cell lines towards an anchorage-independent phenotype is associated with decreased Bim expression.
Figure 6. Bim expression is reduced during the adenoma-carcinoma sequence in two in vitro models of colorectal tumour progression.
The expression of Bim and other members of the Bcl-2 protein family in adenoma-derived and transformed adenoma cell lines. Bim expression is reduced during the in vitro progression of the anchorage-dependent and non-tumorigenic adenoma cell line AA/C1 (C1) to the anchorage-independent and tumorigenic variant AA/C1/SB10C (10C). Similar results were observed in the RG/C2 (C2) to RG/GV (GV) progression model. Decreased Bim expression correlated with increased p-ERK1/2 levels and increased COX-2 expression in the AA series; decreased Bim expression correlated with increased p-ERK1/2, COX-2 and p-Akt levels in the RG series. The expression of other Bcl-2 family members did not change significantly as a function of the adenoma-carcinoma progression in either of the cell line models.
Bim expression is reduced in a subset of colorectal carcinomas in vivo
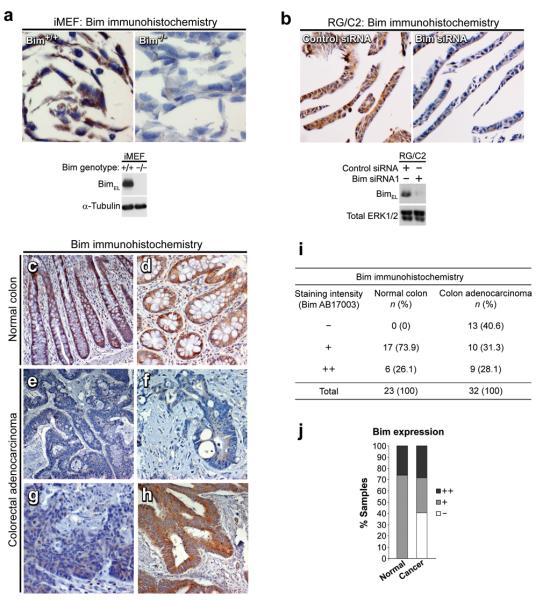
Having shown that Bim expression is reduced during colorectal tumour progression in vitro, we went on to investigate Bim expression levels in vivo in normal and cancerous human colorectal tissue. To validate the Bim antibody for immunohistochemistry (IHC), we prepared paraffin embedded pellets of Bim+/+ and Bim−/− mouse embryonic fibroblasts cells (iMEFs) and human colorectal adenoma cells (RG/C2) transfected with control or Bim siRNA. Whereas Bim+/+ cells stained positive for Bim, staining was absent in Bim−/− cells; this was confirmed by western blotting (Fig. 7a). Bim staining was also markedly reduced in Bim siRNA treated RG/C2 cells (Fig. 7b). Bim staining was cytoplasmic, in accordance with the expected localisation of Bim, as previously reported (O'Reilly, et al., 2000). We then performed IHC analyses on normal human colon (n=23) and human colorectal carcinoma (n=32) tissue samples. In the normal colonic epithelium, cytoplasmic Bim staining was present in all of the samples tested (n=23/23; Fig. 7c, d, i & j). Conversely, a significant proportion (approximately 40%, n=13/32) of colorectal carcinomas had markedly reduced Bim expression in the epithelium (examples shown Fig. 7e–g, i & j). Interestingly, high levels of Bim expression (++) were observed in a number of normal (n=6/23) and carcinoma (n=9/32) samples. Given that Bim is expressed in the normal colorectal epithelium but is reduced in a proportion of colorectal carcinomas, these data suggest that decreased Bim expression may contribute to the development of a subset of colorectal carcinomas.
Figure 7. Bim expression is present in normal colonic epithelium, but reduced in a proportion of colorectal carcinomas.
(a & b) Validation of the Bim antibody for use in immunohistochemistry. (a) Wild-type and Bim null iMEF cell lines were grown under serum-free conditions with 10μM U0126 for 8 hours. Cells were formalin fixed, paraffin embedded and stained with the Bim antibody as described in materials and methods. Bim staining was present in wild-type iMEFs but absent in Bim null iMEFs; Bim expression status was confirmed by western blotting. (b) Control or Bim siRNA transfected RG/C2 cells were prepared as in (a). Bim staining was observed in control siRNA treated RG/C2 cells, but markedly reduced in Bim siRNA treated RG/C2 cells. Knockdown was confirmed by western blotting. (c – h) Bim staining in human colorectal tissue: (c & d) Bim staining was invariably present in the normal colonic epithelium. (e – g) Bim staining was reduced in a subset (40.6%) of colorectal carcinomas (images of three different tumours shown). (h) Example of a colorectal carcinoma displaying high levels of Bim immunoreactivity. (i & j) Summary of Bim expression levels in human colorectal tissue (normal and adenocarcinoma).
Discussion
The acquired ability of tumour cells to evade apoptosis is a central step during tumorigenesis and promotes resistance to cancer therapy (Hanahan & Weinberg, 2000, Johnstone, et al., 2002). Overexpression of COX-2 occurs in the majority of colorectal carcinomas (and in a subset of adenomas) and leads to elevated levels of PGE2. The aim of current study was to gain a further insight into the mechanisms underlying COX-2/PGE2-mediated apoptosis suppression, which to date are not fully understood, particularly in the context of early-stage human colorectal tumorigenesis. Here, we have shown for the first time that the COX-2/PGE2 pathway can exert an inhibitory effect on the intrinsic cell death machinery via repression of the proapoptotic BH3-only protein Bim. Furthermore, our results indicate that Bim expression is reduced in a proportion of human colorectal carcinomas in vivo. Our findings are important for the following reasons: first, they implicate Bim as an important mediator of apoptosis in colorectal adenoma cells; second, they provide a novel mechanism for Bim repression and suggest that Bim is an important target of COX-2/PGE2 signalling in colorectal tumorigenesis; third, they suggest that the chemopreventive properties of NSAIDs may involve de-repression of Bim expression following inhibition of tumour- and/or stromal-cell-derived PGE2 synthesis; finally, they demonstrate that decreased Bim expression is associated with in vitro tumour progression and occurs in a significant proportion of human colorectal carcinomas in vivo, suggesting that a selection pressure for reduced Bim expression occurs during colorectal tumorigenesis.
Although it has been known for some time that Bim expression is negatively regulated by the PI3K-Akt (Dijkers, et al., 2000) and Raf-MEK-ERK1/2 pathways (Reginato, et al., 2003, Weston, et al., 2003), only recently have studies begun to uncover the mechanisms that tumour cells evolve in order to repress or tolerate Bim. For example, Cook and colleagues (Wickenden, et al., 2008) have recently demonstrated that a single mutant allele of BRAFV600E — a genetic lesion that occurs commonly in melanoma and colorectal carcinoma (Davies, et al., 2002) — is sufficient to repress Bim expression and promote growth factor-independent survival (Wickenden, et al., 2008). Furthermore, certain BRAFV600E-mutant melanomas and colorectal carcinomas appear to develop a dependence or ‘addiction’ to Bim repression, since inhibition of mutant BRAF-driven ERK1/2 signalling results in Bim accumulation and Bim-mediated apoptosis (Cartlidge, et al., 2008, Cragg, et al., 2008, Wickenden, et al., 2008). Addiction to Bim repression is not restricted to BRAF-mutant melanomas and colorectal carcinomas. Haematopoietic tumour cells expressing Bcr-Abl+ are addicted to activation of the PI3K-Akt and Raf-MEK-ERK1/2 pathways for survival; this is at least in part dependent on Bim repression by both transcriptional (via FoxO3a) and posttranslational mechanisms (via ERK1/2), but can be overcome by the Bcr-Abl tyrosine kinase inhibitor imatinib (Essafi, et al., 2005, Kuroda, et al., 2006). Similarly, lung cancer cell lines harbouring mutant EGFR are also addicted to Bim repression; this is reversible by EGFR inhibition with, for example, gefitinib (Costa, et al., 2007, Cragg, et al., 2007, Gong, et al., 2007). These studies not only place Bim downstream of multiple oncogenic signalling modules, but also indicate that the negative regulation of Bim activity or expression is — at least in some malignancies — essential for cell survival. Nevertheless, overcoming Bim repression can sensitise tumour cells to apoptosis, highlighting Bim as an important target for cancer therapy. Our findings suggest that the COX-2/PGE2 pathway can be added to the list of oncogenic pathways that promote Bim repression and allow tumour cells to evade apoptosis.
Prompted by our findings and by other studies suggesting an important role for Bim during colorectal tumorigenesis, we compared Bim expression in human normal and cancerous colorectal tissue. Although Bim was expressed in all normal colorectal tissue samples, Bim expression was reduced in approximately 40% of colorectal carcinomas. Whilst our study was underway, Sinicrope and colleagues used tissue microarray analysis to study the expression of BH3-only proteins in colorectal carcinomas, and found that elevated Bim or Puma expression correlated with favourable prognoses (Sinicrope, et al., 2008). These data complement our findings and suggest that Bim may be an important target for therapeutic intervention in colorectal cancer.
Loss of Bim expression has previously been shown to occur via epigenetic silencing in renal cell carcinoma (Zantl, et al., 2007), and homozygous deletion of the Bim gene (BCL2L11) has also been reported in mantle cell lymphoma (Tagawa, et al., 2005). However, it is tempting to speculate that as Bim expression is negatively regulated by pathways which are frequently hyperactivated in colorectal cancers, a proportion of the tumours with reduced Bim expression observed here are likely to possess deregulated Ras-Raf-MEK-ERK1/2 and/or PI3K-Akt-FoxO pathways. Nevertheless, Bim levels per se may not be the only indicator of tumours where Bim activity is kept in check; posttranslational mechanisms that negatively regulate Bim activity (Ewings, et al., 2007) may allow cells to accumulate and tolerate high levels of ‘inactive’ Bim (i.e., hyperphosphorylated Bim) without undergoing cell death, thereby promoting resistance to apoptosis. This might explain the small number of high Bim-expressing tumours observed in this study and in a screen of a panel of colorectal tumour cell lines (A.G. and C.P., unpublished observations). However, it is also important to point out that colorectal tumours may evolve alternative apoptosis-suppressive mechanisms, for example by increasing expression of Bcl-2 (Hague, et al., 1994) or Bcl-xL (Krajewska, et al., 1996). Nevertheless, in these scenarios additional steps (i.e., repression of Bim or other BH3-only proteins) may also be required for effective apoptosis suppression. Indeed, it is interesting to note while the levels of Bcl-2 and Bcl-xL did not change as a function of the adenoma-carcinoma sequence in the in vitro tumour progression models used in this study, there were striking differences in the expression of these proteins between the two model series. Therefore, one might speculate that these differences represent alternative ‘options’ for apoptosis suppression co-opted by different tumours to tolerate BH3-only proteins; nonetheless, they may be insufficient for apoptosis suppression without additional events. Thus, Bim repression in combination with other apoptosis-suppressive events may be necessary for tumour progression.
Recent clinical trials have brought to light the increased risk of adverse cardiovascular events associated with the use of highly selective COX-2 inhibitors for long-term colorectal cancer prevention (Bresalier, et al., 2005). Notwithstanding, the biggest impact to date in terms of colorectal cancer chemoprevention has been through targeting cyclooxygenase-prostaglandin signalling (for a review, see Wang & Dubois, 2009). Our findings implicate Bim as a novel downstream target of COX-2/PGE2 signalling and suggest that Bim expression is reduced in a significant proportion of human colorectal carcinomas. Future studies should seek to determine whether targeting PGE2 production could be used in combination with agents that inhibit (or reduce the expression of) prosurvival Bcl-2 family members (e.g., ABT-737) to promote synergistic apoptosis, and whether loss of Bim expression in colorectal tumours can serve as a biomarker for therapeutic responses to agents targeting the Raf-MEK-ERK1/2 pathway.
Acknowledgements
We would like to thank Debbie Martin and Gini Tilly for help with histology; David Huang for provision of Bim wild-type and null iMEF cells; Andy Silver and Nirosha Suraweera for sequencing analysis; Andy Herman for help with flow cytometry, and members of the CP group for useful discussion. This work was funded by the Citrina Foundation, the John James Bristol Foundation, and by a Cancer Research UK programme grant.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- Balmanno K, Cook SJ. Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ. 2009;16:368–77. doi: 10.1038/cdd.2008.148. [DOI] [PubMed] [Google Scholar]
- Bedi A, Pasricha PJ, Akhtar AJ, Barber JP, Bedi GC, Giardiello FM, et al. Inhibition of apoptosis during development of colorectal cancer. Cancer Res. 1995;55:1811–6. [PubMed] [Google Scholar]
- Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–8. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005;23:2840–55. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Cartlidge RA, Thomas GR, Cagnol S, Jong KA, Molton SA, Finch AJ, et al. Oncogenic BRAF(V600E) inhibits BIM expression to promote melanoma cell survival. Pigment Cell Melanoma Res. 2008;21:534–44. doi: 10.1111/j.1755-148X.2008.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–10. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- Certo M, Del Gaizo, Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–65. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Chan TA, Morin PJ, Vogelstein B, Kinzler KW. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc Natl Acad Sci U S A. 1998;95:681–6. doi: 10.1073/pnas.95.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chell SD, Witherden IR, Dobson RR, Moorghen M, Herman AA, Qualtrough D, et al. Increased EP4 receptor expression in colorectal cancer progression promotes cell growth and anchorage independence. Cancer Res. 2006;66:3106–13. doi: 10.1158/0008-5472.CAN-05-3702. [DOI] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–11. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- Clemo NK, Collard TJ, Southern SL, Edwards KD, Moorghen M, Packham G, et al. BAG-1 is up-regulated in colorectal tumour progression and promotes colorectal tumour cell survival through increased NF-kappaB activity. Carcinogenesis. 2008;29:849–57. doi: 10.1093/carcin/bgn004. [DOI] [PubMed] [Google Scholar]
- Costa DB, Halmos B, Kumar A, Schumer ST, Huberman MS, Boggon TJ, et al. BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med. 2007;4:1669–79. doi: 10.1371/journal.pmed.0040315. discussion 1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg MS, Jansen ES, Cook M, Harris C, Strasser A, Scott CL. Treatment of BRAF mutant human tumor cells with a MEK inhibitor requires Bim and is enhanced by a BH3 mimetic. J Clin Invest. 2008;118:3651–9. doi: 10.1172/JCI35437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg MS, Kuroda J, Puthalakath H, Huang DC, Strasser A. Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PLoS Med. 2007;4:1681–89. doi: 10.1371/journal.pmed.0040316. discussion 1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crew TE, Elder DJ, Paraskeva C. A cyclooxygenase-2 (COX-2) selective non-steroidal anti-inflammatory drug enhances the growth inhibitory effect of butyrate in colorectal carcinoma cells expressing COX-2 protein: regulation of COX-2 by butyrate. Carcinogenesis. 2000;21:69–77. doi: 10.1093/carcin/21.1.69. [DOI] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Dehan E, Bassermann F, Guardavaccaro D, Vasiliver-Shamis G, Cohen M, Lowes KN, et al. betaTrCP- and Rsk1/2-mediated degradation of BimEL inhibits apoptosis. Mol Cell. 2009;33:109–16. doi: 10.1016/j.molcel.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10:1201–4. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- Egle A, Harris AW, Bouillet P, Cory S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci U S A. 2004;101:6164–9. doi: 10.1073/pnas.0401471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder DJ, Baker JA, Banu NA, Moorghen M, Paraskeva C. Human colorectal adenomas demonstrate a size-dependent increase in epithelial cyclooxygenase-2 expression. J Pathol. 2002;198:428–34. doi: 10.1002/path.1232. [DOI] [PubMed] [Google Scholar]
- Elder DJ, Hague A, Hicks DJ, Paraskeva C. Differential growth inhibition by the aspirin metabolite salicylate in human colorectal tumor cell lines: enhanced apoptosis in carcinoma and in vitro-transformed adenoma relative to adenoma relative to adenoma cell lines. Cancer Res. 1996;56:2273–6. [PubMed] [Google Scholar]
- Elder DJ, Paraskeva C. COX-2 inhibitors for colorectal cancer. Nat Med. 1998;4:392–3. doi: 10.1038/nm0498-392. [DOI] [PubMed] [Google Scholar]
- Essafi A, Fernandez de Mattos S, Hassen YA, Soeiro I, Mufti GJ, Thomas NS, et al. Direct transcriptional regulation of Bim by FoxO3a mediates STI571-induced apoptosis in Bcr-Abl-expressing cells. Oncogene. 2005;24:2317–29. doi: 10.1038/sj.onc.1208421. [DOI] [PubMed] [Google Scholar]
- Ewings KE, Hadfield-Moorhouse K, Wiggins CM, Wickenden JA, Balmanno K, Gilley R, et al. ERK1/2-dependent phosphorylation of BimEL promotes its rapid dissociation from Mcl-1 and Bcl-xL. Embo J. 2007;26:2856–67. doi: 10.1038/sj.emboj.7601723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallenne T, Gautier F, Oliver L, Hervouet E, Noel B, Hickman JA, et al. Bax activation by the BH3-only protein Puma promotes cell dependence on antiapoptotic Bcl-2 family members. J Cell Biol. 2009;185:279–90. doi: 10.1083/jcb.200809153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–81. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136:1136–47. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Somwar R, Politi K, Balak M, Chmielecki J, Jiang X, et al. Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS Med. 2007;4:e294. doi: 10.1371/journal.pmed.0040294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhough A, Patsos HA, Williams AC, Paraskeva C. The cannabinoid delta(9)-tetrahydrocannabinol inhibits RAS-MAPK and PI3K-AKT survival signalling and induces BAD-mediated apoptosis in colorectal cancer cells. Int J Cancer. 2007;121:2172–80. doi: 10.1002/ijc.22917. [DOI] [PubMed] [Google Scholar]
- Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, et al. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–86. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- Grosch S, Tegeder I, Niederberger E, Brautigam L, Geisslinger G. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. Faseb J. 2001;15:2742–4. doi: 10.1096/fj.01-0299fje. [DOI] [PubMed] [Google Scholar]
- Hague A, Moorghen M, Hicks D, Chapman M, Paraskeva C. BCL-2 expression in human colorectal adenomas and carcinomas. Oncogene. 1994;9:3367–70. [PubMed] [Google Scholar]
- Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107(Pt 12):3569–77. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanif R, Pittas A, Feng Y, Koutsos MI, Qiao L, Staiano-Coico L, et al. Effects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem Pharmacol. 1996;52:237–45. doi: 10.1016/0006-2952(96)00181-5. [DOI] [PubMed] [Google Scholar]
- Hawcroft G, Ko CW, Hull MA. Prostaglandin E2-EP4 receptor signalling promotes tumorigenic behaviour of HT-29 human colorectal cancer cells. Oncogene. 2007;26:3006–19. doi: 10.1038/sj.onc.1210113. [DOI] [PubMed] [Google Scholar]
- Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–64. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Kaidi A, Qualtrough D, Williams AC, Paraskeva C. Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Res. 2006;66:6683–91. doi: 10.1158/0008-5472.CAN-06-0425. [DOI] [PubMed] [Google Scholar]
- Kaidi A, Williams AC, Paraskeva C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol. 2007;9:210–7. doi: 10.1038/ncb1534. [DOI] [PubMed] [Google Scholar]
- Krajewska M, Moss SF, Krajewski S, Song K, Holt PR, Reed JC. Elevated expression of Bcl-X and reduced Bak in primary colorectal adenocarcinomas. Cancer Res. 1996;56:2422–7. [PubMed] [Google Scholar]
- Kuroda J, Puthalakath H, Cragg MS, Kelly PN, Bouillet P, Huang DC, et al. Bim and Bad mediate imatinib-induced killing of Bcr/Abl+ leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic. Proc Natl Acad Sci U S A. 2006;103:14907–12. doi: 10.1073/pnas.0606176103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–92. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J Biol Chem. 2003;278:18811–6. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- Ley R, Ewings KE, Hadfield K, Cook SJ. Regulatory phosphorylation of Bim: sorting out the ERK from the JNK. Cell Death Differ. 2005;12:1008–14. doi: 10.1038/sj.cdd.4401688. [DOI] [PubMed] [Google Scholar]
- Ley R, Ewings KE, Hadfield K, Howes E, Balmanno K, Cook SJ. Extracellular signal-regulated kinases 1/2 are serum-stimulated "Bim(EL) kinases" that bind to the BH3-only protein Bim(EL) causing its phosphorylation and turnover. J Biol Chem. 2004;279:8837–47. doi: 10.1074/jbc.M311578200. [DOI] [PubMed] [Google Scholar]
- Lovell JF, Billen LP, Bindner S, Shamas-Din A, Fradin C, Leber B, et al. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135:1074–84. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Loveridge CJ, MacDonald AD, Thoms HC, Dunlop MG, Stark LA. The proapoptotic effects of sulindac, sulindac sulfone and indomethacin are mediated by nucleolar translocation of the RelA(p65) subunit of NF-kappaB. Oncogene. 2008;27:2648–55. doi: 10.1038/sj.onc.1210891. [DOI] [PubMed] [Google Scholar]
- Luciano F, Jacquel A, Colosetti P, Herrant M, Cagnol S, Pages G, et al. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene. 2003;22:6785–93. doi: 10.1038/sj.onc.1206792. [DOI] [PubMed] [Google Scholar]
- Nishihara H, Kizaka-Kondoh S, Insel PA, Eckmann L. Inhibition of apoptosis in normal and transformed intestinal epithelial cells by cAMP through induction of inhibitor of apoptosis protein (IAP)-2. Proc Natl Acad Sci U S A. 2003;100:8921–6. doi: 10.1073/pnas.1533221100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly LA, Cullen L, Visvader J, Lindeman GJ, Print C, Bath ML, et al. The proapoptotic BH3-only protein bim is expressed in hematopoietic, epithelial, neuronal, and germ cells. Am J Pathol. 2000;157:449–61. doi: 10.1016/S0002-9440(10)64557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskeva C, Finerty S, Mountford RA, Powell SC. Specific cytogenetic abnormalities in two new human colorectal adenoma-derived epithelial cell lines. Cancer Res. 1989;49:1282–6. [PubMed] [Google Scholar]
- Pozzi A, Yan X, Macias-Perez I, Wei S, Hata AN, Breyer RM, et al. Colon carcinoma cell growth is associated with prostaglandin E2/EP4 receptor-evoked ERK activation. J Biol Chem. 2004;279:29797–804. doi: 10.1074/jbc.M313989200. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–49. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J, et al. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol. 2003;5:733–40. doi: 10.1038/ncb1026. [DOI] [PubMed] [Google Scholar]
- Rice PL, Beard KS, Driggers LJ, Ahnen DJ. Inhibition of extracellular-signal regulated kinases 1/2 is required for apoptosis of human colon cancer cells in vitro by sulindac metabolites. Cancer Res. 2004;64:8148–51. doi: 10.1158/0008-5472.CAN-04-1517. [DOI] [PubMed] [Google Scholar]
- Schmelzle T, Mailleux AA, Overholtzer M, Carroll JS, Solimini NL, Lightcap ES, et al. Functional role and oncogene-regulated expression of the BH3-only factor Bmf in mammary epithelial anoikis and morphogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3787–3792. doi: 10.1073/pnas.0700115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng H, Shao J, Washington MK, DuBois RN. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J Biol Chem. 2001;276:18075–81. doi: 10.1074/jbc.M009689200. [DOI] [PubMed] [Google Scholar]
- Sinicrope FA, Rego RL, Okumura K, Foster NR, O'Connell MJ, Sargent DJ, et al. Prognostic impact of bim, puma, and noxa expression in human colon carcinomas. Clin Cancer Res. 2008;14:5810–8. doi: 10.1158/1078-0432.CCR-07-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa H, Karnan S, Suzuki R, Matsuo K, Zhang X, Ota A, et al. Genome-wide array-based CGH for mantle cell lymphoma: identification of homozygous deletions of the proapoptotic gene BIM. Oncogene. 2005;24:1348–58. doi: 10.1038/sj.onc.1208300. [DOI] [PubMed] [Google Scholar]
- Tan TT, Degenhardt K, Nelson DA, Beaudoin B, Nieves-Neira W, Bouillet P, et al. Key roles of BIM-driven apoptosis in epithelial tumors and rational chemotherapy. Cancer Cell. 2005;7:227–38. doi: 10.1016/j.ccr.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Wang D, Buchanan FG, Wang H, Dey SK, DuBois RN. Prostaglandin E2 enhances intestinal adenoma growth via activation of the Ras-mitogen-activated protein kinase cascade. Cancer Res. 2005;65:1822–9. doi: 10.1158/0008-5472.CAN-04-3671. [DOI] [PubMed] [Google Scholar]
- Wang D, Dubois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 2009 doi: 10.1038/onc.2009.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Wang H, Shi Q, Katkuri S, Walhi W, Desvergne B, et al. Prostaglandin E(2) promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor delta. Cancer Cell. 2004;6:285–95. doi: 10.1016/j.ccr.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Weston CR, Balmanno K, Chalmers C, Hadfield K, Molton SA, Ley R, et al. Activation of ERK1/2 by deltaRaf-1:ER* represses Bim expression independently of the JNK or PI3K pathways. Oncogene. 2003;22:1281–93. doi: 10.1038/sj.onc.1206261. [DOI] [PubMed] [Google Scholar]
- Wickenden JA, Jin H, Johnson M, Gillings AS, Newson C, Austin M, et al. Colorectal cancer cells with the BRAF(V600E) mutation are addicted to the ERK1/2 pathway for growth factor-independent survival and repression of BIM. Oncogene. 2008;27:7150–61. doi: 10.1038/onc.2008.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AC, Harper SJ, Paraskeva C. Neoplastic transformation of a human colonic epithelial cell line: in vitro evidence for the adenoma to carcinoma sequence. Cancer Res. 1990;50:4724–30. [PubMed] [Google Scholar]
- Williams AC, Smartt H, AM HZ, Macfarlane M, Paraskeva C, Collard TJ. Insulin-like growth factor binding protein 3 (IGFBP-3) potentiates TRAIL-induced apoptosis of human colorectal carcinoma cells through inhibition of NF-kappaB. Cell Death Differ. 2007;14:137–45. doi: 10.1038/sj.cdd.4401919. [DOI] [PubMed] [Google Scholar]
- Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–9. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- Yang SY, Sales KM, Fuller B, Seifalian AM, Winslet MC. Apoptosis and colorectal cancer: implications for therapy. Trends Mol Med. 2009;15:225–33. doi: 10.1016/j.molmed.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Zantl N, Weirich G, Zall H, Seiffert BM, Fischer SF, Kirschnek S, et al. Frequent loss of expression of the pro-apoptotic protein Bim in renal cell carcinoma: evidence for contribution to apoptosis resistance. Oncogene. 2007;26:7038–48. doi: 10.1038/sj.onc.1210510. [DOI] [PubMed] [Google Scholar]