Abstract
Fc receptors for immunoglobulins are found on many cells and are important in host defense. We transfected Fc gamma RIIIA, present on macrophages and natural killer (NK) cells, into COS-1 cells to study its role in phagocytosis and calcium mobilization in the absence of other Fc gamma receptors. Human Fc gamma RIIIA-alpha (CD16) was cotransfected with its associated chains, either Fc gamma RIIIA gamma or zeta. Both gamma and zeta were observed to induce a phagocytic signal, but gamma was at least sixfold more effective than zeta. Conservative substitution by phenylalanine of either one of the two cytoplasmic tyrosine residues in the gamma chain resulted in markedly diminished phagocytosis and calcium mobilization. Tyrphostin 23, an inhibitor of tyrosine kinases, reversibly inhibited phagocytosis. Further, in vitro kinase assays with the wild type and mutant gamma chains demonstrated that the wild type gamma chain, but not the mutant gamma chains, is phosphorylated. These results suggest that the cytoplasmic tyrosine residues and tyrosine phosphorylation are required for Fc gamma RIIIA to mediate two signal transduction events: phagocytosis and calcium mobilization.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amigorena S., Salamero J., Davoust J., Fridman W. H., Bonnerot C. Tyrosine-containing motif that transduces cell activation signals also determines internalization and antigen presentation via type III receptors for IgG. Nature. 1992 Jul 23;358(6384):337–341. doi: 10.1038/358337a0. [DOI] [PubMed] [Google Scholar]
- Anderson C. L., Shen L., Eicher D. M., Wewers M. D., Gill J. K. Phagocytosis mediated by three distinct Fc gamma receptor classes on human leukocytes. J Exp Med. 1990 Apr 1;171(4):1333–1345. doi: 10.1084/jem.171.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Caligiuri M., O'Brien C., Manley T., Ritz J., Schlossman S. F. Fc gamma receptor type III (CD16) is included in the zeta NK receptor complex expressed by human natural killer cells. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2274–2278. doi: 10.1073/pnas.87.6.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank U., Ra C., Miller L., White K., Metzger H., Kinet J. P. Complete structure and expression in transfected cells of high affinity IgE receptor. Nature. 1989 Jan 12;337(6203):187–189. doi: 10.1038/337187a0. [DOI] [PubMed] [Google Scholar]
- Clark M. R., Campbell K. S., Kazlauskas A., Johnson S. A., Hertz M., Potter T. A., Pleiman C., Cambier J. C. The B cell antigen receptor complex: association of Ig-alpha and Ig-beta with distinct cytoplasmic effectors. Science. 1992 Oct 2;258(5079):123–126. doi: 10.1126/science.1439759. [DOI] [PubMed] [Google Scholar]
- Einspahr K. J., Abraham R. T., Binstadt B. A., Uehara Y., Leibson P. J. Tyrosine phosphorylation provides an early and requisite signal for the activation of natural killer cell cytotoxic function. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6279–6283. doi: 10.1073/pnas.88.14.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Horton R. M., Cai Z. L., Ho S. N., Pease L. R. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques. 1990 May;8(5):528–535. [PubMed] [Google Scholar]
- Huang M. M., Indik Z., Brass L. F., Hoxie J. A., Schreiber A. D., Brugge J. S. Activation of Fc gamma RII induces tyrosine phosphorylation of multiple proteins including Fc gamma RII. J Biol Chem. 1992 Mar 15;267(8):5467–5473. [PubMed] [Google Scholar]
- Indik Z., Kelly C., Chien P., Levinson A. I., Schreiber A. D. Human Fc gamma RII, in the absence of other Fc gamma receptors, mediates a phagocytic signal. J Clin Invest. 1991 Nov;88(5):1766–1771. doi: 10.1172/JCI115496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving B. A., Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991 Mar 8;64(5):891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Samelson L. E. T cell antigen receptor activation pathways: the tyrosine kinase connection. Cell. 1991 Mar 8;64(5):875–878. doi: 10.1016/0092-8674(91)90310-u. [DOI] [PubMed] [Google Scholar]
- Kruskal B. A., Sastry K., Warner A. B., Mathieu C. E., Ezekowitz R. A. Phagocytic chimeric receptors require both transmembrane and cytoplasmic domains from the mannose receptor. J Exp Med. 1992 Dec 1;176(6):1673–1680. doi: 10.1084/jem.176.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane P. J., Ledbetter J. A., McConnell F. M., Draves K., Deans J., Schieven G. L., Clark E. A. The role of tyrosine phosphorylation in signal transduction through surface Ig in human B cells. Inhibition of tyrosine phosphorylation prevents intracellular calcium release. J Immunol. 1991 Jan 15;146(2):715–722. [PubMed] [Google Scholar]
- Lanier L. L., Yu G., Phillips J. H. Analysis of Fc gamma RIII (CD16) membrane expression and association with CD3 zeta and Fc epsilon RI-gamma by site-directed mutation. J Immunol. 1991 Mar 1;146(5):1571–1576. [PubMed] [Google Scholar]
- Letourneur O., Kennedy I. C., Brini A. T., Ortaldo J. R., O'Shea J. J., Kinet J. P. Characterization of the family of dimers associated with Fc receptors (Fc epsilon RI and Fc gamma RIII). J Immunol. 1991 Oct 15;147(8):2652–2656. [PubMed] [Google Scholar]
- McGillis J. P., Sudduth-Klinger J., Harrowe G., Mitsuhashi M., Payan D. G. Transient expression of the angiotensin II receptor: a rapid and functional analysis of a calcium-mobilizing seven-transmembrane domain receptor in COS-7 cells. Biochem Biophys Res Commun. 1989 Dec 29;165(3):935–941. doi: 10.1016/0006-291x(89)92693-4. [DOI] [PubMed] [Google Scholar]
- Odin J. A., Edberg J. C., Painter C. J., Kimberly R. P., Unkeless J. C. Regulation of phagocytosis and [Ca2+]i flux by distinct regions of an Fc receptor. Science. 1991 Dec 20;254(5039):1785–1788. doi: 10.1126/science.1837175. [DOI] [PubMed] [Google Scholar]
- Paolini R., Jouvin M. H., Kinet J. P. Phosphorylation and dephosphorylation of the high-affinity receptor for immunoglobulin E immediately after receptor engagement and disengagement. Nature. 1991 Oct 31;353(6347):855–858. doi: 10.1038/353855a0. [DOI] [PubMed] [Google Scholar]
- Park J. G., Isaacs R. E., Chien P., Schreiber A. D. In the absence of other Fc receptors, Fc gamma RIIIA transmits a phagocytic signal that requires the cytoplasmic domain of its gamma subunit. J Clin Invest. 1993 Oct;92(4):1967–1973. doi: 10.1172/JCI116790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ra C., Jouvin M. H., Blank U., Kinet J. P. A macrophage Fc gamma receptor and the mast cell receptor for IgE share an identical subunit. Nature. 1989 Oct 26;341(6244):752–754. doi: 10.1038/341752a0. [DOI] [PubMed] [Google Scholar]
- Ra C., Jouvin M. H., Blank U., Kinet J. P. A macrophage Fc gamma receptor and the mast cell receptor for IgE share an identical subunit. Nature. 1989 Oct 26;341(6244):752–754. doi: 10.1038/341752a0. [DOI] [PubMed] [Google Scholar]
- Ra C., Jouvin M. H., Kinet J. P. Complete structure of the mouse mast cell receptor for IgE (Fc epsilon RI) and surface expression of chimeric receptors (rat-mouse-human) on transfected cells. J Biol Chem. 1989 Sep 15;264(26):15323–15327. [PubMed] [Google Scholar]
- Ravetch J. V., Kinet J. P. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- Reth M. Antigen receptor tail clue. Nature. 1989 Mar 30;338(6214):383–384. doi: 10.1038/338383b0. [DOI] [PubMed] [Google Scholar]
- Romeo C., Amiot M., Seed B. Sequence requirements for induction of cytolysis by the T cell antigen/Fc receptor zeta chain. Cell. 1992 Mar 6;68(5):889–897. doi: 10.1016/0092-8674(92)90032-8. [DOI] [PubMed] [Google Scholar]
- Rosales C., Brown E. J. Two mechanisms for IgG Fc-receptor-mediated phagocytosis by human neutrophils. J Immunol. 1991 Jun 1;146(11):3937–3944. [PubMed] [Google Scholar]
- Titus J. A., Perez P., Kaubisch A., Garrido M. A., Segal D. M. Human K/natural killer cells targeted with hetero-cross-linked antibodies specifically lyse tumor cells in vitro and prevent tumor growth in vivo. J Immunol. 1987 Nov 1;139(9):3153–3158. [PubMed] [Google Scholar]
- Tuijnman W. B., Capel P. J., van de Winkel J. G. Human low-affinity IgG receptor Fc gamma RIIa (CD32) introduced into mouse fibroblasts mediates phagocytosis of sensitized erythrocytes. Blood. 1992 Apr 1;79(7):1651–1656. [PubMed] [Google Scholar]
- Unkeless J. C., Scigliano E., Freedman V. H. Structure and function of human and murine receptors for IgG. Annu Rev Immunol. 1988;6:251–281. doi: 10.1146/annurev.iy.06.040188.001343. [DOI] [PubMed] [Google Scholar]
- Weissman A. M., Baniyash M., Hou D., Samelson L. E., Burgess W. H., Klausner R. D. Molecular cloning of the zeta chain of the T cell antigen receptor. Science. 1988 Feb 26;239(4843):1018–1021. doi: 10.1126/science.3278377. [DOI] [PubMed] [Google Scholar]
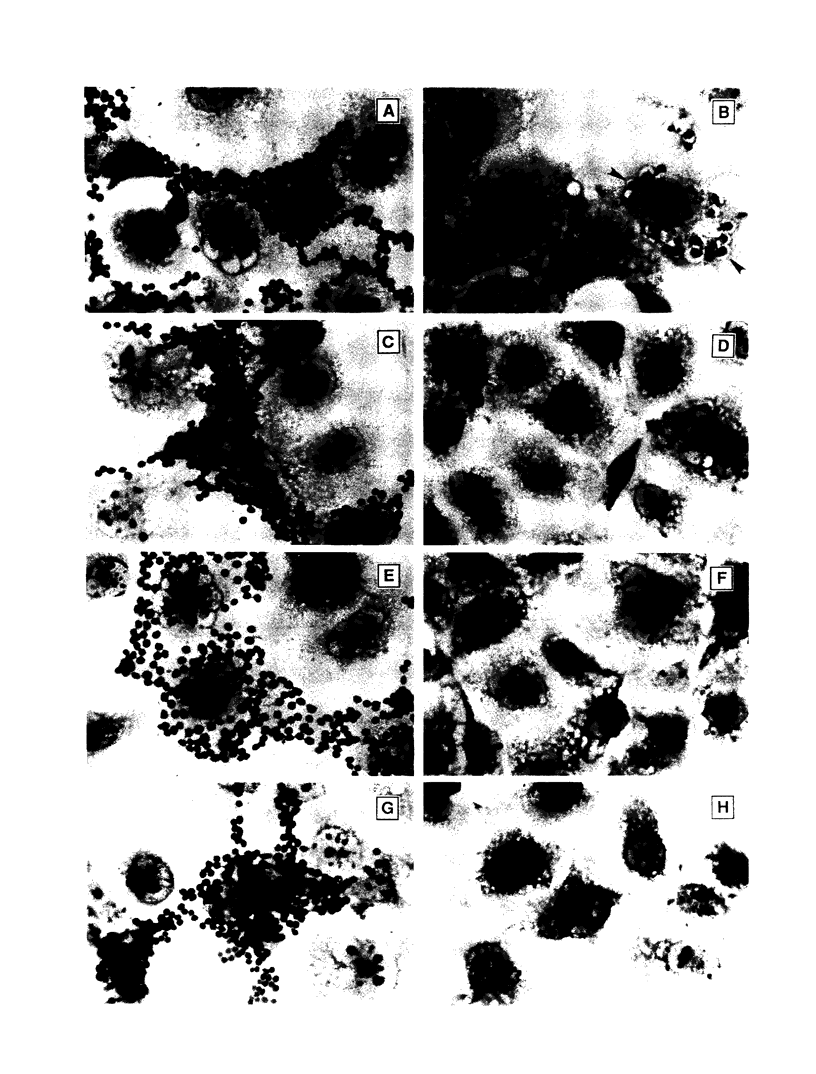
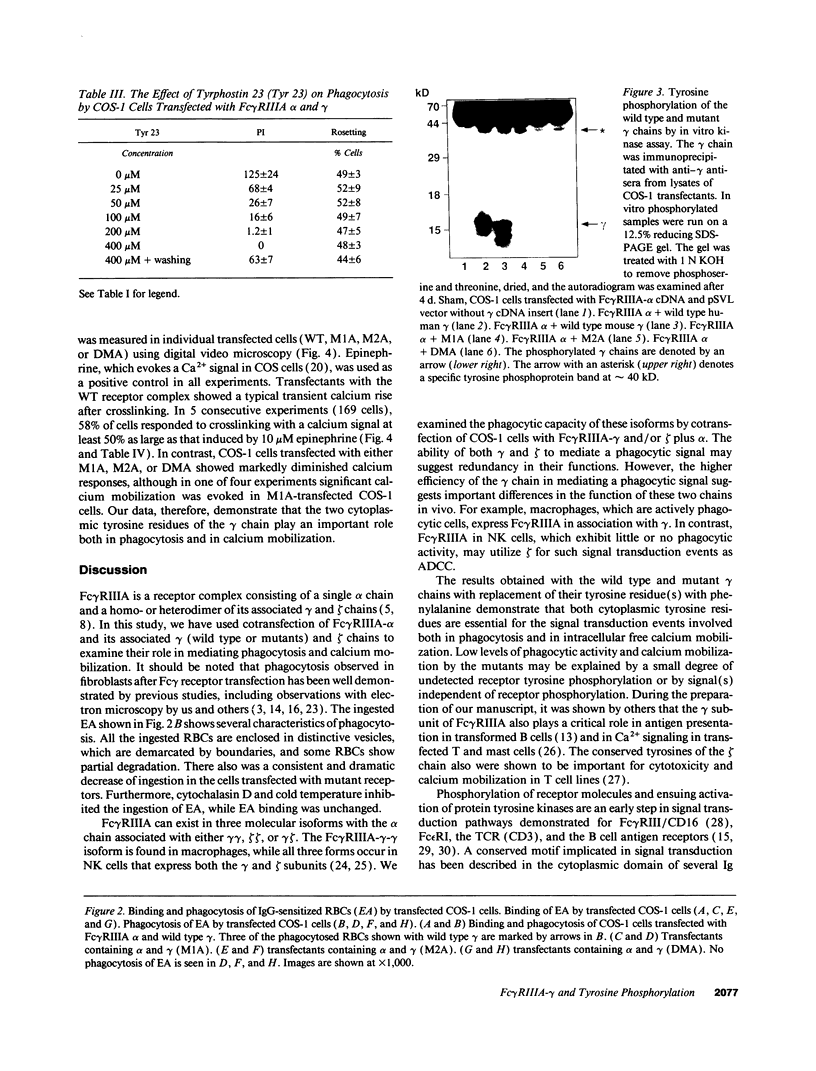
- Wirthmueller U., Kurosaki T., Murakami M. S., Ravetch J. V. Signal transduction by Fc gamma RIII (CD16) is mediated through the gamma chain. J Exp Med. 1992 May 1;175(5):1381–1390. doi: 10.1084/jem.175.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaish P., Gazit A., Gilon C., Levitzki A. Blocking of EGF-dependent cell proliferation by EGF receptor kinase inhibitors. Science. 1988 Nov 11;242(4880):933–935. doi: 10.1126/science.3263702. [DOI] [PubMed] [Google Scholar]