Abstract
Background
Type 2 diabetes has been associated with diminished late-life cognition; less is known about relations of insulin levels and insulin secretion to cognitive change among persons without diabetes. We examined prospectively relations of fasting insulin levels and insulin secretion to cognitive decline among healthy, community-dwelling older men without diabetes.
Methods
Fasting plasma insulin and C-peptide (insulin secretion) levels were measured in 1,353 nondiabetic men, aged 60–92 years (mean = 71.3 years), in the Physicians’ Health Study II, who participated in cognitive testing an average of 3.3 years later. Two assessments were administered 2 years apart (range = 1.5–4.0 years) using telephone-based tests (general cognition, verbal memory and category fluency). Primary outcomes were the Telephone Interview for Cognitive Status (TICS), global cognition (averaging all tests) and verbal memory (averaging 4 verbal tests). Multivariable linear regression models were used to estimate the relations of insulin and C-peptide to cognitive decline.
Results
Higher fasting insulin was associated with a greater decline on all tests, after adjustment. Findings were statistically significant for the TICS and category fluency, e.g. the multivariable-adjusted mean difference (95% CI) in decline for men with the highest versus lowest insulin levels was −0.62 (−1.15, −0.09) points on the TICS (p for trend = 0.04); this difference was similar to that between men 7 years apart in age. Similarly, there was a greater decline across all tests with increasing C-peptide, but the findings were statistically significant only for the global score (p for trend = 0.03).
Conclusions
Higher fasting insulin and greater insulin secretion in older men may be related to overall cognitive decline, even in the absence of diabetes.
Key Words: Insulin, C-Peptide, Cognitive decline, Dementia, Diabetes
Introduction
Type 2 diabetes, initially characterized by insulin resistance and hyperinsulinemia, has been identified as a risk factor for cognitive decline and dementia [1]. However, it is difficult to disentangle the impact of hyperinsulinemia from the effect of sequelae that accompany diabetes itself. Thus, in prior work, we addressed the relation of elevated insulin secretion and fasting insulin levels to cognition among community-dwelling, healthy elders without diabetes. We recently found that both elevated midlife fasting insulin and C-peptide levels were significantly associated with late-life cognitive decline in a large, random sample of nondiabetic women in the Nurses’ Health Study. These relations were not affected by adjustment for vascular factors such as hypertension, dyslipidemia and heart disease. However, since some of the deleterious effects of diabetes are more serious in women than men [2,3], our findings in women are not necessarily generalizable to men. Thus, in the current study, we measured fasting plasma levels of insulin and C-peptide in a random sample of 1,353 older men without diabetes, from the well-characterized Physicians’ Health Study II (PHS-II) [4,5] cohort, and then related these measures to subsequent cognitive change.
Methods
Physicians’ Health Study
The PHS-I was a randomized, double-blind, placebo-controlled, 2 × 2 factorial trial of aspirin and β-carotene for the primary prevention of cardiovascular disease (CVD) and cancer in men. Beginning in 1982, 22,071 US male licensed physicians, then aged 40–84 years, were randomized to 1 of 4 factorial groups. Thereafter, they were followed annually, via mailed self-reported questionnaires, to ascertain endpoints, lifestyle and health-related factors [6,7]. The PHS-II [4,5] randomized trial began in 1997 and was completed on August 31, 2007. An extension of the PHS-I, it examined the prevention of CVD, cancer and age-related eye diseases with vitamin supplements in 14,641 men, of whom 7,641 were original participants of the PHS-I. Morbidity and mortality follow-up outcomes in the PHS-II were extremely high at 95.3% and 97.7%, respectively [4,5].
Between 1995 and 2001, blood samples were obtained from 11,133 (76%) of the 14,641 PHS-II participants. The participants were sent blood collection kits containing the following: (a) 3 EDTA and 3 citrate tubes; (b) a gel-filled freezer pack; (c) a completed overnight courier air bill, and (d) written instructions and other supplies needed for venipuncture. Specimens were sent to our laboratory in freezer packs within 24 h of blood draw. Once received, the samples were fractionated into plasma, red blood cells and buffy coat, and then frozen at −170°C. The entire process was completed swiftly to ensure that the samples were frozen within 30–36 h after venipuncture; precautions were taken to prevent the thawing or warming of specimens during storage [8,9].
Ascertainment of Plasma Insulin and C-Peptide Levels
Using stored blood samples obtained after a ≥8-hour fast, we measured plasma insulin, using a radioimmunoassay specific for insulin (Linco, St. Louis, Mo., USA) based on an antiserum with <1% cross-reactivity for proinsulin and des-31,32-proinsulin, and plasma C-peptide, using antiserum M1230 in an alcohol precipitation nonequilibrium assay [10] with reagents provided by Diagnostic Systems Laboratory (Webster, Tex., USA). The assays were conducted in a single batch. Aliquots from a pool of quality-control plasma were randomly inserted into the batch of samples. The intra-assay coefficients of variation for insulin and C-peptide from these 57 blinded quality-control pairs were 6.8 and 7.2%, respectively.
Cognitive Function Assessment
Starting in 1998, cognitive testing began among 6,773 eligible PHS-II participants aged 65 years or older. There was an 88% participation (5,953 men) among those eligible. A second wave of testing was conducted 2 years later, with an 88% follow-up achieved among those who completed the baseline testing [11].
The cognitive interview consisted of 5 tests: (1) the Telephone Interview for Cognitive Status (TICS); (2) the immediate-recall and (3) delayed-recall trials of the East Boston Memory Test (EBMT); (4) the delayed-recall trial of the TICS 10-word list, and (5) category fluency. The TICS (scores range from 0 to 41 points) [12] is a telephone-administered instrument similar to the Mini-Mental State Examination [13]; it has a high reliability and validity for measuring general cognition [12]. The EBMT [14] is a verbal memory (paragraph recall) task and involves immediate and 15- to 20-min-delayed recalls (scores range from 0 to 12 points). The 20-min-delayed recall of the TICS 10-word list also assesses verbal memory (scores range from 0 to 10 points). Lastly, in the category fluency test, subjects name as many different animals as possible in 1 min. Category fluency captures language, and because it involves abstract conceptualization and strategy, it also maps to the domain of executive function [15]. All tests were conducted over the phone by trained interviewers blind to the study hypotheses.
The reliability and validity of this telephone method have been established [16]. The 30-day test-retest reliability (r = 0.7) and interrater reliability (r ≥ 0.95 for each test) are high. The global score from the telephone battery correlated strongly (r = 0.81) with a global score generated from 21 in-person neuropsychological tests. Furthermore, recent validation work has demonstrated that low telephone-based global scores are significantly associated with an 8-fold risk of dementia diagnosis by blinded neurologists.
Measurement of Covariates and Potential Confounders
Information on a variety of medical conditions (e.g. hypertension and diabetes), lifestyle factors (e.g. smoking, exercise and alcohol use) and medications (e.g. elevated cholesterol treatment) was obtained from the annually mailed self-report questionnaires [17]. Self-reports have proven highly accurate among male health professional participants [17,18].
Determination of Sample for Analysis
We measured insulin and C-peptide in a random sample of 1,353 PHS-II cognitive study participants who had provided blood samples and had no self-reported history of diabetes as of the time of their first cognitive interview. For the analyses of fasting insulin, the sample included 1,314 participants as 39 men had insulin levels below the assay limit of detection (2 μIU/ml).
Informed consent was obtained from all participants. The current study and the overall PHS-II study were approved by the Institutional Review Board of Brigham and Women's Hospital (Boston, Mass., USA).
Statistical Analysis
Study Outcomes
The primary outcomes were general cognitive function and verbal memory, a strong predictor of Alzheimer disease [19,20]. For assessing general cognition, we considered the TICS and the global score combining all 5 cognitive tests. This global score was calculated by averaging the Z scores from each test. Verbal memory was calculated by combining the results of the verbal memory tests (immediate and delayed recalls of the EBMT and 10-word list), also using Z scores. Composite scores are regularly used in cognitive research [21,22] as they integrate information from a variety of sources.
We examined category fluency as a secondary outcome, as executive function is related to vascular factors [23] and is of interest in considering possible cognitive effects of higher levels of insulin or C-peptide.
Overall Analysis
The distributions of insulin and C-peptide were found to be severely right-skewed. Of note, we had previously identified only a mild skew of C-peptide in the PHS cohort when blood samples were taken at a mean age of 57 years [24]. Thus, the distributions in the present study of PHS men with a mean age of 71.3 years (range = 60–92 years) at blood draw were consistent with those of men in the Honolulu-Asia Aging Study [25], who had a mean age of 77 years at blood draw.
Given these nonnormal distributions, the insulin and C-peptide values were natural log transformed. For the analysis, we then created 4 categories according to the distance (log SD) from the log mean [25]: (1) values <1 log SD below the log mean; (2) values below the log mean but within 1 log SD; (3) values above the log mean but within 1 log SD, and (4) values >1 log SD above the log mean. We used linear regression models to estimate the age- and multivariable-adjusted mean differences in cognitive change across these 4 categories. The categorical analyses allowed us to examine whether an inverse U-shaped relation may exist between insulin measures and cognitive change, which has been reported [25]. Since no U-shaped relations were observed, we tested for linear trends by examining insulin and C-peptide as continuous variables, where the unit of analysis was a 1-log-SD increase in insulin or C-peptide.
All statistical analyses were performed using SAS© version 9.1 (SAS Institute Inc., Cary, N.C., USA). Statistical tests were two-sided with α set at 0.05.
Analyses of Covariates and Potential Confounders
In the linear regression models, we included the following: age (years), history of hypertension (yes, no), history of dyslipidemia (yes, no), history of confirmed major CVD event (heart attack or stroke; yes, no), history of depression (self-reported diagnosis of depression or antidepressant treatment; yes, no), cigarette smoking (never, current, past), alcohol intake (never/rare, 1–6 drinks per week, ≥1 drinks per day), body mass index and frequency of vigorous exercise (never/rare, 1–4 times per week, ≥5 times per week). We did not adjust for education as this was identical across our sample of physicians. Information on covariates was determined from questionnaires nearest to the time of blood collection – except age, CVD and depression, which were determined as of the start of cognitive testing.
Results
Sample Characteristics by Insulin and C-Peptide Levels
The participants’ characteristics demonstrated expected associations with both plasma fasting insulin and C-peptide (tables 1, 2). Increasing insulin and C-peptide were both associated with an increasing prevalence of hypertension, dyslipidemia and heart disease. Daily alcohol intake was most prominent among those with the lowest fasting insulin levels. Trends of increasing age, depression prevalence, lower physical activity and obesity were more consistent for increasing C-peptide than for increasing insulin; only 2 men in the lowest C-peptide group were obese.
Table 1.
Characteristics of sample at blood draw, by fasting insulin levels
| <3.3 μIU/ml | 3.3–5.9 μIU/ml | 5.9–10.3 μIU/ml | >10.3 μIU/ml | |
|---|---|---|---|---|
| Participants, n | 188 (14.3) | 557 (42.4) | 384 (29.2) | 185 (14.1) |
| Geometric mean insulin level, μIU/ml | 2.82 | 4.51 | 7.47 | 16.23 |
| Geometric mean C-peptide level, nmol/1 | 0.41 | 0.52 | 0.70 | 1.14 |
| Mean age ± SD, years | 71.5 ±5.9 | 71.1 ±5.8 | 71.5 ±5.8 | 71.2 ±5.6 |
| History of hypertension, % | 37.8 | 43.4 | 45.8 | 61.1 |
| History of elevated total cholesterol, % | 16.7 | 28.1 | 31.4 | 34.1 |
| Current smoking, % | 2.1 | 2.3 | 2.6 | 3.2 |
| Past smoking, % | 49.5 | 44.3 | 47.7 | 47.6 |
| Depression, % | 10.6 | 9.2 | 8.6 | 9.7 |
| History of cardiovascular disease, % | 6.9 | 7.9 | 10.7 | 15.1 |
| Physical activity, % | ||||
| ≥5 times per week | 16.8 | 19.6 | 19.0 | 19.7 |
| 1–4 times per week | 51.4 | 41.3 | 42.6 | 32.2 |
| Alcohol, % | ||||
| ≥1 drinks per day | 47.9 | 41.5 | 37.3 | 36.6 |
| 1–6 drinks per week | 27.7 | 31.8 | 31.3 | 33.9 |
| Mean body mass index score ± SD | 23.8 ±2.6 | 25.1 ±2.7 | 26.5 ±3.4 | 28.2 ±4.2 |
| Body mass index ≥30, % | 4.3 | 3.8 | 13.0 | 22.8 |
Groups are based on 1-SD distances from the sample log mean (geometric sample log mean = 5.9 μIU/ml), and cutoff values are presented after natural log back-transformation. Values in parentheses denote percentages.
Table 2.
Characteristics of sample at blood draw, by fasting C-peptide levels
| <0.38 nmol/1 | 0.38–0.60 nmol/1 | 0.60–0.94 nmol/1 | >0.94 nmol/1 | |
|---|---|---|---|---|
| Participants, n | 187 (13.8) | 509 (37.6) | 458 (33.9) | 199 (14.7) |
| Geometric mean C-peptide level, nmol/1 | 0.31 | 0.48 | 0.73 | 1.27 |
| Geometric mean insulin level, jxIU/ml | 3.48 | 4.52 | 6.54 | 13.18 |
| Mean age ± SD, years | 71.1 ±6.0 | 71.3 ±5.7 | 71.1 ±5.8 | 71.9 ±6.0 |
| History of hypertension, % | 31.0 | 40.2 | 48.0 | 62.3 |
| History of elevated total cholesterol, % | 20.1 | 26.2 | 29.5 | 35.7 |
| Current smoking, % | 1.1 | 2.8 | 2.6 | 2.5 |
| Past smoking, % | 44.1 | 44.0 | 48.1 | 52.3 |
| Depression, % | 8.6 | 7.7 | 10.9 | 11.1 |
| History of cardiovascular disease, % | 7.5 | 6.7 | 11.4 | 14.6 |
| Physical activity, % | ||||
| ≥5 times per week | 22.2 | 17.9 | 21.0 | 15.2 |
| 1–4 times per week | 51.4 | 43.9 | 39.5 | 35.5 |
| Alcohol, % | ||||
| ≥1 drinks per day | 42.5 | 41.5 | 40.2 | 39.1 |
| 1–6 drinks per week | 30.1 | 30.8 | 30.6 | 34.5 |
| Mean body mass index score ± SD | 23.4 ±2.7 | 25.0 ±2.5 | 26.4 ±3.4 | 27.8 ±4.2 |
| Body mass index ≥30, % | 1.1 | 3.9 | 12.3 | 21.7 |
Groups are based on 1-SD distances from the sample log mean (geometric sample log mean = 0.60 nmol/1), and cutoff values are presented after natural log back-transformation. Values in parentheses denote percentages.
Prospective Analyses of Cognitive Decline
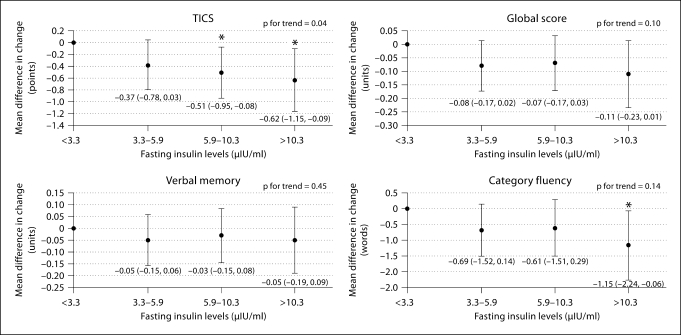
Figure 1 depicts the results for cognitive change according to fasting insulin levels. Overall, a greater cognitive decline was observed at higher versus lower levels of insulin. There were significant multivariable-adjusted associations between higher fasting insulin and decline on the TICS scale (p for trend = 0.04). To help interpret the mean difference of −0.62 points (95% CI: −1.15, −0.09) between those with the highest versus lowest levels of insulin, we contrasted it with the estimate for age. Each additional year of age was associated with a mean decline of 0.09 points (p < 0.0001) on the TICS scale; thus, those in the group with the highest fasting insulin had declines equivalent to those we observed for subjects approximately 7 years older. There was a nonsignificant trend between higher fasting insulin and decline on the global score (p = 0.10), and there was a borderline significant mean difference (95% CI) comparing extreme insulin quartiles of −0.11 units (−0.23, 0.01; p = 0.08). On category fluency, there was a significantly greater decline comparing men with the highest versus lowest fasting insulin levels (mean difference again comparable to subjects 7 years older); however, the association was slightly stronger when adjusted only for age, i.e. a mean difference (95% CI) of −1.29 words (−2.29, −0.28; data not shown in table), compared to a mean difference of −1.15 (−2.24, −0.06) after control for CVD and risk factors. An attenuation after adjustment for vascular factors was only observed for the category fluency outcome. There was no association between fasting insulin and verbal memory.
Fig. 1.
Multivariable-adjusted mean differences in change in cognitive performance, by fasting insulin levels. Adjusted for age (years), hypertension (yes, no), history of dyslipidemia (yes, no), history of heart disease (yes, no), history of depression (yes, no), cigarette smoking (current, past, never), body mass index (score), alcohol intake (never/rare, weekly, daily) and physical activity (high, moderate, low/never). Group cutoff values are presented after natural log back-transformation. ∗ Mean difference is statistically significant.
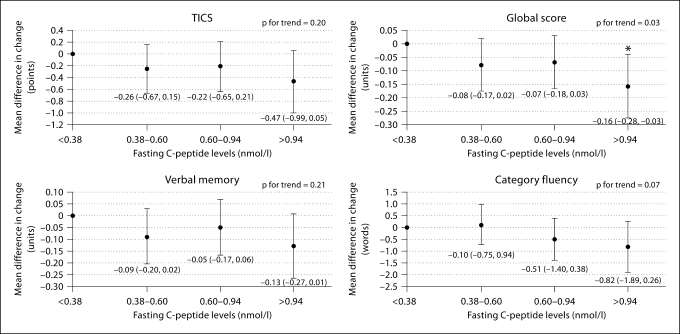
Differences in cognitive change according to C-peptide levels are shown in figure 2. As observed with fasting insulin, there was an overall pattern of greater cognitive decline comparing those with the highest to those with the lowest C-peptide levels. The mean difference in decline, comparing the extreme C-peptide groups, was statistically significant for the global score (p for trend = 0.03); this was cognitively equivalent to aging by about 5.5 years. The mean decline in verbal memory associated with the highest C-peptide group was borderline significant compared to those with the lowest C-peptide (mean difference = −0.13; p = 0.07). As with fasting insulin, there was a notable attenuation of estimates relating C-peptide to change in category fluency scores after adjustment for vascular factors. There was, for instance, a significant age-adjusted trend to a greater decline in category fluency with increasing C-peptide (p for trend = 0.02); however, the trend was borderline significant (p for trend = 0.07) after multivariable adjustment.
Fig. 2.
Multivariable-adjusted mean differences in change in cognitive performance, by fasting C-peptide levels. Adjusted for age (years), hypertension (yes, no), history of dyslipidemia (yes, no), history of heart disease (yes, no), history of depression (yes, no), cigarette smoking (current, past, never), body mass index (score), alcohol intake (never/rare, weekly, daily) and physical activity (high, moderate, low/never). Group cutoff values are presented after natural log back-transformation. ∗ Mean difference is statistically significant.
Discussion
In this random sample of 1,353 community-dwelling older men without diabetes, higher late-life levels of fasting insulin and C-peptide were associated with a greater subsequent decline in general cognition. For example, the multivariable-adjusted mean difference (95% CI) in decline for men with the highest versus lowest fasting insulin was cognitively equivalent to that observed between men 7 years apart in age. Similarly, the decline on the global score for men with the highest versus lowest C-peptide levels was cognitively comparable to the effect of aging by 5.5 years.
Several observational studies [24,26,27,28,29,30] have identified cross-sectional associations between hyperinsulinemia (or measures of insulin resistance) and impaired late-life cognitive performance. However, only few large studies [25,31,32,33,34] have prospectively addressed relations of insulin to cognitive change over time among older adults. Luchsinger et al. [31] reported that higher fasting insulin was associated with a significant decline in memory scores in a cohort of older men and women. Similarly, Yaffe et al. [32] found that older women without diabetes but with impaired fasting glucose (an indicator of growing insulin resistance) had a relative risk of 1.64 (95% CI: 1.03, 2.61) for developing significant cognitive impairment (defined as dementia, mild cognitive impairment or a Short Blessed [35] score of >6) after 4 years of follow-up compared to those with normal glucose; however, impaired fasting glucose was not associated with a significantly greater decline on individual cognitive tests. Finally, recent work involving over 1,000 women from the Nurses’ Health Study has focused on midlife biomarkers, but has also found that higher fasting insulin [34] and C-peptide [33] were associated with increased rates of decline in late-life overall cognition and verbal memory. A notable contrast between these prior results [33,34] and those from the current study was the strong relation of elevated levels of both insulin and C-peptide to verbal memory decline among women. This may suggest a possible gender difference worth further scrutiny in studies involving direct comparisons (i.e. whether verbal memory is more sensitive to insulin-dysregulation-related cognitive decline among women vs. men). Overall, the results of the current study are consistent with emerging evidence suggesting that higher insulin levels are related to general cognitive decline in both older women and men, even in the absence of diabetes.
Higher insulin levels may impact cognition through a variety of mechanisms including vascular damage associated with insulin resistance [29,36]; our results for category fluency are consistent with this potential explanation. Specifically, we observed that estimates of associations between insulin and category fluency decline were substantively attenuated after adjustment for CVD and other vascular factors. As a task that maps to the executive function domain, category fluency is most likely among the tests in our battery to be affected by vascular factors; in fact, executive dysfunction is a prominent feature of vascular cognitive impairment and/or dementia [23]. However, we did not observe a similar attenuation of estimates for the other cognitive outcomes, suggesting that an intermediary influence of vascular pathology is unlikely to be the major explanation for our findings relating insulin and C-peptide levels to overall cognitive decline. Indeed, growing biological evidence suggests more direct effects of higher insulin. Insulin is a competitive inhibitor for insulin-degrading enzyme [37]; therefore, persistent elevations in insulin may interfere with peripheral Aβ clearance, and this could lead to higher Aβ concentrations in the brain [38,39]. Another possibility is that chronically high insulin in the periphery may paradoxically lead to a relatively hypoinsulinized state in the brain [40]: an excessive insulin production and hyperinsulinemia could actually result in cognitive dysfunction by impairing the insulin-mediated utilization of glucose by cells in the brain – including the cells of the hippocampus, which is particularly enriched with insulin receptors [41].
This study has several strengths including the large sample size, prospective design, high rate of follow-up, and abundant information on potential confounders. In addition, we utilized a well-validated method for assessing cognition. There were also strengths pertaining to our measurement of fasting plasma insulin and C-peptide. The assay coefficients of variation show that we measured both peptides with high precision. Furthermore, examining fasting insulin and C-peptide in the same set of random participants provided a test of the robustness of our hypothesized relations between insulin and late-life cognitive decline.
Potential limitations of our study should also be considered. First, we relied on self-reports to exclude diabetic men. However, the high validity of health professional participants’ reports of diabetes has been established [42]; thus, it is unlikely that a strong association between undiagnosed diabetes and cognitive decline would explain the findings. Second, there was an average lag of 3.3 years between blood draw and initial cognitive testing. However, the majority of the blood samples were obtained within 4 years of cognitive testing. Also, prior reliability work showed a within-person correlation of 0.57 between C-peptide levels measured 4 years apart among male health professionals [43]; thus, insulin and C-peptide levels in this study likely are good representations of blood levels at the time of cognitive testing. Third, generalizability is a potential concern. Although basic biological relations observed in this largely Caucasian sample of well-educated men are likely comparable to those seen among men in the general population, further research involving men of ethnic minorities would be necessary to address potential cognitive effects of insulin levels in these populations. Finally, although we were able to adjust for numerous potential confounders, residual confounding is still possible. Nevertheless, the relative homogeneity of our cohort reduces the potential influences of some unmeasured confounders such as health knowledge and access to care.
In conclusion, our study provides further evidence for an association between higher insulin production and insulin resistance – in the absence of diabetes – and cognitive decline in older men. These results are timely as the growing epidemic of obesity in the USA places a substantial percentage of Americans at high risk for developing chronically high insulin levels and insulin resistance. Addressing risk factors for elevated blood insulin, as well as exploring potential interactions involving blood insulin levels and population genetic factors (e.g. apolipoprotein E), could play a key role in an overall public health strategy to combat cognitive decline and dementia.
Acknowledgements
This work was supported by grants AG15933, AG24215, CA34944, CA40360, CA42182, CA97193, HL26490 and HL34595 from the National Institutes of Health. Dr. Okereke's effort was supported by an R01 Minority Supplement to grant AG24215.
References
- 1.Coker LH, Shumaker SA. Type 2 diabetes mellitus and cognition: an understudied issue in women's health. J Psychosom Res. 2003;54:129–139. doi: 10.1016/s0022-3999(02)00523-8. [DOI] [PubMed] [Google Scholar]
- 2.Legato MJ, Gelzer A, Goland R, Ebner SA, Rajan S, Villagra V, Kosowski M, Writing Group for the Partnership for Gender-Specific Medicine Gender-specific care of the patient with diabetes: review and recommendations. Gend Med. 2006;3:131–158. doi: 10.1016/s1550-8579(06)80202-0. [DOI] [PubMed] [Google Scholar]
- 3.Avogaro A, Giorda C, Maggini M, Mannucci E, Raschetti R, Lombardo F, Spila-Alegiani S, Turco S, Velussi M, Ferrannini E, Diabetes and Informatics Study Group, Association of Clinical Diabetologists, Istituto Superiore di Sanità Incidence of coronary heart disease in type-2-diabetic men and women: impact of microvascular complications, treatment, and geographic location. Diabetes Care. 2007;30:1241–1247. doi: 10.2337/dc06-2558. [DOI] [PubMed] [Google Scholar]
- 4.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2008;300:2123–2133. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Sesso HD, Buring JE. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2009;301:52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steering Committee of the Physicians' Health Study Research Group Preliminary report: findings from the aspirin component of the ongoing Physicians' Health Study. N Engl J Med. 1988;318:262–264. doi: 10.1056/NEJM198801283180431. [DOI] [PubMed] [Google Scholar]
- 7.Steering Committee of the Physicians' Health Study Research Group Final report on the aspirin component of the ongoing Physicians' Health Study. N Engl J Med. 1989;321:129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 8.Djoussé L, Kurth T, Gaziano JM. Cystatin C and risk of heart failure in the Physicians' Health Study (PHS) Am Heart J. 2008;155:82–86. doi: 10.1016/j.ahj.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen BH, Song Y, Ding EL, Roberts CK, Manson JE, Rifai N, Buring JE, Gaziano JM, Liu S. Circulating levels of resistin and risk of type 2 diabetes in men and women: results from two prospective cohorts. Diabetes Care. 2009;32:329–334. doi: 10.2337/dc08-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faber OK, Binder C, Markussen J, Heding LG, Naithani VK, Kuzuya H, Blix P, Horwitz DL, Rubenstein AH. Characterization of seven C-peptide antisera. Diabetes. 1978;27:170–177. doi: 10.2337/diab.27.1.s170. [DOI] [PubMed] [Google Scholar]
- 11.Okereke OI, Kang JH, Cook NR, Gaziano JM, Manson JE, Buring JE, Grodstein F. Type 2 diabetes and cognitive decline in two large cohorts of community-dwelling older adults. J Am Geriatr Soc. 2008;56:1028–1036. doi: 10.1111/j.1532-5415.2008.01686.x. [DOI] [PubMed] [Google Scholar]
- 12.Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer's disease. Int J Neurosci. 1991;57:167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- 15.Royall DR, Lauterbach EC, Cummings JL, Reeve A, Rummans TA, Kaufer DI, LaFrance WC, Jr, Coffey CE. Executive control function: a review of its promise and challenges for clinical research. A report from the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 2002;14:377–405. doi: 10.1176/jnp.14.4.377. [DOI] [PubMed] [Google Scholar]
- 16.Kang JH, Cook N, Manson J, Buring JE, Grodstein F. A randomized trial of vitamin E supplementation and cognitive function in women. Arch Intern Med. 2006;166:2462–2468. doi: 10.1001/archinte.166.22.2462. [DOI] [PubMed] [Google Scholar]
- 17.Ajani UA, Gaziano JM, Lotufo PA, Liu S, Hennekens CH, Buring JE, Manson JE. Alcohol consumption and risk of coronary heart disease by diabetes status. Circulation. 2000;102:500–505. doi: 10.1161/01.cir.102.5.500. [DOI] [PubMed] [Google Scholar]
- 18.Cho E, Rimm EB, Stampfer MJ, Willett WC, Hu FB. The impact of diabetes mellitus and prior myocardial infarction on mortality from all causes and from coronary heart disease in men. J Am Coll Cardiol. 2002;40:954–956. doi: 10.1016/s0735-1097(02)02044-2. [DOI] [PubMed] [Google Scholar]
- 19.Small BJ, Fratiglioni L, Viitanen M, Winblad B, Backman L. The course of cognitive impairment in preclinical Alzheimer disease: three- and six-year follow-up of a population-based sample. Arch Neurol. 2000;57:839–844. doi: 10.1001/archneur.57.6.839. [DOI] [PubMed] [Google Scholar]
- 20.Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M. Patterns of cognitive decline in presymptomatic Alzheimer disease: a prospective community study. Arch Gen Psychiatry. 2001;58:853–858. doi: 10.1001/archpsyc.58.9.853. [DOI] [PubMed] [Google Scholar]
- 21.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 22.Wilson RS, Schneider JA, Barnes LL, Beckett LA, Aggarwal NT, Cochran EJ, Berry-Kravis E, Bach J, Fox JH, Evans DA, Bennett DA. The apolipoprotein E epsilon 4 allele and decline in different cognitive systems during a 6-year period. Arch Neurol. 2002;59:1154–1160. doi: 10.1001/archneur.59.7.1154. [DOI] [PubMed] [Google Scholar]
- 23.Román GC, Sachdev P, Royall DR, Bullock RA, Orgogozo JM, López-Pousa S, Arizaga R, Wallin A. Vascular cognitive disorder: a new diagnostic category updating vascular cognitive impairment and vascular dementia. J Neurol Sci. 2004;226:81–87. doi: 10.1016/j.jns.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Okereke O, Kang JH, Gaziano JM, Ma J, Stampfer MJ, Grodstein F. Plasma C-peptide and cognitive performance in older men without diabetes. Am J Geriatr Psychiatry. 2006;14:1041–1050. doi: 10.1097/01.JGP.0000240983.25359.00. [DOI] [PubMed] [Google Scholar]
- 25.Peila R, Rodriguez BL, White LR, Launer LJ. Fasting insulin and incident dementia in an elderly population of Japanese-American men. Neurology. 2004;63:228–233. doi: 10.1212/01.wnl.0000129989.28404.9b. [DOI] [PubMed] [Google Scholar]
- 26.Kalmijn S, Feskens EJM, Launer LJ, Stijnen T, Kromhout D. Glucose intolerance, hyperinsulinaemia and cognitive function in a general population of elderly men. Diabetologia. 1995;38:1096–1102. doi: 10.1007/BF00402181. [DOI] [PubMed] [Google Scholar]
- 27.Stolk RP, Breteler MMB, Ott A, Pols HA, Lamberts SW, Grobbee DE, Hofman A. Insulin and cognitive function in an elderly population: the Rotterdam study. Diabetes Care. 1997;20:792–795. doi: 10.2337/diacare.20.5.792. [DOI] [PubMed] [Google Scholar]
- 28.Abbatecola AM, Paolisso G, Lamponi M, Bandinelli S, Lauretani F, Launer L, Ferrucci L. Insulin resistance and executive dysfunction in older persons. J Am Geriatr Soc. 2004;52:1713–1718. doi: 10.1111/j.1532-5415.2004.52466.x. [DOI] [PubMed] [Google Scholar]
- 29.Geroldi C, Frisoni GB, Paolisso G, Bandinelli S, Lamponi M, Abbatecola AM, Zanetti O, Guralnik JM, Ferrucci L. Insulin resistance in cognitive impairment: the InCHIANTI Study. Arch Neurol. 2005;62:1067–1072. doi: 10.1001/archneur.62.7.1067. [DOI] [PubMed] [Google Scholar]
- 30.Okereke O, Hankinson SE, Hu FB, Grodstein F. Plasma C-peptide and cognitive function among older women without diabetes. Arch Intern Med. 2005;165:1651–1656. doi: 10.1001/archinte.165.14.1651. [DOI] [PubMed] [Google Scholar]
- 31.Luchsinger J, Tang M-X, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63:1187–1192. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- 32.Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63:658–663. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- 33.Okereke OI, Pollak MN, Hu FB, Hankinson SE, Selkoe DJ, Grodstein F. Plasma C-peptide levels and rates of cognitive decline in older, community-dwelling women without diabetes. Psychoneuroendocrinology. 2008;33:455–461. doi: 10.1016/j.psyneuen.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Oijen M, Okereke OI, Kang JH, Pollak MN, Hu FB, Hankinson SE, Grodstein F. Fasting insulin levels and cognitive decline in older women without diabetes. Neuroepidemiology. 2008;30:174–179. doi: 10.1159/000126909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatry. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 36.Hsueh WA, Quinones MJ. Role of endothelial dysfunction in insulin resistance. Am J Cardiol. 2003;92:10J–17J. doi: 10.1016/s0002-9149(03)00611-8. [DOI] [PubMed] [Google Scholar]
- 37.Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr Rev. 1998;19:608–624. doi: 10.1210/edrv.19.5.0349. [DOI] [PubMed] [Google Scholar]
- 38.DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM. Brain-to-plasma amyloid-beta efflux: a measure of brain amyloid burden in a mouse model of Alzheimer's disease. Science. 2002;295:2264–2267. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- 39.Fishel MA, Watson GS, Montine TJ, Wang Q, Green PS, Kulstad JJ, Cook DG, Peskind ER, Baker LD, Goldgaber D, Nie W, Asthana S, Plymate SR, Schwartz MW, Craft S. Hyperinsulinemia provokes synchronous increases in central inflammation and beta-amyloid in normal adults. Arch Neurol. 2005;62:1539–1544. doi: 10.1001/archneur.62.10.noc50112. [DOI] [PubMed] [Google Scholar]
- 40.Craft S. Insulin resistance syndrome and Alzheimer's disease: age- and obesity-related effects on memory, amyloid, and inflammation. Neurobiol Aging. 2005;26:65–69. doi: 10.1016/j.neurobiolaging.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 41.Zhao WQ, Alkon DL. Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol. 2001;177:125–134. doi: 10.1016/s0303-7207(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 42.Manson JE, Stampfer MJ, Colditz GA, Willett WC, Rosner B, Hennekens CH, Speizer FE, Rimm EB, Krolewski AS. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;338:774–778. doi: 10.1016/0140-6736(91)90664-b. [DOI] [PubMed] [Google Scholar]
- 43.Ma J, Giovannucci E, Pollak M, Leavitt A, Tao Y, Gaziano JM, Stampfer MJ. A prospective study of plasma C-peptide and colorectal cancer risk in men. J Natl Cancer Inst. 2004;96:546–553. doi: 10.1093/jnci/djh082. [DOI] [PubMed] [Google Scholar]