Abstract
Pattern recognition receptors (PRRs) detect conserved microbial structures generally absent from eukaryotes. Bacterial pathogens commonly utilize pore-forming toxins or specialized secretion systems to deliver virulence factors that promote bacterial replication by modulating host cell physiology. Detection of these secretion systems or toxins by nucleotide-binding oligomerization domain leucine-rich-repeat proteins (NLRs) triggers the assembly of multiprotein complexes, termed inflammasomes, necessary for caspase-1 activation. Here we demonstrate that caspase-1 activation in response to the Yersinia type III secretion system (T3SS) requires the adapter ASC, and involves both NLRP3 and NLRC4 inflammasomes. We further identify a Yersinia type III secreted effector protein, YopK, which prevents inflammasome activation by preventing cellular recognition of the T3SS. Inflammasome-mediated sensing of the T3SS promotes bacterial clearance from infected tissues in vivo. These data demonstrate that a class of bacterial proteins interferes with cellular recognition of bacterial secretion systems, which contributes to bacterial survival within host tissues.
Introduction
The innate immune response to microbial pathogens is critical for host defense. Evolutionarily conserved pattern recognition receptors (PRRs) recognize microbial components, termed pathogen associated molecular patterns (PAMPs), present in all microbes of a given class, but absent from the host (Janeway and Medzhitov, 2002). Two classes of PRRs include the Toll-like receptors (TLRs), and the nucleotide binding oligomerization domain-leucine-rich-repeat receptors (NLRs). TLRs detect either extracellular or endosomally localized PAMPs, such as bacterial LPS or lipoteichoic acid, or viral nucleic acids, and induce pro-inflammatory gene expression through NF-κB, IRF, and MAP kinase (MAPK) signaling pathways (Akira et al., 2001). In contrast, NLRs detect microbial components within the cytosol or microbial infection-associated activities, and, depending on the NLR, can signal either induction of proinflammatory gene expression, or assembly of caspase-1 activating protein complexes termed inflammasomes (Chen et al., 2008; Martinon et al., 2002).
Inflammasome assembly is necessary for the activation of pro-caspase-1 in response to bacterial, fungal and viral pathogens as well as certain non-infectious stimuli (Martinon et al., 2009). Caspase-1 activation is necessary for cleavage and secretion of the active forms of the pro-inflammatory cytokines IL-1β and IL-18 (Mariathasan and Monack, 2007), as well as for unconventional secretion of a variety of other proteins (Keller et al., 2008). Caspase-1 activation in response to bacterial infections also induces a rapid pro-inflammatory cell death termed pyroptosis (Cookson and Brennan, 2001). Distinct stimuli are thought to trigger assembly of different inflammasomes defined by particular NLRs and the adapter protein ASC. Specifically, the NLRP3 inflammasome is activated in response to a wide variety of stimuli, such as bacterial pore-forming toxins, ionophores, and non-infectious crystals (Cassel et al., 2008; Mariathasan et al., 2006; Martinon et al., 2006). The NLRP3 inflammasome can be induced via potassium efflux (Petrilli et al., 2007), and requires generation of reactive oxygen species (Dostert et al., 2008), or lysosomal disruption (Hornung et al., 2008). In contrast, the NLRC4 inflammasome is activated in response to bacterial flagellin delivered into the cytosol of the infected cell during Salmonella or Legionella infection (Franchi et al., 2006; Miao et al., 2006; Molofsky et al., 2006; Ren et al., 2006), while flagellin is dispensable for activation of the NLRC4 inflammasome in response to Shigella and some Pseudomonas infections (Sutterwala et al., 2007; Suzuki et al., 2007).
Although a common feature of bacterial stimuli that trigger inflammasome activation is the presence of either pore-forming toxins or virulence-associated secretion systems, the precise signals that lead to inflammasome assembly during bacterial infection are not clear. Bacterial type III and type IV secretion systems (T3SS and T4SS, respectively) deliver effector proteins into the host cell that disrupt cellular processes in order to promote bacterial replication (Galan and Wolf-Watz, 2006). Disruption of membrane integrity by bacterial toxins and secretion systems, as well as activities of virulence factors themselves, have been proposed as a signal of pathogen presence that induces membrane repair and innate immune responses (Gurcel et al., 2006; Vance et al., 2009). Bacterial virulence strategies can involve interference with essential components of immune signaling pathways, such as MyD88 and NF-κB (reviewed in (Brodsky and Medzhitov, 2009)), or evasion of innate immune recognition by modifying LPS (Montminy et al., 2006). However, how bacterial pathogens interfere with inflammasome-based detection of their secretion systems is poorly understood.
Pathogenic Yersiniae, which comprise Y. pestis, the causative agent of plague, and the two enteric pathogens, Y. pseudotuberculosis and Y. enterocolitica, express a T3SS encoded on a common virulence plasmid (Cornelis and Wolf-Watz, 1997). The Yersinia effectors, termed Yops, disrupt key cellular signaling pathways of both the innate and adaptive immune response (reviewed in (Viboud and Bliska, 2005)). Inhibition of NF-κB and MAPK signaling by Yersinia YopJ/YopP during infection of macrophages induces caspase-1-independent apoptosis (Denecker et al., 2001). Nonetheless, caspase-1 is activated during YopJ-induced apoptosis in macrophages infected with Y. pestis (Lilo et al., 2008). In addition, macrophage activation can shift the balance from YopJ-dependent apoptosis to a YopJ-independent, caspase-1-mediated cell death following Yersinia infection (Bergsbaken and Cookson, 2007). However, the role of different inflammasomes during Yersinia infection and the contribution of caspase-1 to anti-Yersinia host defense is unknown.
Because a common feature of caspase-1 activation in response to bacterial infection is the presence of virulence-associated secretion systems, we considered the possibility that the Yersinia T3SS itself might act as a trigger of caspase-1 activation. Indeed, pore formation by the Yersinia T3SS can trigger secretion of IL-1β from infected cells (Bergsbaken and Cookson, 2007; Shin and Cornelis, 2007). We also hypothesized that lack of caspase-1 activation in naïve macrophages infected by YopJ-deficient bacteria could be due to inhibition of inflammasome activation by another Yersinia virulence factor. Here we demonstrate that the Yersinia T3SS triggers ASC-dependent inflammasome activation, and that both NLRP3 and NLRC4 contribute separately to caspase-1 activation in response to the T3SS. We further show that a T3SS secreted protein, YopK, interacts with the T3SS translocon and prevents T3SS-induced inflammasome activation. Importantly, preventing inflammasome activation results in decreased serum IL-18 and enhanced survival of YopK-expressing bacteria during infection in vivo. Our data demonstrate that recognition of the T3SS contributes to bacterial clearance by the innate immune system, and define a virulence factor that promotes bacterial survival in vivo by preventing T3SS recognition. Preventing inflammasome activation by interfering with recognition of secretion systems appears to be a novel strategy for bacterial evasion of host immune responses.
Results
Yersinia T3SS triggers inflammasome activation in the absence of either NLRC4 or NLRP3
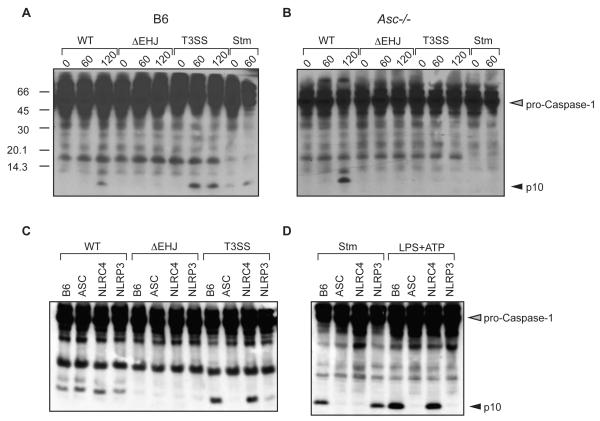
Death of naïve macrophages infected by pathogenic Yersiniae is independent of caspase-1, and requires inhibition of NF-κB and MAPK dependent gene expression by the Yersinia virulence factor YopJ (Denecker et al., 2001; Lilo et al., 2008; Zhang et al., 2005). However, naïve macrophages infected with yopJ mutant Yersinia do not undergo cell death despite the presence of a functional bacterial T3SS. We therefore investigated whether a Yersinia virulence factor might prevent caspase-1 activation in response to the T3SS. Accordingly, Y. pseudotuberculosis containing a modified mCD1 virulence plasmid from Y. pestis that contains all of the genes necessary to assemble a functional type III secretion system, but lacks all other translocated virulence factors (Bartra et al., 2006) induced caspase-1 activation in primary bone marrow-derived macrophages (BMMs). In contrast, YopJ-, YopEJ-, or YopEHJ-deficient bacteria failed to induce caspase-1 activation (Figure 1A and Figure S1A). We subsequently refer to the Y. pseudotuberculosis strain containing mCD1 as T3SS. These data indicated that the Yersinia T3SS triggers caspase-1 activation and that a Yersinia protein distinct from YopE and YopH prevents T3SS-induced inflammasome activation. Both the Yersinia T3SS strain and Salmonella enterica serovar Typhimurium (S. typhimurium) triggered rapid caspase-1 activation in infected cells; in contrast, YopJ-dependent activation of caspase-1 by wild-type Yersinia (WT) did not occur until at least 2 hours post-infection (Figure 1A and Figure S1A).
Figure 1. Macrophages require ASC for caspase-1 activation in response to the Yersinia T3SS.
(A) BMMs derived from WT (B6), or (B) Asc-/- mice were infected with WT, ΔyopEHJ, or type III secretion system only (T3SS) strains of Y. pseudotuberculosis, as indicated, or with S. typhimurium (Stm) as control. Cell lysates were harvested at indicated times post-infection and probed with anti-caspase-1 antibodies. (C) BMMs from indicated mouse strains were harvested 120 minutes post-infection with WT, ΔyopEHJ, or T3SS Yersinia strains, or (D) 60 minutes post-infection with Salmonella enterica serovar Typhimurium (Stm). Cells were treated with LPS for 3 hours followed by 60 minutes ATP treatment (LPS+ATP). Data are representative of at least 3 independent experiments.
To determine the nature of the inflammasomes activated by Yersinia infection, we infected bone marrow-derived macrophages (BMMs) from mice deficient in various inflammasome components. YopJ-dependent activation of caspase-1 did not require ASC, whereas absence of ASC abrogated caspase-1 activation in response to both the T3SS and S. typhimurium (Figure 1B). Caspase-1 activation in response to either T3SS or WT Yersinia also did not require NOD1, NOD2, RIP2, or P2X7 (data not shown). Caspase-1 was still activated in WT and T3SS Yersinia-infected BMMs from Nlrc4-/- or Nlrp3-/- mice, indicating that inflammasome activation in response to YopJ and the T3SS could occur in the absence of either NLRC4 or NLRP3 (Figure S1B). However, direct comparison of caspase-1 activation by the Yersinia T3SS in cells deficient in ASC, NLRC4, and NLRP3 revealed significantly less caspase-1 activation in Nlrp3-/- BMMs than in either B6 or Nlrc4-/- BMMs, suggesting a key role for NLRP3 in Yersinia T3SS-induced inflammasome activation (Figure 1C).
YopE can limit T3SS-induced pore formation in infected cells (Schotte et al., 2004; Viboud and Bliska, 2001). However, we did not observe a role for YopE in inducing or inhibiting caspase-1 activation (Figure S1A). Additionally, in-frame deletion of the flagellin encoding gene fliC did not diminish caspase-1 activation in Yersinia-infected cells (Figure S2), consistent with observations that T3SS-inducing conditions repress Yersinia flagellar gene expression (Minnich and Rohde, 2007). These data suggested that two distinct pathways trigger caspase-1 activation in Yersinia infected cells: one pathway is YopJ-dependent, but is independent of ASC, NLRC4, or NLRP3, while the other pathway responds to the Yersinia T3SS, requires ASC and involves NLRP3 as well as potentially other inflammasomes.
Yersinia T3SS-induced macrophage cell death requires caspase-1
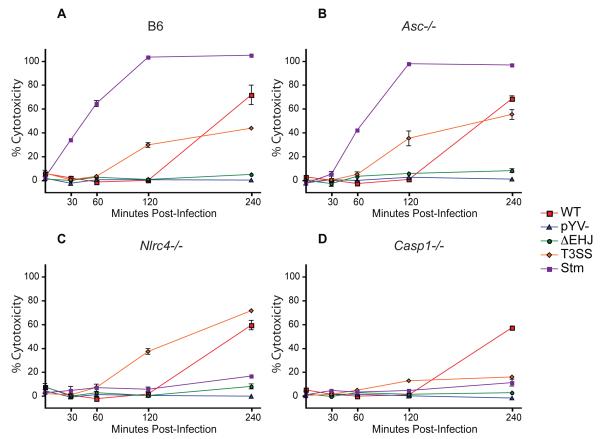
Consistent with the rapid activation of caspase-1 by the T3SS, we observed significant cell death, as measured by LDH release, in T3SS-infected cells within two hours post-infection, whereas WT-infected cells did not release measurable levels of LDH until four hours post-infection (Figure 2A). Bacteria lacking YopJ or YopEHJ did not induce LDH release, consistent with the lack of caspase-1 activation in these cells (Figure 2A and data not shown). Interestingly, ASC was not required for LDH release from either T3SS- or WT-infected cells (Figure 2B). NLRC4 was also dispensable for LDH release in cells infected with either T3SS or WT bacteria, in contrast to those infected with S. typhimurium, (Figure 2C). Caspase-1 was necessary for LDH release in cells infected with the T3SS, in contrast to WT-infected cells (Figure 2D), which undergo a caspase-1-independent, YopJ-dependent cell death (Denecker et al., 2001; Lilo et al., 2008). These data indicate that the Yersinia T3SS induces macrophage death through a caspase-1 dependent, ASC-independent pathway distinct from the YopJ-dependent death induced by WT Yersinia.
Figure 2. Caspase-1 is required for cell death triggered in response to the Yersinia T3SS but not YopJ.
Percent cytotoxicity was measured by release of lactate dehydrogenase (LDH) in supernatants of (A) WT, (B) Asc-/-, (C) Nlrc4-/-, (D) Casp1-/- BMMs collected at indicated times following infection with indicated bacteria. pYV- - Y. pseudotuberculosis lacking Yersinia virulence plasmid. Data are representative of at least three independent experiments.
YopK prevents inflammasome activation in response to the T3SS
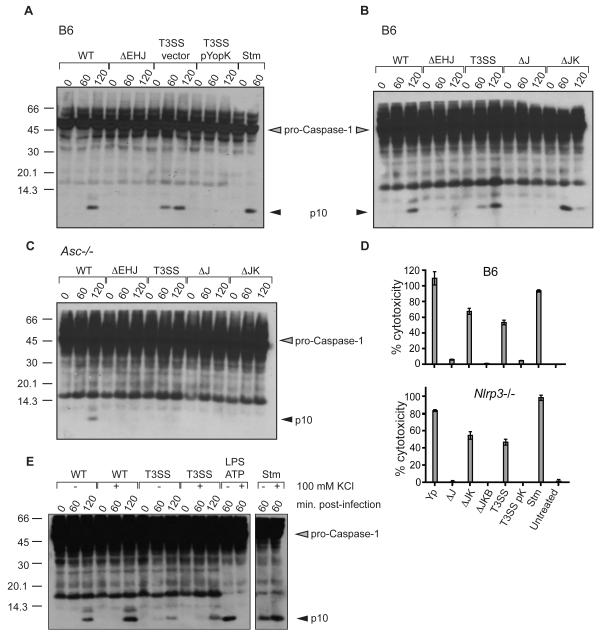
The absence of caspase-1 activation and LDH release in cells infected with either YopJ-deficient or YopEHJ-deficient bacteria suggested that a protein lacking in the T3SS strain prevents inflammasome activation in cells infected with YopJ-deficient bacteria. We hypothesized that YopK, previously suggested to modulate the size of the T3SS-induced pore (Holmstrom et al., 1997), might fulfill this role. Indeed, BMMs infected with a T3SS strain expressing YopK failed to induce caspase-1 activation upon bacterial infection (Figure 3A). Conversely, deletion of both yopJ and yopK resulted in robust activation of caspase-1, in contrast to bacteria lacking either yopJ alone or yopEHJ, confirming that YopK is necessary and sufficient to inhibit inflammasome activation triggered by the T3SS (Figure 3B). The yopJK strain also induced an ASC-dependent caspase-1 activation, similar to the T3SS strain (Figures 3C). Furthermore, YopK prevented caspase-1 dependent pyroptosis in response to the T3SS, as no LDH release occurred in cells infected by bacteria expressing YopK (Figure 3D, ΔJ vs. ΔJK, and T3SS vs. T3SS pYopK). Consistent with ASC-independent release of LDH in T3SS-infected cells, LDH release was also independent of NLRP3 (Figure 3D, compare upper and lower panels). Addition of extracellular potassium or the reactive oxygen scavenger NAC, both of which can prevent activation of the NLRP3 inflammasome, did not prevent activation of caspase-1 in response to infection with either the T3SS or the WT Yersinia strains (Figure 3E and data not shown). Consistent with our previous observations, in-frame deletion of fliC in the ΔyopJK strain or absence of NLRC4 also did not prevent caspase-1 activation in response to the T3SS (Figure S2B and S2C).
Figure 3. Caspase-1 activation and cell death triggered by macrophages in response to the Yersinia T3SS are inhibited by YopK.
(A) WT (B6) BMMs were harvested at indicated times post-infection and assayed for caspase-1 activation by western blotting for cleaved p10 caspase-1 subunit. pYopK – YopK expression plasmid, Vector – vector control plasmid. (B) WT or (C) Asc-/- BMMs were infected with wild-type (WT), ΔJ – yopJ mutant, or ΔJK – yopJK mutant Y. pseudotuberculosis and assayed for caspase-1 activation by western blotting (D) Percent cytotoxicity in WT and isogenic Nlrp3-/- macrophages infected with indicated bacterial strains was assayed 4 hours post-infection (E) WT BMM lysates were harvested at indicated minutes post-infection and assayed for caspase-1 activation following infection with either WT or T3SS strains or S. typhimurium (Stm), or treated with LPS+ATP in the presence or absence of 100 mM KCl, as indicated. Data are representative of two to three independent experiments.
YopK prevents NLRP3-dependent processing and release of IL-1β
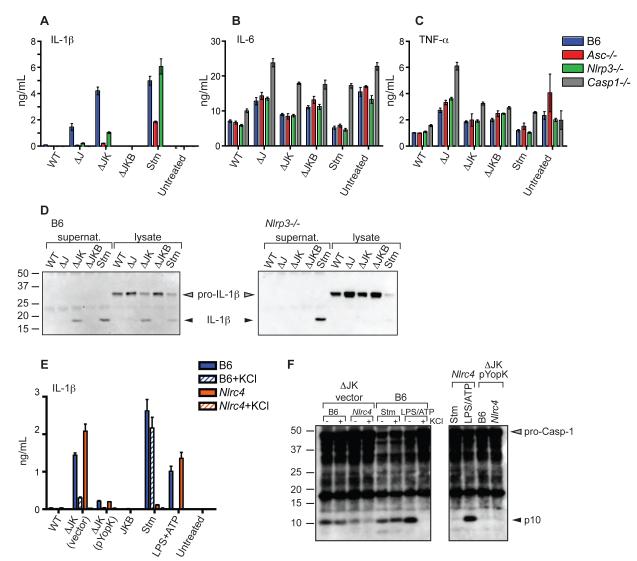
We next examined whether YopK-mediated interference with inflammasome activation in response to the T3SS would also prevent IL-1β secretion Synthesis of pro-IL-1β requires pro-inflammatory stimuli such as LPS or TNF-α (Mariathasan and Monack, 2007). In the absence of LPS pretreatment, we did not observe robust levels of IL-1β release from cells infected with any of the bacterial strains (data not shown). This was most likely due to inhibition of NF-κB and MAPK signaling by WT bacteria and the rapid caspase-1-dependent cell death induced by T3SS bacteria. However, LPS pretreatment of macrophages allowed a robust IL-1β secretion in response to both the T3SS strain and the ΔJK strain, which required YopB-dependent pore formation and was substantially reduced in cells infected with T3SS pYopK or ΔJ strains (Figure 4A and Figure S3A). As expected, IL-6 and TNF-α secretion was independent of both the T3SS and YopK (Figures 4B and 4C). Both NLRP3- and ASC-deficient macrophages secreted substantially lower amounts of IL-1β, in accordance with their reduced levels of active caspase-1 (Figure 4A). As expected, secretion of IL-18 by Yersinia-infected cells was similarly to that of IL-1β (Figure S3B). Furthermore, mature IL-1β was detected in macrophage supernatants from WT macrophages infected with the ΔJK strain but not the ΔJ or ΔJKB strain, which lacks the translocon component YopB (Figure 4D, left panel). In contrast, only S. typhimurium and not the Yersinia T3SS induced processing and secretion of IL-1β in NLRP3-deficient macrophages, despite the presence of substantial amounts of pro-IL-1β protein in these cells (Figure 4D, right panel). Collectively, these data demonstrate that the NLRP3 inflammasome plays an important role in processing and secretion of IL-1β in response to the Yersinia T3SS.
Figure 4. Processing and secretion of IL-1β in response to the Yersinia T3SS is inhibited by YopK and requires NLRP3 and ASC.
(A, B, and C) BMMs were treated with LPS for 3 hours prior to infection, and supernatants harvested 2 hours post-infection and analyzed by ELISA for presence of (A) IL-1β (B) IL-6 or (C) TNF-α (D) WT or Nlrp3-/- BMMs were infected with indicated bacterial strains and supernatants and whole-cell lysates were immunoprecipitated with anti-IL-1β antibodies followed by western blotting for IL-1β (E) WT or Nlrc4-/- BMMs were treated with 100 mM KCl as indicated 15 minutes prior to infection and supernatants analyzed for IL-1β (F) BMMs were treated with 100 mM KCl prior to infection and cell lysates analyzed by western blotting with anti-caspase-1 antibodies.
Secretion of IL-1β in response to the Yersinia T3SS also did not require NLRC4, and was unaltered by the absence of fliC, consistent with the observation that NLRC4 and flagellin were not required for caspase-1 activation in Yersinia-infected cells (Figure S3C). However, in cells treated with extracellular KCl to inhibit the NLRP3 inflammasome, WT BMMs infected with the ΔJK strain still secreted some IL-1β, whereas IL-1β was undetectable in supernatants of NLRC4-deficient cells or cells treated with LPS+ATP (Figure 4E). This suggested that NLRC4 might play a role in inflammasome activation and IL-1β release during Yersinia infection. Consistently, infection of cells with ΔJK bacteria indicated that while robust caspase-1 activation was observed in WT BMMs treated with KCl or untreated NLRC4-deficient cells, active caspase-1 was nearly absent in NLRC4-deficient cells treated with KCl (Figure 4F). Together these data suggest that NLRP3 and NLRC4 inflammasomes play independent or additive roles in caspase-1 activation in response to the Yersinia T3SS. Interestingly, despite the presence of detectable caspase-1 activation in macrophages infected with WT Yersinia, no active caspase-1 or secreted IL-1β was detected in WT Yersinia infected macrophages that were pretreated with LPS (Figure 4A and S4).
YopK interacts with the T3SS translocon
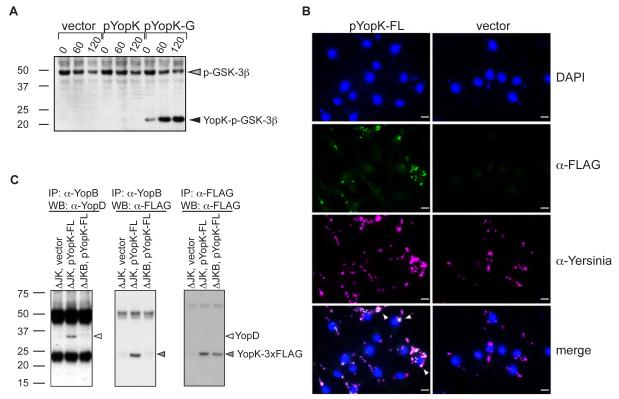
YopK could be translocated into the cytoplasm of infected cells and interfere directly with inflammasome activation, or could modify the structure of the T3SS or translocon itself, thereby preventing host detection of the T3SS. Co-infection of cells with T3SS and T3SS pYopK bacteria did not prevent caspase-1 activation, suggesting that YopK does not prevent caspase-1 activation by another T3SS in trans (Figure S5A). YopK is reported to be both translocated as well as tightly associated with the bacteria during infection (Garcia et al., 2006; Holmstrom et al., 1997). We therefore generated reporter constructs to assess the translocation and intracellular location of YopK. A YopK-GSK-3β fusion that is phosphorylated only upon delivery of the tagged protein into infected cells demonstrated that YopK was indeed translocated into the cytosol of infected cells by the T3SS (Figure 5A and Figure S5B). However, fluorescence microscopy analysis of cells infected with bacteria expressing YopK-FLAG showed that YopK was not detected within the cytoplasm of the host cell (Figure 5B). Neither of these C-terminal tags affected YopK function in blocking caspase-1 activation (Figure S5C and S5D). Together these data support a model in which YopK is translocated through the type III secretion system and interacts with the intracellular portion of the translocon to either modulate or block subsequent translocation events or to modify the structure of the translocon itself so as to prevent its detection by the immune system. Consistent with YopK being associated with the translocon, both YopD and YopK co-immunoprecipitated with YopB (Figure 5C). This immunoprecipitation was specific, as neither YopD nor YopK were immunoprecipitated in the absence of YopB. The inability to immunoprecipitate YopK in the absence of YopB was not due to lack of YopK expression, as equal levels of YopK were observed in both strains (Figure 5C, right panel). Together these data formally demonstrate that YopK associates with the T3SS translocon.
Figure 5. YopK is translocated into cells and physically interacts with T3SS translocon.
(A) BMMs were infected with T3SS expressing either empty vector control (vector), YopK expression plasmid (pYopK), or a GSK-3β tagged YopK expression vector (pYopK-G). Whole cell lysates were harvested and blotted for phospho-GSK-3β to determine the extent of translocation. (B) T3SS containing either pYopK-3XFLAG or vector control were used to infect BMMs on glass cover slides and stained with DAPI (top row), anti-FLAG (2nd row), or anti-total Yersinia (3rd row) antibodies. Bottom 2 panels are merged images. Arrows indicate colozalization of FLAG and Yersinia staining. Bar = 10 μM. (C) HeLa cells were infected with bacterial ΔyopJK bacteria containing vector control or YopK-3XFLAG plasmid, or infected with ΔyopJKB bacteria containing YopK-3XFLAG plasmid as additional control. Cell lysates were prepared, immunoprecipitated with anti-YopB antibodies (left and middle panels) or anti-FLAG M2 antibodies (right panel). Immunoprecipitated samples were run on SDS-PAGE and probed with antibodies against YopD (left panel) or YopK-FLAG (middle and right panels). Data are representative of two to three independent experiments.
Inflammasome-mediated responses to the Yersinia T3SS promote bacterial clearance
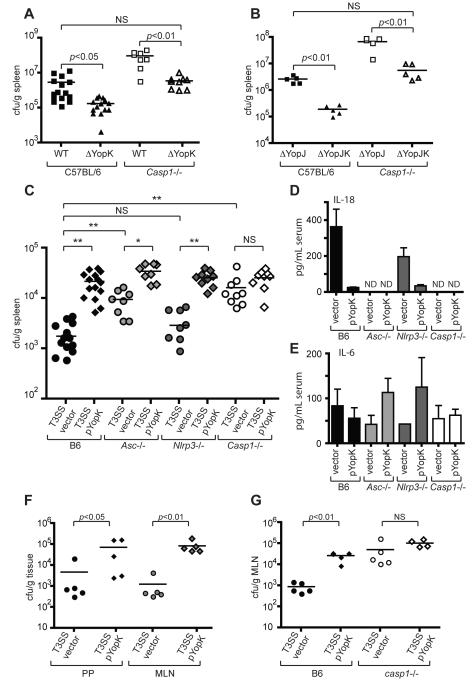
YopK-deficient Yersinia are attenuated following both oral and intraperitoneal infection of mice (Holmstrom et al., 1995). We therefore investigated whether prevention of inflammasome activation by YopK might play a role in Yersinia virulence in vivo. Consistent with previous reports, mice infected intraperitoneally with YopK-deficient bacteria had reduced bacterial loads in the spleen compared to mice infected with bacteria expressing YopK (Figure 6A and 6B). Caspase-1-deficient mice had substantially higher levels of YopK-deficient bacteria, suggesting that YopK interference with caspase-1 activation might contribute to bacterial survival in host tissues. However, WT and YopJ-deficient bacteria also showed higher bacterial loads in Casp1-/- mice than isogenic yopK mutants, suggesting that in the context of other virulence factors YopK plays further roles in virulence in addition to preventing caspase-1 activation. Importantly, the increased bacterial load of yopK mutant bacteria observed in the Casp1-/- mice was not the result of a generalized defect in anti-bacterial responses, as WT and Casp1-/- mice had equivalent levels of phoP mutant Yersinia in the spleen at four days post-infection (Figure S6A). We therefore investigated how inflammasome-mediated recognition of the T3SS itself might contribute to bacterial clearance. WT mice infected with a T3SS vector control strain had markedly lower levels of bacteria in the spleen 4 days post-infection than the T3SS pYopK strain (Figure 6C). This was not due to a difference in initial colonization, as equivalent levels of bacteria were found in the spleens of infected mice 24 hours post-infection (Figure S6B). The T3SS strain replicated to significantly higher levels in mice lacking ASC, although the bacterial loads of the T3SS vector strain in Asc-/- mice were still lower than those of T3SS pYopK. However, in Casp1-/- mice T3SS and T3SS pYopK infections resulted in equivalent bacterial loads, which were similar to those observed for the T3SS pYopK strain in WT mice (Figure 6C). These data demonstrate that caspase-1-dependent responses against the Yersinia T3SS are critical for bacterial clearance, and preventing this response enhances bacterial survival within infected tissues. The data further suggest that ASC plays an important role in the response against the T3SS strain, but that caspase-1-dependent, ASC-independent responses also contribute to bacterial clearance. Consistent with increased inflammasome activation, mice infected with the T3SS strain had substantially higher levels of serum IL-18 than mice infected with T3SS pYopK, which was ASC- and caspase-1 dependent (Figure 6D). This contrasted with serum IL-6, which was independent of ASC and caspase-1 (Figure 6E). Interestingly, although NLRP3 was important for IL-1β and IL-18 secretion by BMMs in vitro, NLRP3 was not essential for bacterial clearance, as the bacterial load of the T3SS strain in Nlrp3-/- mice was indistinguishable from that of WT mice (Figure 6A). These data suggest that although NLRP3 is responsible for the majority of caspase-1 activation in cultured macrophages, additional inflammasomes, potentially the NLRC4 inflammasome, contribute to the response against the T3SS in vivo. Levels of T3SS pYopK bacteria were also significantly higher than the T3SS vector strain in Peyer’s patches and mesenteric lymph nodes following oral infection, and this difference was also absent in caspase-1-deficient mice (Figures 6F and 6G). Together, these data demonstrate that caspase-1 plays an important role in the recognition and clearance of bacteria expressing the T3SS in vivo, and show that interference with inflammasome recognition of the T3SS by bacterial pathogens promotes bacterial survival.
Figure 6. YopK inhibits caspase-1 dependent bacterial clearance in vivo.
(A) WT and Casp1-/- mice were infected intraperitoneally with WT or yopK mutant bacteria, or (B) yopJ or yopJK mutant bacteria, and spleen homogenates were plated 4 days post-infection to determine bacterial CFU per gram of tissue. (C) WT, Asc-/-, Nrlp3-/-, and Casp1-/- mice were infected as in (A) with either T3SS vector control or T3SS pYopK bacteria, and bacterial CFU per gram of tissue were determined. (D) Sera from mice in (C) were harvested and analyzed by ELISA for levels of circulating IL-18 or (E) IL-6. (F) WT mice were infected orally with indicated bacterial strains, and CFU per gram of Peyer’s patches and mesenteric lymph nodes was determined. (G) WT or Casp1-/- mice were infected as in (F) and bacterial load in mesenteric lymph nodes was determined. Statistical significance was analyzed by Student’s two-tailed t-test. * p<0.0002, ** p<0.0001. Data represent 2 to 3 independent pooled experiments (A, B, C) or at least three independent experiments for C57BL/6 mice and two independent experiments for knockout mice (F, G).
Discussion
A key feature of mammalian bacterial pathogens is the expression of pore-forming toxins or virulence-associated secretion systems, which deliver virulence factors that modulate or disrupt host cell physiology. An important aspect of the host response to these secretion systems involves assembly of inflammasomes, multiprotein complexes necessary for activation of caspase-1, secretion of caspase-1-dependent proteins, and triggering of a caspase-1-dependent cell death termed pyroptosis. Here we demonstrate that the bacterial pathogen Yersinia utilizes the virulence protein YopK to promote bacterial survival in vivo by interfering with detection of the Yersinia type III secretion system (T3SS) by the inflammasome. We further find that YopK functions not by interacting with host proteins, but by interacting with the T3SS itself, thereby preventing recognition of the T3SS by the host cell.
The presence of a T3SS appears to be preferentially associated with pathogenesis among bacteria that colonize mammals, although in plants and invertebrates, T3SS are associated with both pathogenic and mutualistic interactions (Coombes, 2009). Although Toll-like Receptors (TLRs) that detect bacterial structures such as LPS and lipoteichoic acid are essential for host defense against bacterial infection, they also play an important role in the homeostasis and development of mucosal tissues (Rakoff-Nahoum et al., 2004). It is therefore possible that additional signaling pathways respond to specific features of pathogens. As the T3SS is often required for pathogenesis, its detection by the host might serve to induce pathogen-specific responses. Recent work has highlighted the existence of specific gene transcription programs induced by bacterial type III and type IV secretion systems (Auerbuch et al., 2009; Shin et al., 2008). However, host mechanisms to detect and eliminate pathogens impose a selection pressure on pathogens to evade immune detection. Our data provide evidence for a mechanism utilized by bacteria to prevent detection of the T3SS by host immune cells and thereby avoid activation of inflammasome-dependent immune responses.
Several bacterial pathogens are reported to interfere with inflammasome activation: The T3SS of Pseudomonas aeruginosa activates the NLRC4 inflammasome, and this activation is blocked by the secreted bacterial phospholipase, ExoU (Sutterwala et al., 2007); M. tuberculosis also activates caspase-1 through mechanisms involving both NLRC4 and NLRP3, and this activation is inhibited by the zinc metalloprotease, Zmp1 (Master et al., 2008). The trigger(s) of inflammasome induction by M. tuberculosis and the mechanism of its inhibition by Zmp1 are not defined. ExoU blocks inflammasome activation in a manner that is dependent on its enzymatic activity but triggers a caspase-1-independent cell death. The T3SS of Pseudomonas and Yersinia share a similar architecture (Cornelis, 2006). However, while inhibition of inflammasome activation by ExoU induces a caspase-1-independent cell death, YopK prevents T3SS-induced caspase-1 activation as well as cell death. Thus, the interaction of the Yersinia T3SS and YopK with the host cell is distinct from both of these mechanisms in that YopK functions to prevent cellular detection of T3SS activity, thereby preventing the host from recognizing the presence of Yersinia as a pathogen. This type of strategy appears analogous to modification of LPS or flagellin to avoid recognition by TLR4 and TLR5, respectively. Indeed, Yersinia LPS is modified during in vivo infection such that it is a poor agonist of TLR4, and artificially enhancing its stimulatory capacity results in TLR4-dependent attenuation of bacterial replication (Montminy et al., 2006). YopK represents a conceptually similar but mechanistically distinct strategy that also enables Yersinia to avoid triggering inflammasome activation.
YopK is unique among bacterial virulence factors in that it has no primary sequence homology to any protein outside of the pathogenic Yersinia species, and once translocated into the host cell, physically associates with the intracellular side of the T3SS translocon. YopK has been implicated in bacterial virulence (Holmstrom et al., 1995), but its role during infection remained undefined. We show that YopK prevents recognition of the Yersinia T3SS by the innate immune system. This represents a novel strategy for inflammasome inhibition, and suggests the existence of a class of bacterial proteins that function to mask the presence of secretion systems from inflammasome machinery in order to promote colonization of host tissues. YopK was previously reported to modulate the size of the Yersinia T3SS pore (Holmstrom et al., 1997), raising the possibility that sensing of the T3SS by the host cell depends on signaling events induced in response to pore formation by the T3SS. Alternatively, YopK could act as a gatekeeper to prevent the unintended translocation of small molecules or peptides whose inappropriate translocation might be a signal for inflammasome activation. The Yersinia inner rod protein YscI could be translocated into the cytosol in the absence of YopK, analogous to that of Salmonella PrgJ (Miao et al., 2010), potentially accounting for the role of NLRC4 in the response to the Yersinia T3SS.
Although no homologues of YopK are currently known, functionally analogous proteins may exist in other pathogens. Flagellin-deficient Salmonella have reduced capacity to induce inflammasome activation, suggesting that Salmonella may also encode a virulence factor that can block T3SS detection (Franchi et al., 2006; Miao et al., 2006). Furthermore, while the T3SS is a feature of mammalian bacterial pathogens, these systems serve as colonization factors in mutualistic interactions between bacterial endosymbionts and plant or insect hosts (Coombes, 2009). Some components of endogenous mammalian microflora may similarly utilize T3SS, necessitating a mechanism analogous to the one illustrated by YopK in Yersinia in order to prevent inappropriately triggering a host immune response.
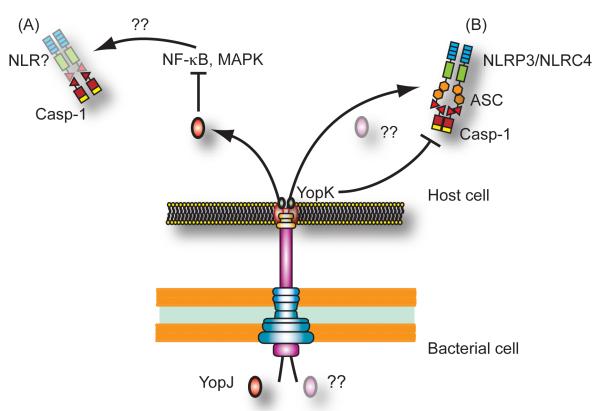
Detection of the Yersinia T3SS triggers ASC-dependent caspase-1 activation primarily involving the NLRP3 inflammasome, with a contribution of NLRC4 to the response as well (Figure 7). This could potentially account for the ASC-independent but caspase-1-dependent cell death triggered by the Yersinia T3SS. Our observations that ASC-deficient mice had lower levels of T3SS bacteria than caspase-1-deficient mice also suggests that NLRC4 may play a greater role in vivo than in vitro. Both NLRC4 and NLRP3 inflammasomes are triggered by Listeria infection, in response to flagellin and listeriolysin O (Warren et al., 2008). ASC-dependent (but NLRP3-independent) and NLRC4-dependent inflammasomes contribute separately to caspase-1 activation in Legionella-infected cells (Case et al., 2009). This suggests that full induction of caspase-1-dependent responses during bacterial infection involves synergy between different inflammasomes responding to the presence of multiple stimuli. Further studies are necessary to determine the precise nature of these stimuli, as well as how activation of the different inflammasomes is coordinated during infection.
Figure 7. Yersinia infection triggers two distinct pathways of caspase-1 activation.
(A) Inhibition of NF-κB and MAPK signaling by Y. pseudotuberculosis YopJ (red oval) activates caspase-1 independently of ASC, NLRP3 or NLRC4. (B) In contrast, the Yersinia T3SS triggers an ASC-dependent pathway of caspase-1 activation involving both NLRP3 and NLRC4. This pathway may be triggered by T3SS-induced pore formation, or by translocation of an unknown protein or small molecule (pink oval) into the cell. YopK (green ovals) interacts with the T3SS translocon and prevents inflammasome activation in response to detection of the T3SS by the host cell.
Our data demonstrate that YopK prevents ASC-dependent inflammasome activation in response to the T3SS, and that WT bacteria induce a distinct ASC-independent pathway of caspase-1 activation that depends on the activity of YopJ (Figure 7). How does preventing inflammasome activation in response to the T3SS by YopK function in the context of wild-type bacteria? Previous studies indicate that while YopJ plays an important role in bacterial colonization following the intestinal stage of infection by Y. psuedotuberculosis (Monack et al., 1998), YopJ activity may be downregulated during the systemic phase of the infection. YopJ is dispensable for Yersinia virulence when the intestinal barrier is bypassed by either subcutaneous or intraperitoneal infection (Auerbuch and Isberg, 2007; Lemaitre et al., 2006). Expression of more cytotoxic isoforms of YopJ causes attenuation of both Y. pestis and Y. pseudotuberculosis in vivo, indicating that YopJ must be tightly regulated (Brodsky and Medzhitov, 2008; Zauberman et al., 2009). Finally, CD8+ T cell-mediated killing of Yersinia-infected cells results in uptake of both the apoptotic cells and the attached extracellular Yersinia by uninfected macrophages, which plays an important role in host resistance to infection (Bergman et al., 2009). That cell-extrinsic killing is necessary for apoptosis of Yersinia-infected cells in vivo suggests that YopJ may not be expressed or translocated during infection of systemic sites, pointing to a situation in which YopK would play an important role in preventing detection of the T3SS. Indeed, YopK-deficient bacteria are attenuated relative to either isogenic WT or YopJ-deficient strains, and this virulence defect is ameliorated in the absence of caspase-1. The data further demonstrate a critical role for caspase-1 in resistance to both WT and YopJ-deficient Yersinia, and also suggest that in the context of other virulence factors YopK plays another role in virulence in addition to preventing inflammasome activation. Collectively these studies suggest that different sets of virulence factors function during different stages of infection. Thus, similar to more complex multicellular parasites, bacterial pathogens may employ unique pathogenicity programs at different stages of infection. Elucidating these pathogenicity programs during infection will provide important insight into the roles of different virulence factors within distinct host environments.
Experimental Procedures
Cell culture and infection conditions
Mice were housed in accordance with NIH and YARC approved guidelines and all studies involving mice were performed in accordance with approved Yale University IACUC protocols and procedures. Knockout mice used in these studies have been previously described (Sutterwala et al., 2007; Sutterwala et al., 2006). Bone marrow cells were grown at 37 °C in a humidified incubator in DMEM containing HEPES, 10% FCS and 30% L929 supernatant for 7-8 days. Differentiated bone marrow-derived macrophages (BMMs) were replated into 12- or 24-well dishes 16-20 hours prior to infection. Bacterial strains are described in Supplemental Table 1. Yersinia were grown overnight with aeration in 2×YT broth at 26 °C. The bacteria were diluted into fresh 2×YT containing 20 mM sodium oxalate and 20 mM MgCl2. Bacteria were grown with aeration for 1 hour at 26 °C followed by 2 hours at 37 °C. Salmonella were grown overnight in LB medium at 37 °C with aeration, diluted into fresh LB containing 300 mM NaCl and grown standing at 37 °C for 3 hours. Bacteria were washed 3 times with pre-warmed DMEM, added to the cells at an MOI of 20:1 and spun onto the cells at 1000 rpm for 5 minutes. Cells were incubated at 37 °C for 1 hour post-infection followed by addition of 100 μg/mL Gentamicin.
Mouse infections
8-10 week old age and sex-matched mice were infected intraperitoneally with 5×104 to 1×105 or orally with 5×108 bacteria. Mice were sacrificed and the tissues and sera harvested at indicated times post-infection. Bacterial load was determined by plating dilutions of tissue homogenates and serum cytokines were measured by sandwich ELISA as described above. Serum IL-18 was detected with anti-IL-18 capture and detection antibodies (MBL International).
Western blotting and antibodies
Cells were lysed in 20 mM HEPES, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM EDTA. Lysates were mixed with protein loading buffer, boiled, centrifuged, and 20% of the total cell lysate was loaded onto 4-12% NuPAGE gels (Invitrogen). Proteins were transferred to PVDF membrane (Millipore) and blotted with rabbit anti-mouse caspase-1 antibody (sc-514, Santa Cruz Biotechnology). Secondary antibodies were goat anti-rabbit or anti-mouse HRP (Jackson Immunoresearch). Rabbit anti-p-GSK-3β (Cell Signaling) was used to detect translocation of GSK-3β–tagged proteins. Processed IL-1β was immunoprecipitated as described (Mariathasan et al., 2004) and detected with with the 3ZD monoclonal antibody (Biological Resources Branch, National Cancer Institute).
Co-immunoprecipitation
Cells were washed 3 times with PBS 2 hrs post-infection. Cells were lysed on ice in 50 mM Tris, 150 mM NaCl, 1% Digitonin with complete protease inhibitors (Roche). Cell lysates were precleared with Protein-G agarose beads (Invitrogen), followed by immunoprecipitation with anti-YopB polyclonal antibodies (Ryndak et al., 2005) and immunoblotting for YopD using mouse anti-YopD monoclonal antibodies (Noel et al., 2009) or anti-FLAG M2 monoclonal antibodies (Sigma). Detection was performed using HRP-conjugated TrueBlot anti-mouse IgG (eBiosciences).
Cell death assays
BMMs were seeded into 96-well plates at a density of 5×104 cells per well. Prior to infection, medium was replaced with DMEM lacking phenol red. All subsequent steps were performed in this medium. Cells were infected as described above, and supernatants harvested at indicated times post-infection. Lactate dehydrogenase release was quantified using the Cytotox96 Assay Kit (Promega) according to manufacturer’s instructions.
Cytokine production
BMMs were pretreated with E. coli LPS (Sigma) for 3 hours prior to bacterial infection as described above, and supernatants were harvested 2 hours post-infection. Release of proinflammatory cytokines was measured by enzyme linked immunosorbent assay (ELISA) using capture and detection antibodies against IL-6, TNF-α, (BD Pharmingen), or IL-1β (eBioscience).
Immunofluorescence
BMMs were seeded onto glass cover slips and infected with indicated bacterial strains as described above. 1 hour post-infection cells were washed, fixed in 4% paraformaldehyde, permeabilized with 0.5% Triton X-100, and blocked with 5% BSA. Cells were stained with anti-FLAG M2 antibodies (Sigma) and anti-total Yersinia antibodies, followed by labeling with anti-mouse Alexa488 and anti-rabbit Alexa594 (Molecular Probes). After antibody labeling, cells were counterstained with DAPI and imaged using an AxioPlan 2 epifluorescence microscope (Carl Zeiss).
Statistical analysis
Plotting of data and statisitical analysis was performed using Graphpad Prism 4.0 software, and statistical significance determined by unpaired two-tailed Student’s t test.
Supplementary Material
Acknowledgements
We thank Greg Plano (University of Miami) for the mCD1 plasmid, Pam Wearsh and members of the Medzhitov lab for scientific discussion, Jelena Bezbradica, Daniel Stetson, Dominik Schenten, and Sunny Shin for critical reading, Chris Case for extra Casp1-/- mice, and Charles Annicelli, Nancy Allen and Margaret Green for technical assistance. This work was supported in part by New England RCE AI057159 (RM), R01 AI043389 (JBB), Northeast Biodefense Center U54-AI057158-Lipkin (JBB) and F32 AI065081 (IB). RM is an investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Auerbuch V, Golenbock DT, Isberg RR. Innate immune recognition of Yersinia pseudotuberculosis type III secretion. PLoS Pathog. 2009;5:e1000686. doi: 10.1371/journal.ppat.1000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbuch V, Isberg RR. Growth of Yersinia pseudotuberculosis in mice occurs independently of Toll-like receptor 2 expression and induction of interleukin-10. Infect Immun. 2007;75:3561–3570. doi: 10.1128/IAI.01497-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra SS, Jackson MW, Ross JA, Plano GV. Calcium-regulated type III secretion of Yop proteins by an Escherichia coli hha mutant carrying a Yersinia pestis pCD1 virulence plasmid. Infect Immun. 2006;74:1381–1386. doi: 10.1128/IAI.74.2.1381-1386.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman MA, Loomis WP, Mecsas J, Starnbach MN, Isberg RR. CD8(+) T cells restrict Yersinia pseudotuberculosis infection: bypass of anti-phagocytosis by targeting antigen-presenting cells. PLoS Pathog. 2009;5:e1000573. doi: 10.1371/journal.ppat.1000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsbaken T, Cookson BT. Macrophage activation redirects Yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog. 2007;3:e161. doi: 10.1371/journal.ppat.0030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky IE, Medzhitov R. Reduced secretion of YopJ by Yersinia limits in vivo cell death but enhances bacterial virulence. PLoS Pathog. 2008;4:e1000067. doi: 10.1371/journal.ppat.1000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky IE, Medzhitov R. Targeting of immune signalling networks by bacterial pathogens. Nat Cell Biol. 2009;11:521–526. doi: 10.1038/ncb0509-521. [DOI] [PubMed] [Google Scholar]
- Case CL, Shin S, Roy CR. Asc and Ipaf Inflammasomes direct distinct pathways for caspase-1 activation in response to Legionella pneumophila. Infect Immun. 2009;77:1981–1991. doi: 10.1128/IAI.01382-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA, Sutterwala FS. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci U S A. 2008;105:9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Shaw MH, Kim YG, Nunez G. Nod-like Receptors: Role in Innate Immunity and Inflammatory Disease. Annu Rev Pathol. 2008;4:365–398. doi: 10.1146/annurev.pathol.4.110807.092239. [DOI] [PubMed] [Google Scholar]
- Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- Coombes BK. Type III secretion systems in symbiotic adaptation of pathogenic and non-pathogenic bacteria. Trends Microbiol. 2009;17:89–94. doi: 10.1016/j.tim.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Cornelis GR. The type III secretion injectisome. Nat Rev Microbiol. 2006;4:811–825. doi: 10.1038/nrmicro1526. [DOI] [PubMed] [Google Scholar]
- Cornelis GR, Wolf-Watz H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol. 1997;23:861–867. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- Denecker G, Declercq W, Geuijen CA, Boland A, Benabdillah R, van Gurp M, Sory MP, Vandenabeele P, Cornelis GR. Yersinia enterocolitica YopP-induced apoptosis of macrophages involves the apoptotic signaling cascade upstream of bid. J Biol Chem. 2001;276:19706–19714. doi: 10.1074/jbc.M101573200. [DOI] [PubMed] [Google Scholar]
- Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in Salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- Galan JE, Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 2006;444:567–573. doi: 10.1038/nature05272. [DOI] [PubMed] [Google Scholar]
- Garcia JT, Ferracci F, Jackson MW, Joseph SS, Pattis I, Plano LR, Fischer W, Plano GV. Measurement of effector protein injection by type III and type IV secretion systems by using a 13-residue phosphorylatable glycogen synthase kinase tag. Infect Immun. 2006;74:5645–5657. doi: 10.1128/IAI.00690-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell. 2006;126:1135–1145. doi: 10.1016/j.cell.2006.07.033. [DOI] [PubMed] [Google Scholar]
- Holmstrom A, Petterson J, Rosqvist R, Hakansson S, Tafazoli F, Fallman M, Magnusson KE, Wolf-Watz H, Forsberg A. YopK of Yersinia pseudotuberculosis controls translocation of Yop effectors across the eukaryotic cell membrane. Mol Microbiol. 1997;24:73–91. doi: 10.1046/j.1365-2958.1997.3211681.x. [DOI] [PubMed] [Google Scholar]
- Holmstrom A, Rosqvist R, Wolf-Watz H, Forsberg A. Virulence plasmid-encoded YopK is essential for Yersinia pseudotuberculosis to cause systemic infection in mice. Infect Immun. 1995;63:2269–2276. doi: 10.1128/iai.63.6.2269-2276.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA, Jr., Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- Lemaitre N, Sebbane F, Long D, Hinnebusch BJ. Yersinia pestis YopJ suppresses tumor necrosis factor alpha induction and contributes to apoptosis of immune cells in the lymph node but is not required for virulence in a rat model of bubonic plague. Infect Immun. 2006;74:5126–5131. doi: 10.1128/IAI.00219-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilo S, Zheng Y, Bliska JB. Caspase-1 activation in macrophages infected with Yersinia pestis KIM requires the type III secretion system effector YopJ. Infect Immun. 2008;76:3911–3923. doi: 10.1128/IAI.01695-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- Master SS, Rampini SK, Davis AS, Keller C, Ehlers S, Springer B, Timmins GS, Sander P, Deretic V. Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe. 2008;3:224–232. doi: 10.1016/j.chom.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, Warren SE, Leaf IA, Aderem A. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci U S A. 2010;107:3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnich SA, Rohde HN. A rationale for repression and/or loss of motility by pathogenic Yersinia in the mammalian host. Adv Exp Med Biol. 2007;603:298–310. doi: 10.1007/978-0-387-72124-8_27. [DOI] [PubMed] [Google Scholar]
- Molofsky AB, Byrne BG, Whitfield NN, Madigan CA, Fuse ET, Tateda K, Swanson MS. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J Exp Med. 2006;203:1093–1104. doi: 10.1084/jem.20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monack DM, Mecsas J, Bouley D, Falkow S. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J Exp Med. 1998;188:2127–2137. doi: 10.1084/jem.188.11.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy SW, Khan N, McGrath S, Walkowicz MJ, Sharp F, Conlon JE, Fukase K, Kusumoto S, Sweet C, Miyake K, et al. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat Immunol. 2006;7:1066–1073. doi: 10.1038/ni1386. [DOI] [PubMed] [Google Scholar]
- Noel BL, Lilo S, Capurso D, Hill J, Bliska J. Yersinia pestis can bypass protective antibodies to LcrV and activation with IFN{gamma} to survive and induce apoptosis in murine macrophages. Clin Vaccine Immunol. 2009 doi: 10.1128/CVI.00172-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2006;2:e18. doi: 10.1371/journal.ppat.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryndak MB, Chung H, London E, Bliska JB. Role of predicted transmembrane domains for type III translocation, pore formation, and signaling by the Yersinia pseudotuberculosis YopB protein. Infect Immun. 2005;73:2433–2443. doi: 10.1128/IAI.73.4.2433-2443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotte P, Denecker G, Van Den Broeke A, Vandenabeele P, Cornelis GR, Beyaert R. Targeting Rac1 by the Yersinia effector protein YopE inhibits caspase-1-mediated maturation and release of interleukin-1beta. J Biol Chem. 2004;279:25134–25142. doi: 10.1074/jbc.M401245200. [DOI] [PubMed] [Google Scholar]
- Shin H, Cornelis GR. Type III secretion translocation pores of Yersinia enterocolitica trigger maturation and release of pro-inflammatory IL-1beta. Cell Microbiol. 2007;9:2893–2902. doi: 10.1111/j.1462-5822.2007.01004.x. [DOI] [PubMed] [Google Scholar]
- Shin S, Case CL, Archer KA, Nogueira CV, Kobayashi KS, Flavell RA, Roy CR, Zamboni DS. Type IV secretion-dependent activation of host MAP kinases induces an increased proinflammatory cytokine response to Legionella pneumophila. PLoS Pathog. 2008;4:e1000220. doi: 10.1371/journal.ppat.1000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med. 2007;204:3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galan JE, Askenase PW, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Franchi L, Toma C, Ashida H, Ogawa M, Yoshikawa Y, Mimuro H, Inohara N, Sasakawa C, Nunez G. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 2007;3:e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance RE, Isberg RR, Portnoy DA. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe. 2009;6:10–21. doi: 10.1016/j.chom.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viboud GI, Bliska JB. A bacterial type III secretion system inhibits actin polymerization to prevent pore formation in host cell membranes. Embo J. 2001;20:5373–5382. doi: 10.1093/emboj/20.19.5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viboud GI, Bliska JB. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu Rev Microbiol. 2005;59:69–89. doi: 10.1146/annurev.micro.59.030804.121320. [DOI] [PubMed] [Google Scholar]
- Warren SE, Mao DP, Rodriguez AE, Miao EA, Aderem A. Multiple Nod-like receptors activate caspase 1 during Listeria monocytogenes infection. J Immunol. 2008;180:7558–7564. doi: 10.4049/jimmunol.180.11.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauberman A, Tidhar A, Levy Y, Bar-Haim E, Halperin G, Flashner Y, Cohen S, Shafferman A, Mamroud E. Yersinia pestis endowed with increased cytotoxicity is avirulent in a bubonic plague model and induces rapid protection against pneumonic plague. PLoS One. 2009;4:e5938. doi: 10.1371/journal.pone.0005938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ting AT, Marcu KB, Bliska JB. Inhibition of MAPK and NF-kappa B pathways is necessary for rapid apoptosis in macrophages infected with Yersinia. J Immunol. 2005;174:7939–7949. doi: 10.4049/jimmunol.174.12.7939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.