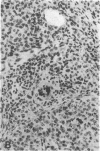
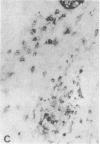
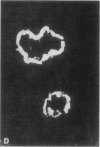
Abstract
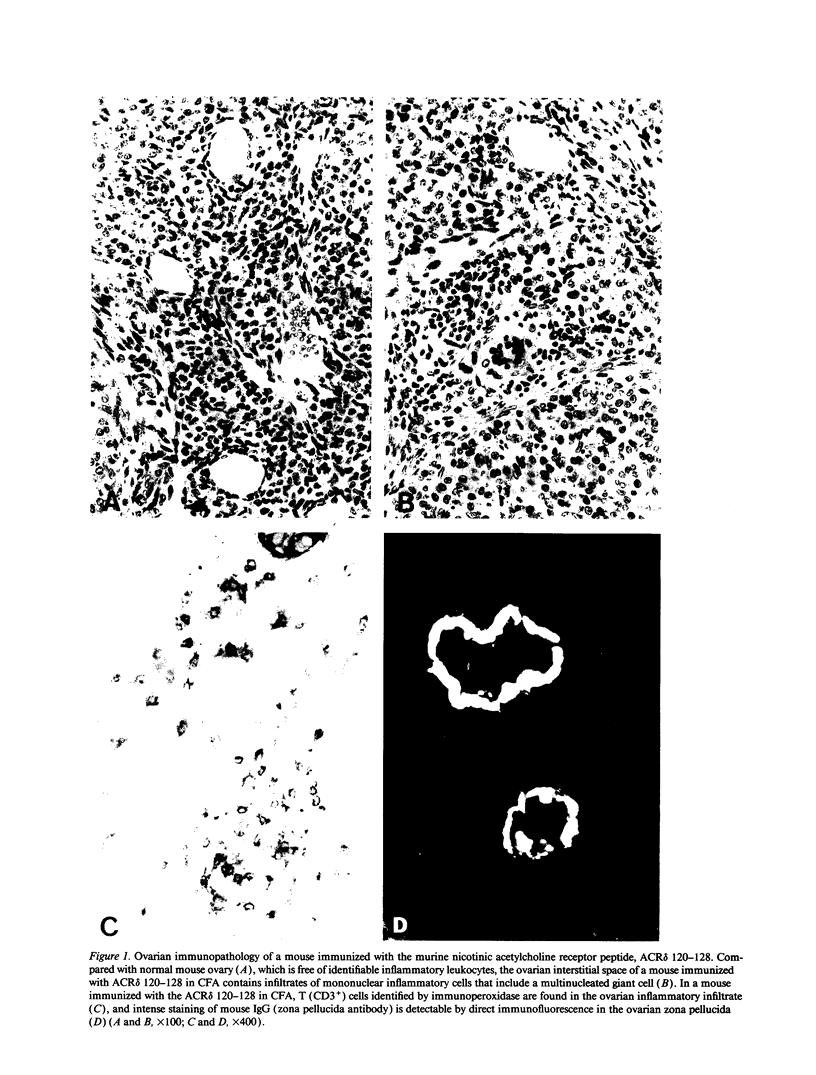
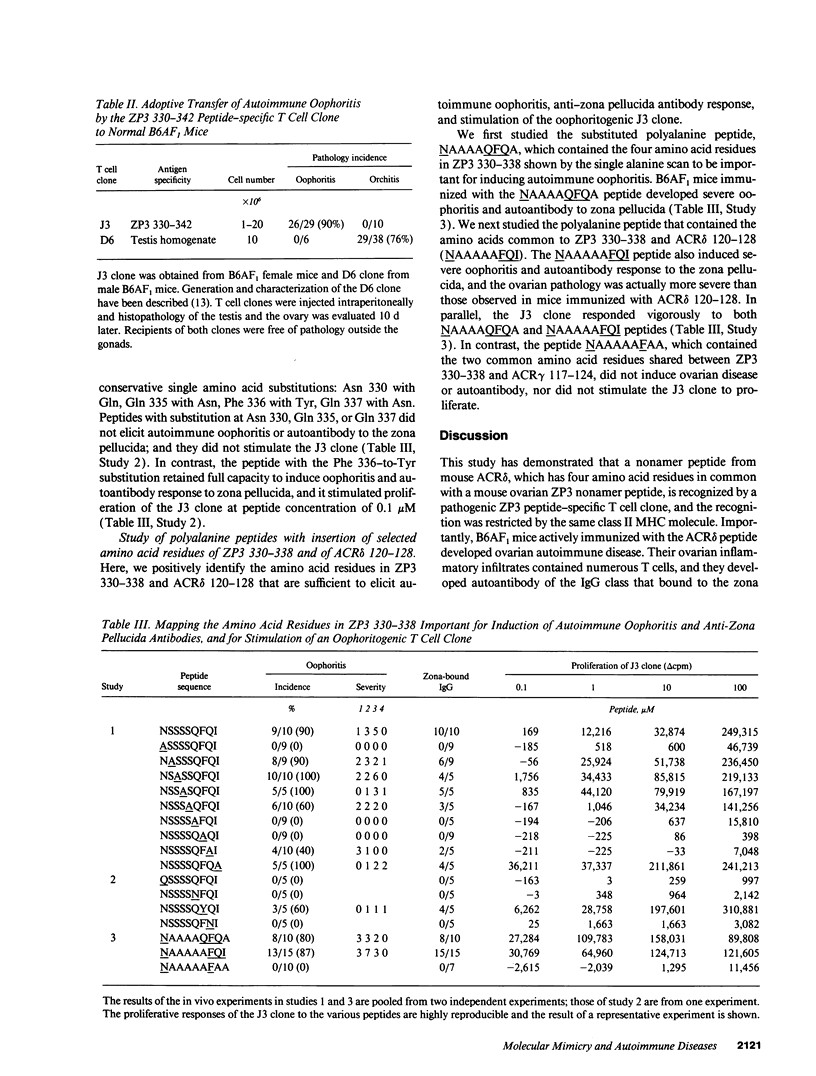
A nonamer peptide from murine nicotinic acetylcholine receptor delta chain (ACR delta), which shared four amino acid residues with a nonamer peptide of murine ovarian zona pellucida glycoprotein ZP3, induced murine autoimmune oophoritis and IgG autoantibody to the zona pellucida. Crossreaction between the ACR delta and ZP3 peptides was established by the response of a ZP3 peptide-specific, oophoritogenic T cell clone to both peptides in association with IA (alpha k beta b). By substituting the ZP3 peptides with a single alanine, four amino acids within the ZP3 peptide were found to be important for ovarian autoimmune disease, autoantibody response, and stimulation of the ZP3-specific T cell clone. Substitution with conservative amino acids of three residues also ablated activity, whereas the fourth, a phenylalanine, was replaceable by tyrosine without loss of activity. Of the four critical amino acids, three were shared between the ZP3 peptide and the ACR delta peptide. Moreover, polyalanine peptides with the four critical ZP3 amino acids or the four amino acids common to the ZP3 and ACR delta peptides induced immune response to ZP3 and elicited severe ovarian autoimmune disease. Thus, organ-specific autoimmune disease can occur through immune response against unrelated self (or foreign) peptides that share with a self-peptide sufficient common amino acid residues critical for activation of pathogenic, autoreactive T cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon R., Horwitz R. J. Synthetic peptides as vaccines. Curr Opin Immunol. 1992 Aug;4(4):449–453. doi: 10.1016/s0952-7915(06)80037-3. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. E., Dean J. Human homolog of the mouse sperm receptor. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6014–6018. doi: 10.1073/pnas.87.16.6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean J. Biology of mammalian fertilization: role of the zona pellucida. J Clin Invest. 1992 Apr;89(4):1055–1059. doi: 10.1172/JCI115684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlanger B. F., Cleveland W. L., Wassermann N. H., Ku H. H., Hill B. L., Sarangarajan R., Rajagopalan R., Cayanis E., Edelman I. S., Penn A. S. Auto-anti-idiotype: a basis for autoimmunity and a strategy for anti-receptor antibodies. Immunol Rev. 1986 Dec;94:23–37. doi: 10.1111/j.1600-065x.1986.tb01162.x. [DOI] [PubMed] [Google Scholar]
- Fairchild P. J., Wraith D. C. Peptide-MHC interaction in autoimmunity. Curr Opin Immunol. 1992 Dec;4(6):748–753. doi: 10.1016/0952-7915(92)90056-k. [DOI] [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B. Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science. 1985 Nov 29;230(4729):1043–1045. doi: 10.1126/science.2414848. [DOI] [PubMed] [Google Scholar]
- Gammon G., Sercarz E. How some T cells escape tolerance induction. Nature. 1989 Nov 9;342(6246):183–185. doi: 10.1038/342183a0. [DOI] [PubMed] [Google Scholar]
- Gautam A. M., Pearson C. I., Smilek D. E., Steinman L., McDevitt H. O. A polyalanine peptide with only five native myelin basic protein residues induces autoimmune encephalomyelitis. J Exp Med. 1992 Aug 1;176(2):605–609. doi: 10.1084/jem.176.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D. F., Henderson R. A., Shabanowitz J., Sakaguchi K., Michel H., Sevilir N., Cox A. L., Appella E., Engelhard V. H. Characterization of peptides bound to the class I MHC molecule HLA-A2.1 by mass spectrometry. Science. 1992 Mar 6;255(5049):1261–1263. doi: 10.1126/science.1546328. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. L., Reay P. A., Ehrich E. W., Davis M. M. Molecular components of T-cell recognition. Annu Rev Immunol. 1992;10:835–873. doi: 10.1146/annurev.iy.10.040192.004155. [DOI] [PubMed] [Google Scholar]
- Kohno S., Munoz J. A., Williams T. M., Teuscher C., Bernard C. C., Tung K. S. Immunopathology of murine experimental allergic orchitis. J Immunol. 1983 Jun;130(6):2675–2682. [PubMed] [Google Scholar]
- LaBarbera A. R., Miller M. M., Ober C., Rebar R. W. Autoimmune etiology in premature ovarian failure. Am J Reprod Immunol Microbiol. 1988 Mar;16(3):115–122. doi: 10.1111/j.1600-0897.1988.tb00180.x. [DOI] [PubMed] [Google Scholar]
- LaPolla R. J., Mayne K. M., Davidson N. Isolation and characterization of a cDNA clone for the complete protein coding region of the delta subunit of the mouse acetylcholine receptor. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7970–7974. doi: 10.1073/pnas.81.24.7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipham W. J., Redmond T. M., Takahashi H., Berzofsky J. A., Wiggert B., Chader G. J., Gery I. Recognition of peptides that are immunopathogenic but cryptic. Mechanisms that allow lymphocytes sensitized against cryptic peptides to initiate pathogenic autoimmune processes. J Immunol. 1991 Jun 1;146(11):3757–3762. [PubMed] [Google Scholar]
- Lorenz R. G., Tyler A. N., Allen P. M. Reconstruction of the immunogenic peptide RNase(43-56) by identification and transfer of the critical residues into an unrelated peptide backbone. J Exp Med. 1989 Jul 1;170(1):203–215. doi: 10.1084/jem.170.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar S. E., Chamow S. M., Baur A. W., Oliver C., Robey F., Dean J. Vaccination with a synthetic zona pellucida peptide produces long-term contraception in female mice. Science. 1989 Nov 17;246(4932):935–938. doi: 10.1126/science.2479101. [DOI] [PubMed] [Google Scholar]
- Ohashi P. S., Oehen S., Buerki K., Pircher H., Ohashi C. T., Odermatt B., Malissen B., Zinkernagel R. M., Hengartner H. Ablation of "tolerance" and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991 Apr 19;65(2):305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B. Molecular mimicry and autoimmune disease. Cell. 1987 Sep 11;50(6):819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Nerenberg M., Southern P., Price J., Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991 Apr 19;65(2):319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- Paliard X., West S. G., Lafferty J. A., Clements J. R., Kappler J. W., Marrack P., Kotzin B. L. Evidence for the effects of a superantigen in rheumatoid arthritis. Science. 1991 Jul 19;253(5017):325–329. doi: 10.1126/science.1857971. [DOI] [PubMed] [Google Scholar]
- Rhim S. H., Millar S. E., Robey F., Luo A. M., Lou Y. H., Yule T., Allen P., Dean J., Tung K. S. Autoimmune disease of the ovary induced by a ZP3 peptide from the mouse zona pellucida. J Clin Invest. 1992 Jan;89(1):28–35. doi: 10.1172/JCI115572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringuette M. J., Chamberlin M. E., Baur A. W., Sobieski D. A., Dean J. Molecular analysis of cDNA coding for ZP3, a sperm binding protein of the mouse zona pellucida. Dev Biol. 1988 Jun;127(2):287–295. doi: 10.1016/0012-1606(88)90315-6. [DOI] [PubMed] [Google Scholar]
- Röcken M., Urban J. F., Shevach E. M. Infection breaks T-cell tolerance. Nature. 1992 Sep 3;359(6390):79–82. doi: 10.1038/359079a0. [DOI] [PubMed] [Google Scholar]
- Singh V. K., Yamaki K., Abe T., Shinohara T. Molecular mimicry between uveitopathogenic site of retinal S-antigen and Escherichia coli protein: induction of experimental autoimmune uveitis and lymphocyte cross-reaction. Cell Immunol. 1989 Aug;122(1):262–273. doi: 10.1016/0008-8749(89)90166-4. [DOI] [PubMed] [Google Scholar]
- Smith H., Lou Y. H., Lacy P., Tung K. S. Tolerance mechanism in experimental ovarian and gastric autoimmune diseases. J Immunol. 1992 Sep 15;149(6):2212–2218. [PubMed] [Google Scholar]
- Tung K. S., Lu C. Y. Immunologic basis of reproductive failure. Monogr Pathol. 1991;(33):308–333. [PubMed] [Google Scholar]
- Van de Rijn I., Bleiweis A. S., Zabriskie J. B. Antigens in Streptococcus mutans cross reactive with human heart muscle. J Dent Res. 1976 Apr;55(Spec No):C59–C64. doi: 10.1177/002203457605500326011. [DOI] [PubMed] [Google Scholar]
- Wraith D. C., Bruun B., Fairchild P. J. Cross-reactive antigen recognition by an encephalitogenic T cell receptor. Implications for T cell biology and autoimmunity. J Immunol. 1992 Dec 1;149(11):3765–3770. [PubMed] [Google Scholar]
- Yu L., LaPolla R. J., Davidson N. Mouse muscle nicotinic acetylcholine receptor gamma subunit: cDNA sequence and gene expression. Nucleic Acids Res. 1986 Apr 25;14(8):3539–3555. doi: 10.1093/nar/14.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]