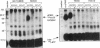Abstract
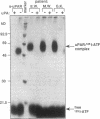
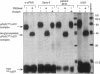
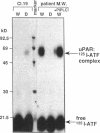
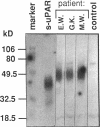
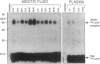
We have identified a soluble form of the human urokinase plasminogen activator (uPA) receptor (uPAR) in the ascitic fluids from patients with ovarian cancer. After purification of uPAR from the ascitic fluids by ligand-affinity chromatography (pro-uPA Sepharose), the uPAR was initially identified by cross-linking to a radiolabeled amino-terminal fragment of human uPA. The uPAR purified from the ascitic fluid has no bound ligand (uPA), as similar amounts can be purified by ligand-affinity chromatography as by immuno-affinity chromatography. uPAR from ascitic fluids partitions in the water phase after a temperature-dependent phase separation of a detergent extract. It therefore lacks at least the lipid moiety of the glycophospholipid anchor present in cellular-bound uPARs. It is highly glycosylated and the deglycosylated form has the same electrophoretic mobility as previously characterized cellular uPAR from other sources. The immunoreactivity of the purified uPAR from the ascitic fluid is indistinguishable from that of characterized uPAR, demonstrated by Western blotting with three different anti-uPAR monoclonal antibodies. The uPAR was found in 11 of 11 ascitic fluids from patients with ovarian cancer and in elevated amounts in the plasma from 2 of 3 patients. The concentration of soluble uPAR in the ascitic fluid was estimated to range between 1 and 10 ng/ml. Human soluble uPAR, derived from the tumor cells, was also found in the ascitic fluid and serum from nude mice xenografted intraperitoneally with three different human ovarian carcinomas.
Full text
PDF

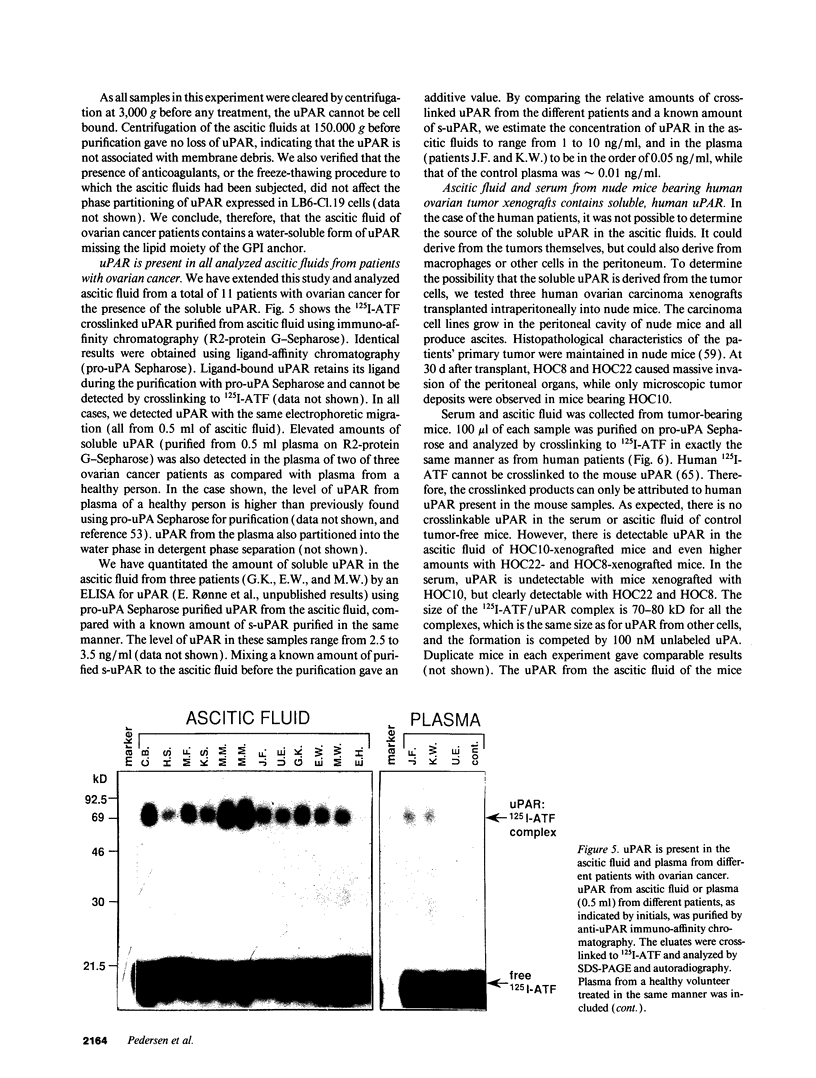
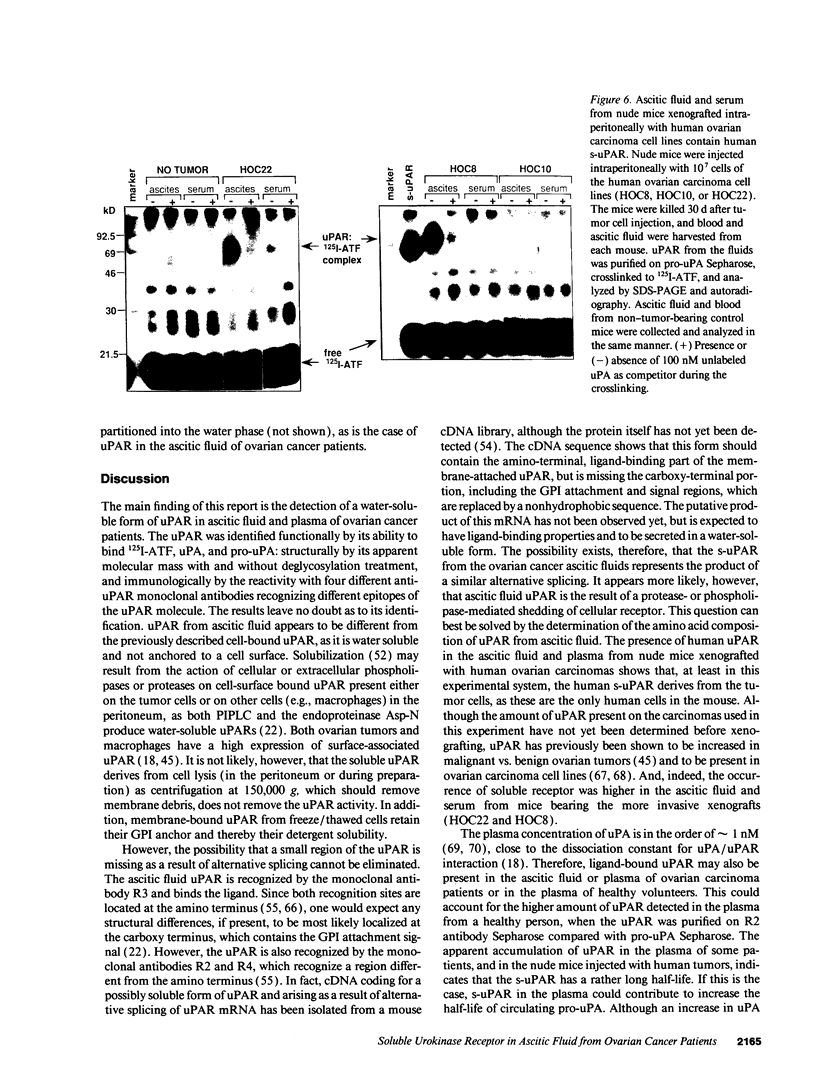


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod J. H., Reich R., Miskin R. Expression of human recombinant plasminogen activators enhances invasion and experimental metastasis of H-ras-transformed NIH 3T3 cells. Mol Cell Biol. 1989 May;9(5):2133–2141. doi: 10.1128/mcb.9.5.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbach W. R., Horner D. L., Logan J. S. The growth hormone-binding protein in rat serum is an alternatively spliced form of the rat growth hormone receptor. Genes Dev. 1989 Aug;3(8):1199–1205. doi: 10.1101/gad.3.8.1199. [DOI] [PubMed] [Google Scholar]
- Behrendt N., Ploug M., Patthy L., Houen G., Blasi F., Danø K. The ligand-binding domain of the cell surface receptor for urokinase-type plasminogen activator. J Biol Chem. 1991 Apr 25;266(12):7842–7847. [PubMed] [Google Scholar]
- Behrendt N., Rønne E., Ploug M., Petri T., Løber D., Nielsen L. S., Schleuning W. D., Blasi F., Appella E., Danø K. The human receptor for urokinase plasminogen activator. NH2-terminal amino acid sequence and glycosylation variants. J Biol Chem. 1990 Apr 15;265(11):6453–6460. [PubMed] [Google Scholar]
- Boyd D. Examination of the effects of epidermal growth factor on the production of urokinase and the expression of the plasminogen activator receptor in a human colon cancer cell line. Cancer Res. 1989 May 1;49(9):2427–2432. [PubMed] [Google Scholar]
- Cajot J. F., Bamat J., Bergonzelli G. E., Kruithof E. K., Medcalf R. L., Testuz J., Sordat B. Plasminogen-activator inhibitor type 1 is a potent natural inhibitor of extracellular matrix degradation by fibrosarcoma and colon carcinoma cells. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6939–6943. doi: 10.1073/pnas.87.18.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajot J. F., Schleuning W. D., Medcalf R. L., Bamat J., Testuz J., Liebermann L., Sordat B. Mouse L cells expressing human prourokinase-type plasminogen activator: effects on extracellular matrix degradation and invasion. J Cell Biol. 1989 Aug;109(2):915–925. doi: 10.1083/jcb.109.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casslén B., Gustavsson B., Astedt B. Cell membrane receptors for urokinase plasminogen activator are increased in malignant ovarian tumours. Eur J Cancer. 1991;27(11):1445–1448. doi: 10.1016/0277-5379(91)90028-c. [DOI] [PubMed] [Google Scholar]
- Cohen R. L., Xi X. P., Crowley C. W., Lucas B. K., Levinson A. D., Shuman M. A. Effects of urokinase receptor occupancy on plasmin generation and proteolysis of basement membrane by human tumor cells. Blood. 1991 Jul 15;78(2):479–487. [PubMed] [Google Scholar]
- Cubellis M. V., Wun T. C., Blasi F. Receptor-mediated internalization and degradation of urokinase is caused by its specific inhibitor PAI-1. EMBO J. 1990 Apr;9(4):1079–1085. doi: 10.1002/j.1460-2075.1990.tb08213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Duffy M. J., Reilly D., O'Sullivan C., O'Higgins N., Fennelly J. J., Andreasen P. Urokinase-plasminogen activator, a new and independent prognostic marker in breast cancer. Cancer Res. 1990 Nov 1;50(21):6827–6829. [PubMed] [Google Scholar]
- Ehlers M. R., Riordan J. F. Membrane proteins with soluble counterparts: role of proteolysis in the release of transmembrane proteins. Biochemistry. 1991 Oct 22;30(42):10065–10074. doi: 10.1021/bi00106a001. [DOI] [PubMed] [Google Scholar]
- Ellis V., Scully M. F., Kakkar V. V. Plasminogen activation initiated by single-chain urokinase-type plasminogen activator. Potentiation by U937 monocytes. J Biol Chem. 1989 Feb 5;264(4):2185–2188. [PubMed] [Google Scholar]
- Estreicher A., Mühlhauser J., Carpentier J. L., Orci L., Vassalli J. D. The receptor for urokinase type plasminogen activator polarizes expression of the protease to the leading edge of migrating monocytes and promotes degradation of enzyme inhibitor complexes. J Cell Biol. 1990 Aug;111(2):783–792. doi: 10.1083/jcb.111.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estreicher A., Wohlwend A., Belin D., Schleuning W. D., Vassalli J. D. Characterization of the cellular binding site for the urokinase-type plasminogen activator. J Biol Chem. 1989 Jan 15;264(2):1180–1189. [PubMed] [Google Scholar]
- Foekens J. A., Schmitt M., van Putten W. L., Peters H. A., Bontenbal M., Jänicke F., Klijn J. G. Prognostic value of urokinase-type plasminogen activator in 671 primary breast cancer patients. Cancer Res. 1992 Nov 1;52(21):6101–6105. [PubMed] [Google Scholar]
- Gower H. J., Barton C. H., Elsom V. L., Thompson J., Moore S. E., Dickson G., Walsh F. S. Alternative splicing generates a secreted form of N-CAM in muscle and brain. Cell. 1988 Dec 23;55(6):955–964. doi: 10.1016/0092-8674(88)90241-3. [DOI] [PubMed] [Google Scholar]
- Grøndahl-Hansen J., Agerlin N., Munkholm-Larsen P., Bach F., Nielsen L. S., Dombernowsky P., Danø K. Sensitive and specific enzyme-linked immunosorbent assay for urokinase-type plasminogen activator and its application to plasma from patients with breast cancer. J Lab Clin Med. 1988 Jan;111(1):42–51. [PubMed] [Google Scholar]
- Grøndahl-Hansen J., Christensen I. J., Rosenquist C., Brünner N., Mouridsen H. T., Danø K., Blichert-Toft M. High levels of urokinase-type plasminogen activator and its inhibitor PAI-1 in cytosolic extracts of breast carcinomas are associated with poor prognosis. Cancer Res. 1993 Jun 1;53(11):2513–2521. [PubMed] [Google Scholar]
- Grøndahl-Hansen J., Ralfkiaer E., Kirkeby L. T., Kristensen P., Lund L. R., Danø K. Localization of urokinase-type plasminogen activator in stromal cells in adenocarcinomas of the colon in humans. Am J Pathol. 1991 Jan;138(1):111–117. [PMC free article] [PubMed] [Google Scholar]
- Hearing V. J., Law L. W., Corti A., Appella E., Blasi F. Modulation of metastatic potential by cell surface urokinase of murine melanoma cells. Cancer Res. 1988 Mar 1;48(5):1270–1278. [PubMed] [Google Scholar]
- Hollas W., Blasi F., Boyd D. Role of the urokinase receptor in facilitating extracellular matrix invasion by cultured colon cancer. Cancer Res. 1991 Jul 15;51(14):3690–3695. [PubMed] [Google Scholar]
- Huizinga T. W., van der Schoot C. E., Jost C., Klaassen R., Kleijer M., von dem Borne A. E., Roos D., Tetteroo P. A. The PI-linked receptor FcRIII is released on stimulation of neutrophils. Nature. 1988 Jun 16;333(6174):667–669. doi: 10.1038/333667a0. [DOI] [PubMed] [Google Scholar]
- Hébert C. A., Baker J. B. Linkage of extracellular plasminogen activator to the fibroblast cytoskeleton: colocalization of cell surface urokinase with vinculin. J Cell Biol. 1988 Apr;106(4):1241–1247. doi: 10.1083/jcb.106.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P. H., Christensen E. I., Ebbesen P., Gliemann J., Andreasen P. A. Lysosomal degradation of receptor-bound urokinase-type plasminogen activator is enhanced by its inhibitors in human trophoblastic choriocarcinoma cells. Cell Regul. 1990 Dec;1(13):1043–1056. doi: 10.1091/mbc.1.13.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänicke F., Schmitt M., Pache L., Ulm K., Harbeck N., Höfler H., Graeff H. Urokinase (uPA) and its inhibitor PAI-1 are strong and independent prognostic factors in node-negative breast cancer. Breast Cancer Res Treat. 1993;24(3):195–208. doi: 10.1007/BF01833260. [DOI] [PubMed] [Google Scholar]
- Kristensen P., Eriksen J., Blasi F., Danø K. Two alternatively spliced mouse urokinase receptor mRNAs with different histological localization in the gastrointestinal tract. J Cell Biol. 1991 Dec;115(6):1763–1771. doi: 10.1083/jcb.115.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lund L. R., Rømer J., Rønne E., Ellis V., Blasi F., Danø K. Urokinase-receptor biosynthesis, mRNA level and gene transcription are increased by transforming growth factor beta 1 in human A549 lung carcinoma cells. EMBO J. 1991 Nov;10(11):3399–3407. doi: 10.1002/j.1460-2075.1991.tb04904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masazza G., Lucchini V., Tomasoni A., Peccatori F., Lampasona V., Giudici G., Mangioni C., Biondi A., Giavazzi R. Malignant behavior and resistance to cisplatin of human ovarian carcinoma xenografts established from the same patient at different stages of the disease. Cancer Res. 1991 Dec 1;51(23 Pt 1):6358–6362. [PubMed] [Google Scholar]
- Massazza G., Tomasoni A., Lucchini V., Allavena P., Erba E., Colombo N., Mantovani A., D'Incalci M., Mangioni C., Giavazzi R. Intraperitoneal and subcutaneous xenografts of human ovarian carcinoma in nude mice and their potential in experimental therapy. Int J Cancer. 1989 Sep 15;44(3):494–500. doi: 10.1002/ijc.2910440320. [DOI] [PubMed] [Google Scholar]
- Masucci M. T., Pedersen N., Blasi F. A soluble, ligand binding mutant of the human urokinase plasminogen activator receptor. J Biol Chem. 1991 May 15;266(14):8655–8658. [PubMed] [Google Scholar]
- Møller L. B., Ploug M., Blasi F. Structural requirements for glycosyl-phosphatidylinositol-anchor attachment in the cellular receptor for urokinase plasminogen activator. Eur J Biochem. 1992 Sep 1;208(2):493–500. doi: 10.1111/j.1432-1033.1992.tb17213.x. [DOI] [PubMed] [Google Scholar]
- Møller L. B., Pöllänen J., Rønne E., Pedersen N., Blasi F. N-linked glycosylation of the ligand-binding domain of the human urokinase receptor contributes to the affinity for its ligand. J Biol Chem. 1993 May 25;268(15):11152–11159. [PubMed] [Google Scholar]
- Nielsen L. S., Kellerman G. M., Behrendt N., Picone R., Danø K., Blasi F. A 55,000-60,000 Mr receptor protein for urokinase-type plasminogen activator. Identification in human tumor cell lines and partial purification. J Biol Chem. 1988 Feb 15;263(5):2358–2363. [PubMed] [Google Scholar]
- Nusrat A. R., Chapman H. A., Jr An autocrine role for urokinase in phorbol ester-mediated differentiation of myeloid cell lines. J Clin Invest. 1991 Mar;87(3):1091–1097. doi: 10.1172/JCI115070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson D., Pöllänen J., Høyer-Hansen G., Rønne E., Sakaguchi K., Wun T. C., Appella E., Danø K., Blasi F. Internalization of the urokinase-plasminogen activator inhibitor type-1 complex is mediated by the urokinase receptor. J Biol Chem. 1992 May 5;267(13):9129–9133. [PubMed] [Google Scholar]
- Ossowski L., Clunie G., Masucci M. T., Blasi F. In vivo paracrine interaction between urokinase and its receptor: effect on tumor cell invasion. J Cell Biol. 1991 Nov;115(4):1107–1112. doi: 10.1083/jcb.115.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowski L. In vivo invasion of modified chorioallantoic membrane by tumor cells: the role of cell surface-bound urokinase. J Cell Biol. 1988 Dec;107(6 Pt 1):2437–2445. doi: 10.1083/jcb.107.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowski L., Reich E. Antibodies to plasminogen activator inhibit human tumor metastasis. Cell. 1983 Dec;35(3 Pt 2):611–619. doi: 10.1016/0092-8674(83)90093-4. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Russo-Payne H., Wilson E. L. Inhibition of urokinase-type plasminogen activator by antibodies: the effect on dissemination of a human tumor in the nude mouse. Cancer Res. 1991 Jan 1;51(1):274–281. [PubMed] [Google Scholar]
- Petch L. A., Harris J., Raymond V. W., Blasband A., Lee D. C., Earp H. S. A truncated, secreted form of the epidermal growth factor receptor is encoded by an alternatively spliced transcript in normal rat tissue. Mol Cell Biol. 1990 Jun;10(6):2973–2982. doi: 10.1128/mcb.10.6.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picone R., Kajtaniak E. L., Nielsen L. S., Behrendt N., Mastronicola M. R., Cubellis M. V., Stoppelli M. P., Pedersen S., Danø K., Blasi F. Regulation of urokinase receptors in monocytelike U937 cells by phorbol ester phorbol myristate acetate. J Cell Biol. 1989 Feb;108(2):693–702. doi: 10.1083/jcb.108.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploug M., Eriksen J., Plesner T., Hansen N. E., Danø K. A soluble form of the glycolipid-anchored receptor for urokinase-type plasminogen activator is secreted from peripheral blood leukocytes from patients with paroxysmal nocturnal hemoglobinuria. Eur J Biochem. 1992 Sep 1;208(2):397–404. doi: 10.1111/j.1432-1033.1992.tb17200.x. [DOI] [PubMed] [Google Scholar]
- Ploug M., Rønne E., Behrendt N., Jensen A. L., Blasi F., Danø K. Cellular receptor for urokinase plasminogen activator. Carboxyl-terminal processing and membrane anchoring by glycosyl-phosphatidylinositol. J Biol Chem. 1991 Jan 25;266(3):1926–1933. [PubMed] [Google Scholar]
- Pyke C., Graem N., Ralfkiaer E., Rønne E., Høyer-Hansen G., Brünner N., Danø K. Receptor for urokinase is present in tumor-associated macrophages in ductal breast carcinoma. Cancer Res. 1993 Apr 15;53(8):1911–1915. [PubMed] [Google Scholar]
- Pyke C., Kristensen P., Ralfkiaer E., Grøndahl-Hansen J., Eriksen J., Blasi F., Danø K. Urokinase-type plasminogen activator is expressed in stromal cells and its receptor in cancer cells at invasive foci in human colon adenocarcinomas. Am J Pathol. 1991 May;138(5):1059–1067. [PMC free article] [PubMed] [Google Scholar]
- Pöllänen J., Hedman K., Nielsen L. S., Danø K., Vaheri A. Ultrastructural localization of plasma membrane-associated urokinase-type plasminogen activator at focal contacts. J Cell Biol. 1988 Jan;106(1):87–95. doi: 10.1083/jcb.106.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quax P. H., Pedersen N., Masucci M. T., Weening-Verhoeff E. J., Danø K., Verheijen J. H., Blasi F. Complementation between urokinase-producing and receptor-producing cells in extracellular matrix degradation. Cell Regul. 1991 Oct;2(10):793–803. doi: 10.1091/mbc.2.10.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbani S. A., Desjardins J., Bell A. W., Banville D., Mazar A., Henkin J., Goltzman D. An amino-terminal fragment of urokinase isolated from a prostate cancer cell line (PC-3) is mitogenic for osteoblast-like cells. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1058–1064. doi: 10.1016/s0006-291x(05)80893-9. [DOI] [PubMed] [Google Scholar]
- Ragno P., Cassano S., Degen J., Kessler C., Blasi F., Rossi G. The receptor for the plasminogen activator of urokinase type is up-regulated in transformed rat thyroid cells. FEBS Lett. 1992 Jul 20;306(2-3):193–198. doi: 10.1016/0014-5793(92)80998-v. [DOI] [PubMed] [Google Scholar]
- Reich R., Thompson E. W., Iwamoto Y., Martin G. R., Deason J. R., Fuller G. C., Miskin R. Effects of inhibitors of plasminogen activator, serine proteinases, and collagenase IV on the invasion of basement membranes by metastatic cells. Cancer Res. 1988 Jun 15;48(12):3307–3312. [PubMed] [Google Scholar]
- Roldan A. L., Cubellis M. V., Masucci M. T., Behrendt N., Lund L. R., Danø K., Appella E., Blasi F. Cloning and expression of the receptor for human urokinase plasminogen activator, a central molecule in cell surface, plasmin dependent proteolysis. EMBO J. 1990 Feb;9(2):467–474. doi: 10.1002/j.1460-2075.1990.tb08132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rønne E., Behrendt N., Ellis V., Ploug M., Danø K., Høyer-Hansen G. Cell-induced potentiation of the plasminogen activation system is abolished by a monoclonal antibody that recognizes the NH2-terminal domain of the urokinase receptor. FEBS Lett. 1991 Aug 19;288(1-2):233–236. doi: 10.1016/0014-5793(91)81042-7. [DOI] [PubMed] [Google Scholar]
- Sappino A. P., Huarte J., Belin D., Vassalli J. D. Plasminogen activators in tissue remodeling and invasion: mRNA localization in mouse ovaries and implanting embryos. J Cell Biol. 1989 Nov;109(5):2471–2479. doi: 10.1083/jcb.109.5.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlechte W., Murano G., Boyd D. Examination of the role of the urokinase receptor in human colon cancer mediated laminin degradation. Cancer Res. 1989 Nov 1;49(21):6064–6069. [PubMed] [Google Scholar]
- Schmitt M., Goretzki L., Jänicke F., Calvete J., Eulitz M., Kobayashi H., Chucholowski N., Graeff H. Biological and clinical relevance of the urokinase-type plasminogen activator (uPA) in breast cancer. Biomed Biochim Acta. 1991;50(4-6):731–741. [PubMed] [Google Scholar]
- Selkoe D. J. Deciphering Alzheimer's disease: the amyloid precursor protein yields new clues. Science. 1990 Jun 1;248(4959):1058–1060. doi: 10.1126/science.2111582. [DOI] [PubMed] [Google Scholar]
- Shih Y. J., Baynes R. D., Hudson B. G., Flowers C. H., Skikne B. S., Cook J. D. Serum transferrin receptor is a truncated form of tissue receptor. J Biol Chem. 1990 Nov 5;265(31):19077–19081. [PubMed] [Google Scholar]
- Solberg H., Løber D., Eriksen J., Ploug M., Rønne E., Behrendt N., Danø K., Høyer-Hansen G. Identification and characterization of the murine cell surface receptor for the urokinase-type plasminogen activator. Eur J Biochem. 1992 Apr 15;205(2):451–458. doi: 10.1111/j.1432-1033.1992.tb16799.x. [DOI] [PubMed] [Google Scholar]
- Stoppelli M. P., Tacchetti C., Cubellis M. V., Corti A., Hearing V. J., Cassani G., Appella E., Blasi F. Autocrine saturation of pro-urokinase receptors on human A431 cells. Cell. 1986 Jun 6;45(5):675–684. doi: 10.1016/0092-8674(86)90782-8. [DOI] [PubMed] [Google Scholar]