Abstract
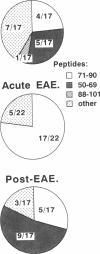
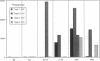
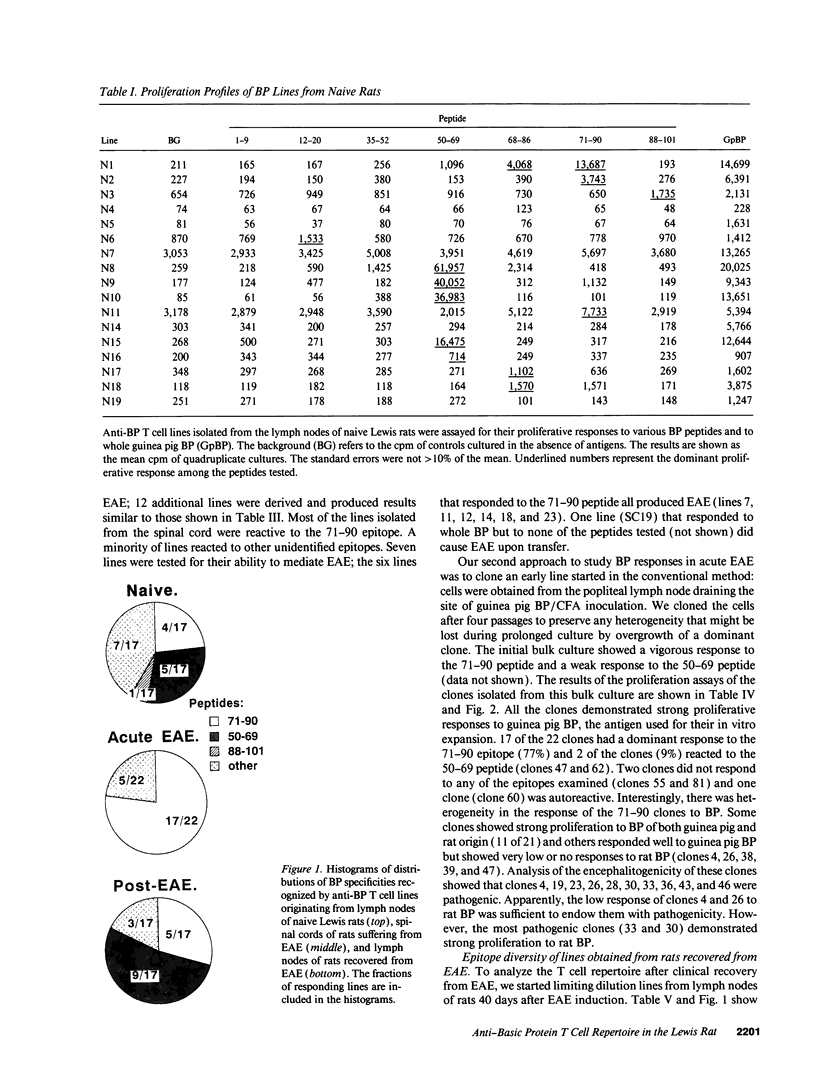
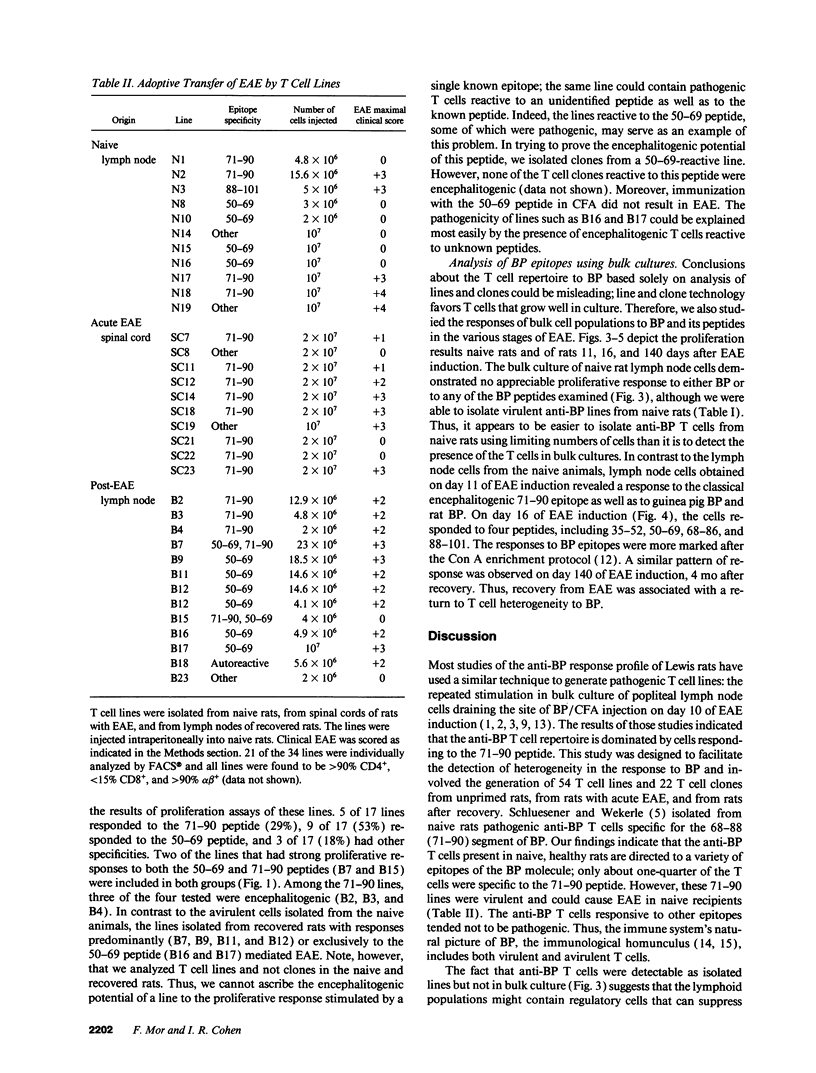
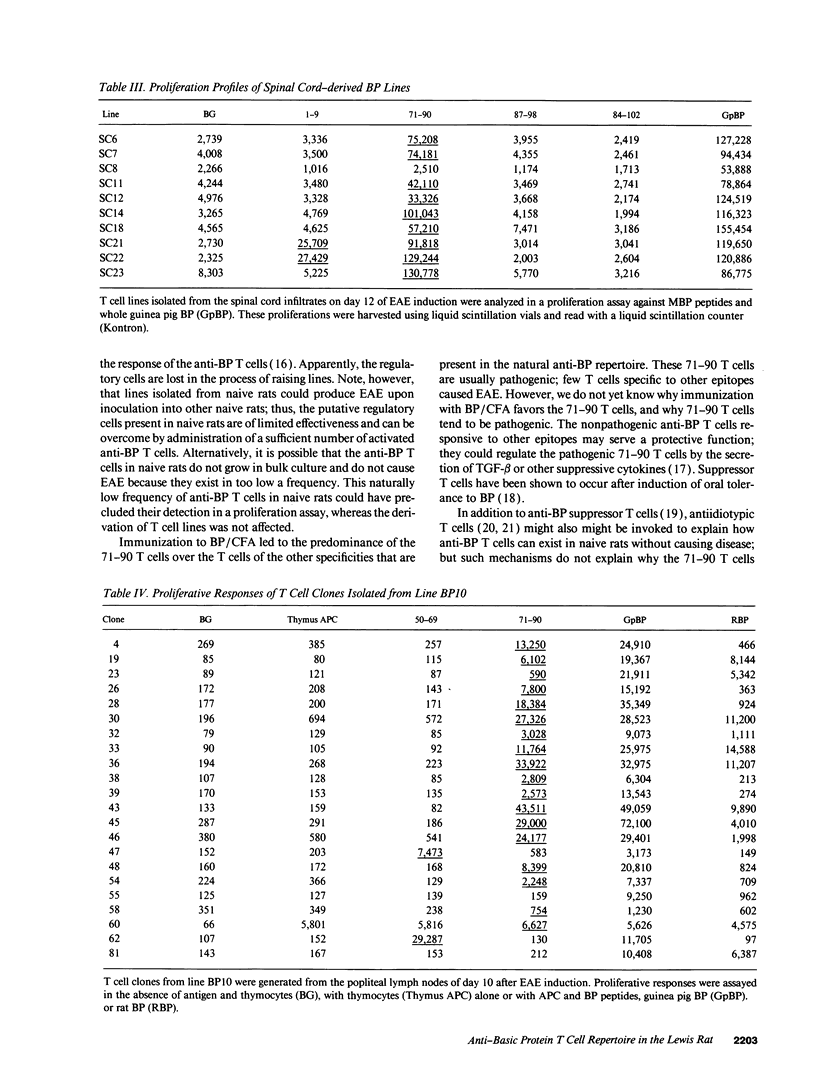
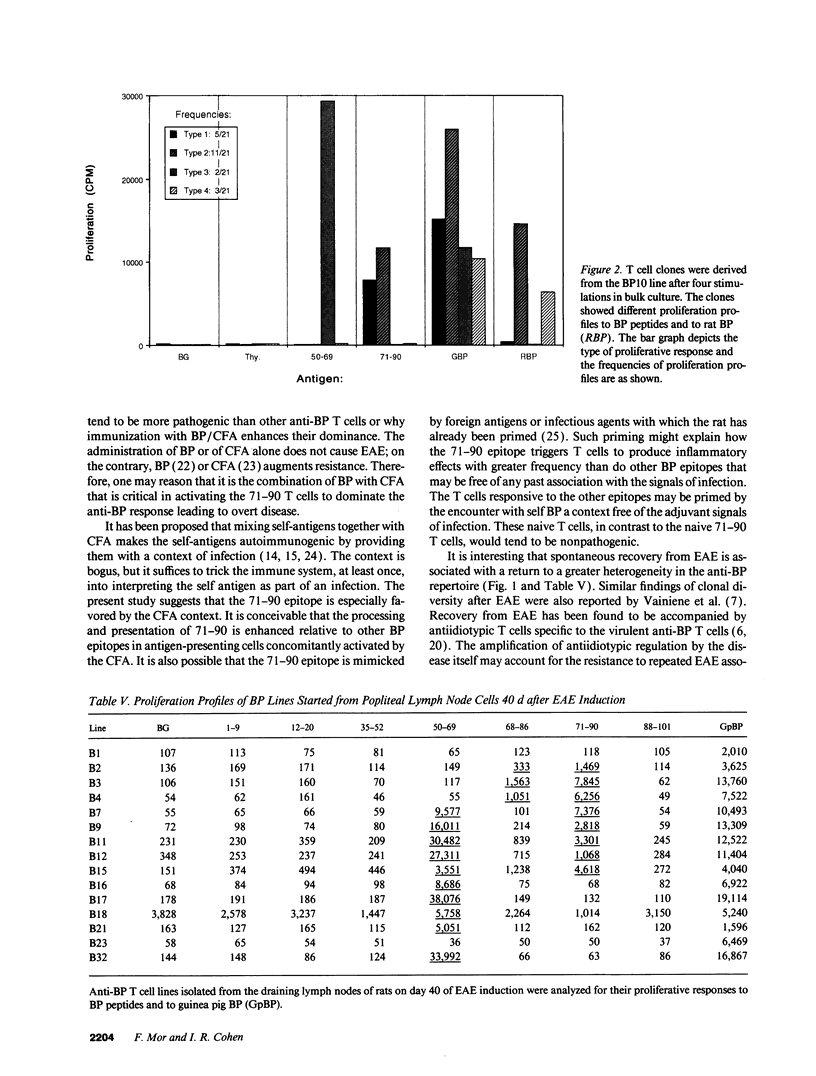
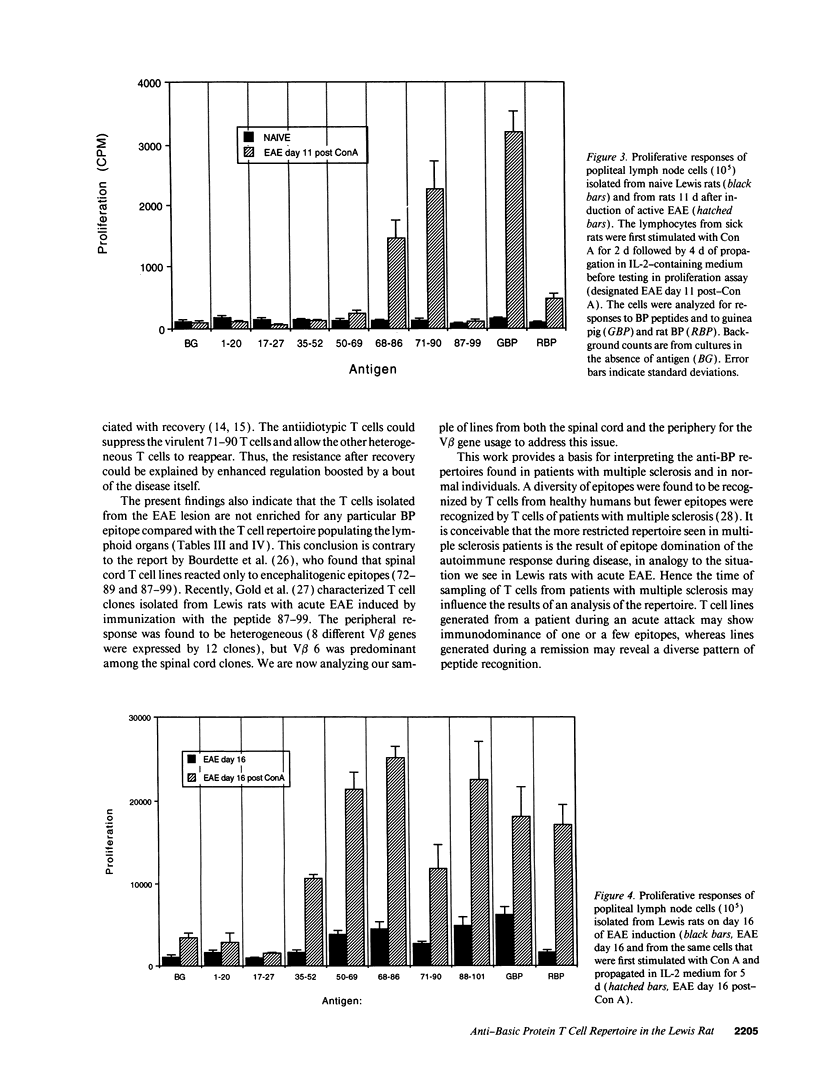
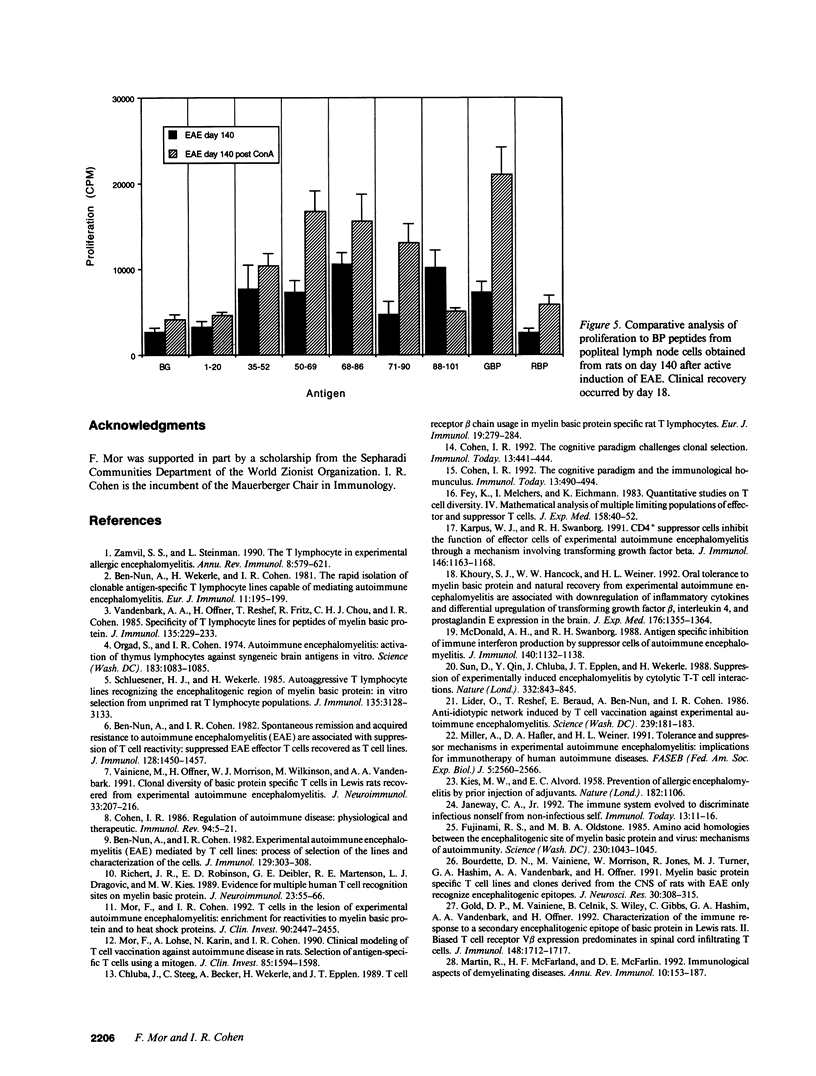
An epitope present in the 71-90 sequence of basic protein (BP) has been identified as the dominant epitope recognized by most Lewis rat encephalitogenic T cells isolated during experimental autoimmune encephalomyelitis (EAE). In the present study, we investigated the BP epitopes recognized by Lewis rat T cells in naive rats, in rats suffering from acute EAE, and in recovered rats. T cells isolated from the spinal cord lesions and from the lymph nodes were studied using T cell lines and bulk cultures. Virulence of the T cells was assayed by adoptive transfer. We now report that naive and recovered Lewis rats are populated with T cells reactive to a variety of BP epitopes and only a minority are specific for the 71-90 epitope. In contrast, the induction of EAE was associated with a predominance of T cells reactive to the 71-90 epitope. T cells recovered from naive, diseased, or recovered rats were found to be virulent upon passive transfer. Some of these virulent T cells were specific to BP epitopes other than the 71-90 epitope. There was no major difference in the BP specificities of T cells isolated from the lesions and from the lymph nodes. Thus, natural T cell reactivity to BP is heterogeneous and pathogenicity is not confined to one particular epitope, active disease is characterized by a dominant response to the 71-90 epitope, and recovery is marked by a return to heterogeneity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Nun A., Cohen I. R. Experimental autoimmune encephalomyelitis (EAE) mediated by T cell lines: process of selection of lines and characterization of the cells. J Immunol. 1982 Jul;129(1):303–308. [PubMed] [Google Scholar]
- Ben-Nun A., Cohen I. R. Spontaneous remission and acquired resistance to autoimmune encephalomyelitis (EAE) are associated with suppression of T cell reactivity: suppressed EAE effector T cells recovered as T cell lines. J Immunol. 1982 Mar;128(3):1450–1457. [PubMed] [Google Scholar]
- Ben-Nun A., Wekerle H., Cohen I. R. The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur J Immunol. 1981 Mar;11(3):195–199. doi: 10.1002/eji.1830110307. [DOI] [PubMed] [Google Scholar]
- Bourdette D. N., Vainiene M., Morrison W., Jones R., Turner M. J., Hashim G. A., Vandenbark A. A., Offner H. Myelin basic protein specific T cell lines and clones derived from the CNS of rats with EAE only recognize encephalitogenic epitopes. J Neurosci Res. 1991 Oct;30(2):308–315. doi: 10.1002/jnr.490300205. [DOI] [PubMed] [Google Scholar]
- Chluba J., Steeg C., Becker A., Wekerle H., Epplen J. T. T cell receptor beta chain usage in myelin basic protein-specific rat T lymphocytes. Eur J Immunol. 1989 Feb;19(2):279–284. doi: 10.1002/eji.1830190210. [DOI] [PubMed] [Google Scholar]
- Cohen I. R. Regulation of autoimmune disease physiological and therapeutic. Immunol Rev. 1986 Dec;94:5–21. doi: 10.1111/j.1600-065x.1986.tb01161.x. [DOI] [PubMed] [Google Scholar]
- Cohen I. R. The cognitive paradigm and the immunological homunculus. Immunol Today. 1992 Dec;13(12):490–494. doi: 10.1016/0167-5699(92)90024-2. [DOI] [PubMed] [Google Scholar]
- Cohen I. R. The cognitive principle challenges clonal selection. Immunol Today. 1992 Nov;13(11):441–444. doi: 10.1016/0167-5699(92)90071-E. [DOI] [PubMed] [Google Scholar]
- Fey K., Melchers I., Eichmann K. Quantitative studies on T cell diversity. IV. Mathematical analysis of multiple limiting populations of effector and suppressor T cells. J Exp Med. 1983 Jul 1;158(1):40–52. doi: 10.1084/jem.158.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B. Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science. 1985 Nov 29;230(4729):1043–1045. doi: 10.1126/science.2414848. [DOI] [PubMed] [Google Scholar]
- Gold D. P., Vainiene M., Celnik B., Wiley S., Gibbs C., Hashim G. A., Vandenbark A. A., Offner H. Characterization of the immune response to a secondary encephalitogenic epitope of basic protein in Lewis rats. II. Biased T cell receptor V beta expression predominates in spinal cord infiltrating T cells. J Immunol. 1992 Mar 15;148(6):1712–1717. [PubMed] [Google Scholar]
- Janeway C. A., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992 Jan;13(1):11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- KIES M. W., ALVORD E. C., Jr [Prevention of allergic encephalomyelitis by prior injection of adjuvants]. Nature. 1958 Oct 18;182(4642):1106–1106. doi: 10.1038/1821106a0. [DOI] [PubMed] [Google Scholar]
- Karpus W. J., Swanborg R. H. CD4+ suppressor cells inhibit the function of effector cells of experimental autoimmune encephalomyelitis through a mechanism involving transforming growth factor-beta. J Immunol. 1991 Feb 15;146(4):1163–1168. [PubMed] [Google Scholar]
- Khoury S. J., Hancock W. W., Weiner H. L. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992 Nov 1;176(5):1355–1364. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lider O., Reshef T., Beraud E., Ben-Nun A., Cohen I. R. Anti-idiotypic network induced by T cell vaccination against experimental autoimmune encephalomyelitis. Science. 1988 Jan 8;239(4836):181–183. doi: 10.1126/science.2447648. [DOI] [PubMed] [Google Scholar]
- Martin R., McFarland H. F., McFarlin D. E. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- McDonald A. H., Swanborg R. H. Antigen-specific inhibition of immune interferon production by suppressor cells of autoimmune encephalomyelitis. J Immunol. 1988 Feb 15;140(4):1132–1138. [PubMed] [Google Scholar]
- Miller A., Hafler D. A., Weiner H. L. Tolerance and suppressor mechanisms in experimental autoimmune encephalomyelitis: implications for immunotherapy of human autoimmune diseases. FASEB J. 1991 Aug;5(11):2560–2566. doi: 10.1096/fasebj.5.11.1868980. [DOI] [PubMed] [Google Scholar]
- Mor F., Cohen I. R. T cells in the lesion of experimental autoimmune encephalomyelitis. Enrichment for reactivities to myelin basic protein and to heat shock proteins. J Clin Invest. 1992 Dec;90(6):2447–2455. doi: 10.1172/JCI116136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor F., Lohse A. W., Karin N., Cohen I. R. Clinical modeling of T cell vaccination against autoimmune diseases in rats. Selection of antigen-specific T cells using a mitogen. J Clin Invest. 1990 May;85(5):1594–1598. doi: 10.1172/JCI114610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgad S., Cohen I. R. Autoimmune encephalomyelitis: activation of thymus lymphocytes against syngeneic brain antigens in vitro. Science. 1974 Mar 15;183(4129):1083–1085. doi: 10.1126/science.183.4129.1083. [DOI] [PubMed] [Google Scholar]
- Richert J. R., Robinson E. D., Deibler G. E., Martenson R. E., Dragovic L. J., Kies M. W. Evidence for multiple human T cell recognition sites on myelin basic protein. J Neuroimmunol. 1989 Jun;23(1):55–66. doi: 10.1016/0165-5728(89)90073-8. [DOI] [PubMed] [Google Scholar]
- Schluesener H. J., Wekerle H. Autoaggressive T lymphocyte lines recognizing the encephalitogenic region of myelin basic protein: in vitro selection from unprimed rat T lymphocyte populations. J Immunol. 1985 Nov;135(5):3128–3133. [PubMed] [Google Scholar]
- Sun D., Qin Y., Chluba J., Epplen J. T., Wekerle H. Suppression of experimentally induced autoimmune encephalomyelitis by cytolytic T-T cell interactions. Nature. 1988 Apr 28;332(6167):843–845. doi: 10.1038/332843a0. [DOI] [PubMed] [Google Scholar]
- Vainiene M., Offner H., Morrison W. J., Wilkinson M., Vandenbark A. A. Clonal diversity of basic protein specific T cells in Lewis rats recovered from experimental autoimmune encephalomyelitis. J Neuroimmunol. 1991 Sep;33(3):207–216. doi: 10.1016/0165-5728(91)90108-j. [DOI] [PubMed] [Google Scholar]
- Vandenbark A. A., Offner H., Reshef T., Fritz R., Chou C. H., Cohen I. R. Specificity of T lymphocyte lines for peptides of myelin basic protein. J Immunol. 1985 Jul;135(1):229–233. [PubMed] [Google Scholar]
- Zamvil S. S., Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]