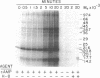
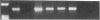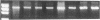Abstract
Human T lymphocytes possess both the type I and II isozymes of protein kinase A (PKA). The type I (PKA-I) isozyme is predominantly associated with the plasma membrane, whereas the type II (PKA-II) isozyme is primarily localized to the cytosol. Because the functions of both PKA-I and PKA-II isozymes in the biochemical events of T lymphocyte activation have not been clearly elucidated, we tested the hypothesis that very early events of normal human T lymphocyte activation are mediated by the PKA-I and/or PKA-II isozyme(s). Fresh normal human T cells and a normal human CD4+ T cell line (GK606) activated with anti-CD3-epsilon and recombinant interleukin 1 alpha (rIL-1 alpha) exhibited a peak six- to sevenfold increase of PKA phosphotransferase activity at 5 min that returned to baseline by 60 min. Similarly, both fresh T cells and the T cell line activated by phorbol myristate acetate and ionomycin demonstrated a peak eightfold increase of PKA activity by 15 min that returned toward baseline by 60 min. Chromatographic separation of the PKA isozymes and quantification of phosphotransferase activities after T cell activation by either agonist pair showed preferential activation of the PKA-I isozyme, resulting in a significant reduction in the ratio of PKA-I to PKA-II isozyme activity from 3.1:1-6.2:1 to 1.1:1-3.2:1. PKA-I isozyme activation resulted in the release of free catalytic (C) subunit, an increase in C subunit phosphotransferase activity, and the phosphorylation of T cell plasma membrane-associated proteins, p14, p17, p20, p21, p38, and p48. However, activation of the PKA-I isozyme did not appear to be required for the transcription of IL-2 mRNA, an event necessary for mitosis. These data indicate that ligand-induced T cell activation is associated with rapid activation of the PKA-I, but not PKA-II, isozyme that results in phosphorylation of plasma membrane-associated proteins. The involvement of the PKA-I isozyme during the very early events of T cell activation suggests that this isozyme may be an antigen- or mitogen-stimulated protein kinase.
Full text
PDF


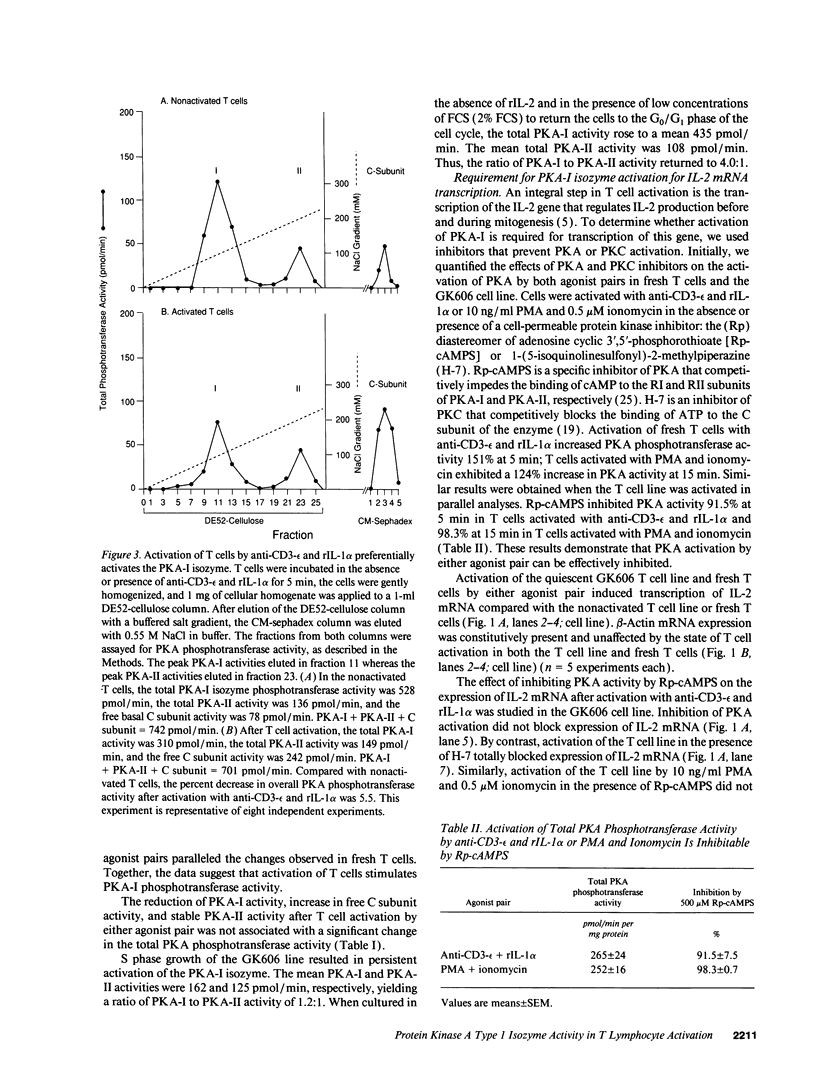
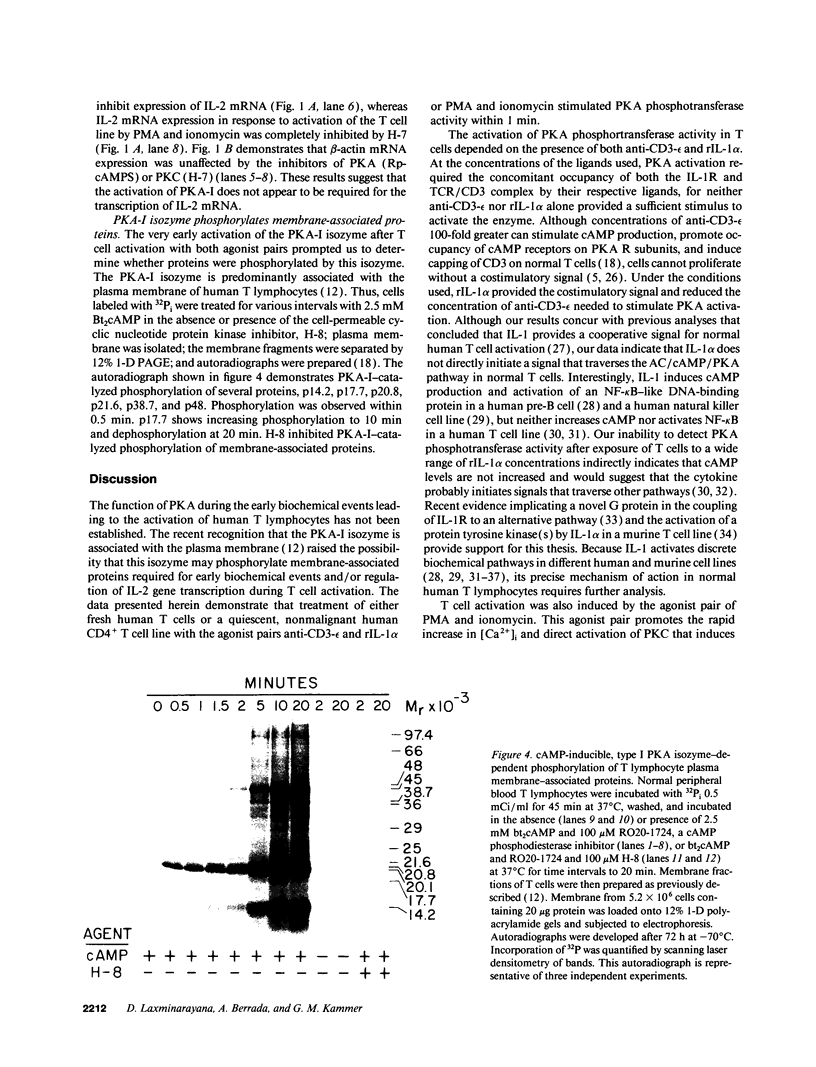


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell J. D., Brunton L. L. Enhancement of adenylate cyclase activity in S49 lymphoma cells by phorbol esters. Withdrawal of GTP-dependent inhibition. J Biol Chem. 1986 Sep 15;261(26):12036–12041. [PubMed] [Google Scholar]
- Berry N., Nishizuka Y. Protein kinase C and T cell activation. Eur J Biochem. 1990 Apr 30;189(2):205–214. doi: 10.1111/j.1432-1033.1990.tb15478.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Braylan R. C., Benson N. A., Nourse V., Kruth H. S. Correlated analysis of cellular DNA, membrane antigens and light scatter of human lymphoid cells. Cytometry. 1982 Mar;2(5):337–343. doi: 10.1002/cyto.990020511. [DOI] [PubMed] [Google Scholar]
- Brenner C. A., Tam A. W., Nelson P. A., Engleman E. G., Suzuki N., Fry K. E., Larrick J. W. Message amplification phenotyping (MAPPing): a technique to simultaneously measure multiple mRNAs from small numbers of cells. Biotechniques. 1989 Nov-Dec;7(10):1096–1103. [PubMed] [Google Scholar]
- Byus C. V., Klimpel G. R., Lucas D. O., Russell D. H. Type I and type II cyclic AMP-dependent protein kinase as opposite effectors of lymphocyte mitogenesis. Nature. 1977 Jul 7;268(5615):63–64. doi: 10.1038/268063a0. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Davies A. A., Crumpton M. J. Activators of protein kinase C down-regulate and phosphorylate the T3/T-cell antigen receptor complex of human T lymphocytes. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8158–8162. doi: 10.1073/pnas.82.23.8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell D., Davies A. A., Londei M., Feldman M., Crumpton M. J. Association of phosphorylation of the T3 antigen with immune activation of T lymphocytes. Nature. 1987 Feb 5;325(6104):540–542. doi: 10.1038/325540a0. [DOI] [PubMed] [Google Scholar]
- Chedid M., Mizel S. B. Involvement of cyclic AMP-dependent protein kinases in the signal transduction pathway for interleukin-1. Mol Cell Biol. 1990 Jul;10(7):3824–3827. doi: 10.1128/mcb.10.7.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedid M., Yoza B. K., Brooks J. W., Mizel S. B. Activation of AP-1 by IL-1 and phorbol esters in T cells. Role of protein kinase A and protein phosphatases. J Immunol. 1991 Aug 1;147(3):867–873. [PubMed] [Google Scholar]
- Crabtree G. R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- Didier M., Aussel C., Pelassy C., Fehlmann M. IL-1 signaling for IL-2 production in T cells involves a rise in phosphatidylserine synthesis. J Immunol. 1988 Nov 1;141(9):3078–3080. [PubMed] [Google Scholar]
- Grove J. R., Deutsch P. J., Price D. J., Habener J. F., Avruch J. Plasmids encoding PKI(1-31), a specific inhibitor of cAMP-stimulated gene expression, inhibit the basal transcriptional activity of some but not all cAMP-regulated DNA response elements in JEG-3 cells. J Biol Chem. 1989 Nov 25;264(33):19506–19513. [PubMed] [Google Scholar]
- Hasler P., Moore J. J., Kammer G. M. Human T lymphocyte cAMP-dependent protein kinase: subcellular distributions and activity ranges of type I and type II isozymes. FASEB J. 1992 Jun;6(9):2735–2741. doi: 10.1096/fasebj.6.9.1319361. [DOI] [PubMed] [Google Scholar]
- Hasler P., Schultz L. A., Kammer G. M. Defective cAMP-dependent phosphorylation of intact T lymphocytes in active systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1978–1982. doi: 10.1073/pnas.87.5.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Kobayashi R. Pharmacology of protein kinase inhibitors. Annu Rev Pharmacol Toxicol. 1992;32:377–397. doi: 10.1146/annurev.pa.32.040192.002113. [DOI] [PubMed] [Google Scholar]
- Hollingsworth E. B., Ukena D., Daly J. W. The protein kinase C activator phorbol-12-myristate-13-acetate enhances cyclic AMP accumulation in pheochromocytoma cells. FEBS Lett. 1986 Feb 3;196(1):131–134. doi: 10.1016/0014-5793(86)80227-7. [DOI] [PubMed] [Google Scholar]
- Kammer G. M., Boehm C. A., Rudolph S. A., Schultz L. A. Mobility of the human T lymphocyte surface molecules CD3, CD4, and CD8: regulation by a cAMP-dependent pathway. Proc Natl Acad Sci U S A. 1988 Feb;85(3):792–796. doi: 10.1073/pnas.85.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammer G. M., Kurrasch R., Scillian J. J. Capping of the surface OKT3 binding molecule prevents the T-cell proliferative response to antigens: evidence that this molecule conveys the activation signal. Cell Immunol. 1984 Aug;87(1):284–294. doi: 10.1016/0008-8749(84)90152-7. [DOI] [PubMed] [Google Scholar]
- Kammer G. M. The adenylate cyclase-cAMP-protein kinase A pathway and regulation of the immune response. Immunol Today. 1988 Jul-Aug;9(7-8):222–229. doi: 10.1016/0167-5699(88)91220-0. [DOI] [PubMed] [Google Scholar]
- Katada T., Gilman A. G., Watanabe Y., Bauer S., Jakobs K. H. Protein kinase C phosphorylates the inhibitory guanine-nucleotide-binding regulatory component and apparently suppresses its function in hormonal inhibition of adenylate cyclase. Eur J Biochem. 1985 Sep 2;151(2):431–437. doi: 10.1111/j.1432-1033.1985.tb09120.x. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., O'Shea J. J., Luong H., Ross P., Bluestone J. A., Samelson L. E. T cell receptor tyrosine phosphorylation. Variable coupling for different activating ligands. J Biol Chem. 1987 Sep 15;262(26):12654–12659. [PubMed] [Google Scholar]
- Klausner R. D., Samelson L. E. T cell antigen receptor activation pathways: the tyrosine kinase connection. Cell. 1991 Mar 8;64(5):875–878. doi: 10.1016/0092-8674(91)90310-u. [DOI] [PubMed] [Google Scholar]
- Livesey S. A., Martin T. J. Selective activation of the cAMP-dependent protein kinase isoenzymes. Methods Enzymol. 1988;159:105–118. doi: 10.1016/0076-6879(88)59012-2. [DOI] [PubMed] [Google Scholar]
- Lohmann S. M., Walter U. Regulation of the cellular and subcellular concentrations and distribution of cyclic nucleotide-dependent protein kinases. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;18:63–117. [PubMed] [Google Scholar]
- Muñoz E., Zubiaga A., Huang C., Huber B. T. Interleukin-1 induces protein tyrosine phosphorylation in T cells. Eur J Immunol. 1992 Jun;22(6):1391–1396. doi: 10.1002/eji.1830220610. [DOI] [PubMed] [Google Scholar]
- Naghshineh S., Noguchi M., Huang K. P., Londos C. Activation of adipocyte adenylate cyclase by protein kinase C. J Biol Chem. 1986 Nov 5;261(31):14534–14538. [PubMed] [Google Scholar]
- Nikula H., Vihko K., Huhtaniemi I. Protein kinase C and Gi-protein mediated modulation of cAMP production in different stages of the rat seminiferous epithelium. Mol Cell Endocrinol. 1990 May 7;70(3):247–253. doi: 10.1016/0303-7207(90)90215-t. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- O'Neill L. A., Bird T. A., Saklatvala J. How does interleukin 1 activate cells? Interleukin 1 signal transduction. Immunol Today. 1990 Nov;11(11):392–394. doi: 10.1016/0167-5699(90)90155-3. [DOI] [PubMed] [Google Scholar]
- Osborn L., Kunkel S., Nabel G. J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R. Mechanisms by which accessory cells contribute in growth of resting T lymphocytes initiated by OKT3 antibody. Eur J Immunol. 1985 Jul;15(7):645–651. doi: 10.1002/eji.1830150702. [DOI] [PubMed] [Google Scholar]
- Pyne N. J., Freissmuth M., Palmer S. Phosphorylation of the spliced variant forms of the recombinant stimulatory guanine-nucleotide-binding regulatory protein (Gs alpha) by protein kinase C. Biochem J. 1992 Jul 1;285(Pt 1):333–338. doi: 10.1042/bj2850333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray K., Thompson N., Kennard N., Rollins P., Grenfell S., Witham S., Smithers N., Solari R. Investigation of guanine-nucleotide-binding protein involvement and regulation of cyclic AMP metabolism in interleukin 1 signal transduction. Biochem J. 1992 Feb 15;282(Pt 1):59–67. doi: 10.1042/bj2820059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins P., Witham S., Ray K., Thompson N., Sadler H., Smithers N., Grenfell S., Solari R. Modification of biological responses to interleukin-1 by agents that perturb signal transduction pathways. Cytokine. 1991 Jan;3(1):42–53. doi: 10.1016/1043-4666(91)90009-3. [DOI] [PubMed] [Google Scholar]
- Rothermel J. D., Jastorff B., Botelho L. H. Inhibition of glucagon-induced glycogenolysis in isolated rat hepatocytes by the Rp diastereomer of adenosine cyclic 3',5'-phosphorothioate. J Biol Chem. 1984 Jul 10;259(13):8151–8155. [PubMed] [Google Scholar]
- Samelson L. E., Patel M. D., Weissman A. M., Harford J. B., Klausner R. D. Antigen activation of murine T cells induces tyrosine phosphorylation of a polypeptide associated with the T cell antigen receptor. Cell. 1986 Sep 26;46(7):1083–1090. doi: 10.1016/0092-8674(86)90708-7. [DOI] [PubMed] [Google Scholar]
- Shirakawa F., Chedid M., Suttles J., Pollok B. A., Mizel S. B. Interleukin 1 and cyclic AMP induce kappa immunoglobulin light-chain expression via activation of an NF-kappa B-like DNA-binding protein. Mol Cell Biol. 1989 Mar;9(3):959–964. doi: 10.1128/mcb.9.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa F., Yamashita U., Chedid M., Mizel S. B. Cyclic AMP--an intracellular second messenger for interleukin 1. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8201–8205. doi: 10.1073/pnas.85.21.8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortora G., Yokozaki H., Pepe S., Clair T., Cho-Chung Y. S. Differentiation of HL-60 leukemia by type I regulatory subunit antisense oligodeoxynucleotide of cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):2011–2015. doi: 10.1073/pnas.88.5.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Allen P. M. The basis for the immunoregulatory role of macrophages and other accessory cells. Science. 1987 May 1;236(4801):551–557. doi: 10.1126/science.2437650. [DOI] [PubMed] [Google Scholar]
- Weiss A., Imboden J. B. Cell surface molecules and early events involved in human T lymphocyte activation. Adv Immunol. 1987;41:1–38. doi: 10.1016/s0065-2776(08)60029-2. [DOI] [PubMed] [Google Scholar]
- Yang S. Y., Chouaib S., Dupont B. A common pathway for T lymphocyte activation involving both the CD3-Ti complex and CD2 sheep erythrocyte receptor determinants. J Immunol. 1986 Aug 15;137(4):1097–1100. [PubMed] [Google Scholar]
- Yatomi Y., Arata Y., Tada S., Kume S., Ui M. Phosphorylation of the inhibitory guanine-nucleotide-binding protein as a possible mechanism of inhibition by protein kinase C of agonist-induced Ca2+ mobilization in human platelet. Eur J Biochem. 1992 May 1;205(3):1003–1009. doi: 10.1111/j.1432-1033.1992.tb16867.x. [DOI] [PubMed] [Google Scholar]
- Yoshimasa T., Sibley D. R., Bouvier M., Lefkowitz R. J., Caron M. G. Cross-talk between cellular signalling pathways suggested by phorbol-ester-induced adenylate cyclase phosphorylation. Nature. 1987 May 7;327(6117):67–70. doi: 10.1038/327067a0. [DOI] [PubMed] [Google Scholar]