Abstract
Purpose
A drug of high potency and reduced immunogenicity is needed to develop a targeted biological drug that when injected systemically can penetrate to malignant B cells. Therefore, a novel deimmunized bispecific ligand directed toxin (BLT) targeted by dual high affinity scFvs spliced to PE38 with a KDEL c-terminus was genetically engineered. The aims were to reduce toxin immunogenicity using mutagenesis, measure the ability of mutated drug to elicit anti-toxin antibody responses, and show that mutated drug was effective against systemic B cell lymphoma in vivo.
Experimental design
Both human anti-CD22 scFv and anti-CD19 scFv were cloned onto the same single chain molecule with truncated pseudomonas exotoxin (PE38) to create the drug. Site specific mutagenesis was used to mutate amino acids in 7 key epitopic toxin regions that dictate B cell generation of neutralizing anti-toxin antibodies. Bioassays were used to determine whether mutation reduced potency and ELISAs were performed to determine whether anti-toxin antibodies were reduced. Finally, a powerful genetically altered luciferase xenograft model was used that could be imaged in real time to determine the effect on systemic malignant human B cell lymphoma, Raji-luc.
Results
Patient B-ALL, B-CLL, and B lymphoma were high in CD22 and CD19 expression. 2219KDEL 7mut was significantly effective against systemic Raji-luc in mice and prevented metastatic spread. Mutagenesis reduced neutralizing anti-toxin antibodies by about 80% with no apparent loss in in vitro or in vivo activity.
Conclusions
Because 2219KDEL7mut immunogenicity was significantly reduced and the drug was highly effective in vivo, we can now give multiple drug treatments with targeted toxins in future clinical trials.
Keywords: immunotoxin, pseudomonas exotoxin a, leukemia, lymphoma, scid model, anti-CD19 scFv, anti-CD22 scFv
INTRODUCTION
Acute leukemia represents 30% of all cancer in American children under the age of 15 years and 12% of cancer cases in those ages 15 to 19 years and is the most common childhood malignancy. Eighty percent of these are B lineage acute lymphoblastic leukemia (B-ALL). Chemotherapy resistance is a frequent cause of treatment failure in relapsed patients (1) and alternative therapies are urgently needed.
One alternative is targeted toxins which are synthesized by coupling an antibody or antibody fragment to a potent, catalytic toxin, such as pseudomonas exotoxin, capable of inhibiting protein synthesis (2). Investigators have continued to develop targeted toxins because these agents are potent, catalytic, and irreversible in their action. Our research group has specialized in enhancing potency even further with the development of certain bispecific targeted toxins (BLT) designed to promote activity (3-7). Despite successes in potency enhancement, immunogenicity is the major problem since effective therapy requires multiple treatments that result in the generation of neutralizing antibodies, mostly against the toxin (8). In the past 20 years, despite great efforts, no solution has been found for this problem. We have made use of the ability to genetically modify biological targeted toxins to address immunogenicity and other problems such as potency and toxicity (6,7) that limit their usefulness for therapy.
Onda and Pastan recently showed that there are only 7 major epitopes in the pseudomonas toxin (PE) recognized by B cells (9). Furthermore, it is possible to remove or replace hydrophilic amino acids at these B cell epitopes to create a “low immunogenic” form of PE that will limit the formation of neutralizing antibodies in mice (10). Therefore, we used these to mutate a truncated form of PE toxin (PE38) selected based on previous research describing a series of internal frame deletion mutations that established the best location for genetic fusion of PE to targeting ligands (11). PE38 contains the A fragment of native PE that catalyzes ADP ribosylation of elongation factor 2 (EF-2) leading to irreversible inhibition of protein synthesis and cell death with as few as 1000 molecules (12). We further modified PE to include Lys-Asp-Glu-Leu (KDEL) as a C-terminal transport signal that enhances EF-2 access and potency (13).
For CD19 targeting, investigators using conventional biochemically linked anti-CD19 IT have reported anti-cancer affects (14-18). However, these have not reached the mainstream because of varied degrees of effectiveness. The 95 kDa CD19 membrane glycoprotein is broadly expressed on B cell leukemia/lymphoma (19) including B-ALL. CD22 is also expressed on B cells and anti-CD22 IT have proven successful in the treatment of rare Hairy Cell Leukemia (HCL) (20). To broaden our toxin delivery and our anti-leukemia affect, we cloned a new molecule by fusing two repeating sFv subunits recognizing human CD19 and human CD22 spliced downstream of truncated DT390 (3) and subsequently used genetic engineering to improve it (21).
DT2219 had better anti-tumor activity than its monospecific counterparts or a mixture of the two, thus indicating an advantage of including both ligands on the same single chain molecule and fulfilling our criteria for a successful BLT. Phase 1 studies are currently underway, but we do anticipate immunogenicity problems with the drug since DT is highly immunogenic, a problem in clinical trials with DT-based targeted toxins (22). This study represents an important improvement in the creation of a new anti-cancer biological combining two advancements “reduced immunogenicity” toxin and the “enhanced potency” BLT. In this study, the recently optimized anti-CD22 and anti-CD19 scFvs were genetically spliced to mutated “low immunogenicity” PE DNA to create a hybrid protein 2219KDEL7mut that had powerful anti-lymphoma effects and produced long-term disease-free survivors. The anti-cancer benefit was demonstrated in a powerful bioluminescence luciferase reporter gene model that permitted real-time imaging.
MATERIALS AND METHODS
Construction of 2219KDEL7mut
The hybrid gene was synthesized using assembly PCR. In its final configuration, the 2219ARLKDEL fusion gene (from 5′ end to 3′ end) consisted of an EcoRI restriction site, then the anti-CD22 scFv gene (Figure 1A). This anti-CD22 scFv gene was oriented with the VL domain preceding the VH domain and was conjoined by a fragment encoding the ARL linker (GSTSGSGKPGSGEGSTKG) (23). Next, a G4S linker (GGGGS) followed by anti-CD19 scFv (in the same VL/VH orientation and same ARL linker) was cloned and then a downstream 7 amino acid EASGGPE linker. The linker was followed by PE38 (360 aa) with its c-terminal REDLK replaced with the ER retention sequence KDEL, and finally, a NotI restriction site at the 3′ end. The resultant 2650 bp EcoRI/NotI fragment gene was spliced into the pET21d bacteria expression vector under control of an isopropyl-b-D-thiogalactopyranoside (IPTG) inducible T7 promoter. DNA sequencing analysis (Biomedical Genomics Center, University of Minnesota) was used to verify that the gene was correct in sequence and cloned in frame. To create a mutated 2219ARLKDEL molecule (2219ARLKDEL7mut) with decreased immunogenicity, 8 amino acids representing the 7 major epitopes on PE38 KDEL were mutated using the QuickChange Multi Site-Directed Mutagenesis Kit (Stratagene. La Jolla CA, USA) and were confirmed by DNA sequencing. The following immunogenic, hydrophilic amino acids were altered: R490A, R513A, R467A, E548S, K590S, R432G, Q332S, R313A (9, 10). An irrelevant control Bic3, recognizing human T cells, was synthesized by fusing two repeating scFvs recognizing human CD3epsilon to DT390 (24). CD3CD3KDEL was another control immunotoxin in which the same anti-CD3 scFvs were partially humanized and then fused to PE38. Both of these killed the CD3+ HPBMLT T leukemia cell line and not the CD− Raji line so they were used interchangeably. Control anti-B cell DT2219ARL was previously reported and contains the exact same 2219 targeting ligands (21).
Figure 1.

Construction of the plasmid containing the 2219KDEL7mut gene. The gene is described in the methods section. The PyMol graphic was generated by downloading the Protein Data Bank (http://www.rcsb.org/pdb/home/) x-ray crystallographic structure of PE (file identification number: 1KLT) into the PyMol 3D molecular modeling program (http://www.pymol.org/). Shown is a frontal view of PE38KDEL and a 180° reverse orientation of the molecule. The 8 surface immunogenic amino acids that were mutated are darkened for enhanced visualization.
Inclusion body isolation, Refolding and purification
Plasmid was transformed into the Escherichia coli strain BL21(DE3)(EMD, Madison WI). Bacteria were grown in 800 ml Luria Broth supplemented with 100 ug/ml carbenicillin in a 2L flask at 37°C with shaking. Expression of the hybrid gene was induced by the addition of isopropyl-b-D-thiogalactopyranoside (IPTG, FisherBiotech Fair Lawn, NJ). Two hours after induction, the bacteria were harvested by centrifugation. The cell pellets were suspended and homogenized using a polytron homogenizer. After sonication and centrifugation, the pellets were extracted with 0.3% sodium Deoxycholate, 5% Triton X-100, 10% Glycerin, 50 mM Tris, 50 mM NaCl, 5 mM EDTA, pH8.0 and washed.
The proteins were refolded using a sodium N-lauroyl-sarcosine (SLS) air oxidation method modified from a previously reported procedure for isolating sFv (24). Refolded 2219KDEL 7mut was purified by FPLC ion exchange chromatography (Q Sepharose Fast Flow, Sigma, St. Louis, MO) using step gradient of 0.3 M NaCl in 20 mM Tris-HCl, pH 9.0 over 4 column volumes followed by gelfiltration chromatography (superdex 200, GE).
Antibodies and cells
The anti-CD19 monoclonal antibodyx (MoAb) hybridoma HD37 that secretes mouse IgG1 kappa has been previously described by Dorken et al. (25) and has been studied as a targeted toxin conjugated to ricin toxin A chain (26). RFB4 (anti-CD22) was generously provided by Dr. Ellen Vitetta, University of Texas Southwestern Medical Center, Dallas TX. The scFv used for 2219KDEL were derived from these Moab. Anti-Ly5.2, a rat IgG2a from clone A20-1.7, generously provided by Dr. Uli Hammerling, Sloan Kettering Cancer Research Center, New York, NY. Anti-Ly5.2 was used as a control since it recognized mouse CD45.1, a hematopoietic cell surface marker not expressed on human cells.
Human cell lines included the CD19−CD22− T cell leukemia HPBMLT (27) and the CD22+19+ Burkitt’s lymphoma Raji (28). Raji was stably transfected with vector containing the firefly luciferase (Luc) and green fluorescent protein (GFP) genes, as well as a blastocidin resistance gene (Clontech Laboratories, Mountain View California, USA). Transfection was performed with Lipofectamine reagent (Invitrogen, Carlsbad California, USA) and stable clones were established using a FACSDiva flow cytometer (University of Minnesota Flow Cytometry Core Facility of the Masonic Cancer Center) to seed individual GFP-positive cells into a 96-well plate. A subclone was chosen that retained identical morphological and biological properties to the specific parental cell line. The CD22+19+ Burkitt’s lymphoma cell line Daudi and the REH B-ALL cell line were also used (29). Lines were obtained from the American Type Tissue Culture Collection (Rockville MD). Patient enriched peripheral blood mononuclear cells were obtained under IRB approval without identifiers and according to HIPAA rules to protect privacy of personal health information.
Bioassays
To determine the effect of 2219KDEL7mut on normal B and malignant B cell function, Raji, Daudi, and REH were used. Flow cytometry shows that they are >90% positive for both CD19 expression and CD22 expression (data not shown). Cells (2 ×104) were plated in a 96-well flat-bottom plate in RPMI supplemented with 10% fetal bovine serum, 2mM L-glutamine, 100 U/ml penicillin, 100 ug/ml streptomycin. Immunotoxin in varying concentrations was added to triplicate wells containing cells. The plates were incubated at 37°C, 5% CO2 for 72 hr. Cells were then incubated with one μCi [methyl-3H]-thymidine (GE Healthcare, UK) per well for eight hours and harvested onto glass fiber filters, washed, dried and counted for ten minutes in a standard scintillation counter. Data were analyzed using Prism 4 (GraphPad Software, Inc) and were presented as “percent control response” calculated by dividing the cpm of untreated cells by the cpm of the immunotoxin-treated cells (× 100).
Blocking studies were conducted to test specificity. Briefly, 0.05, 0.5, 5, or 50 nM RFB4 or HD37 were added to media containing 1 nM 2219KDEL7mut. Resulting mixtures were added to wells containing Daudi cells and proliferation was measured by 3H-thymidine uptake as described. The mouse specific anti-Ly 5.2 was studied as a negative control. Data were presented as percent control response.
Flow Cytometry
BLTs or Moab were conjugated to fluorescein isothiocyanate (FITC) for direct immunofluorescence studies using flow cytometry as previously reported (4). Cells were incubated with FITC-labeled MAbs, washed, and then resuspended for analysis. Irrelevant control 3A1e-FITC was used to determine the degree of background binding. Flow cytometry was performed on a FACScalibur (Becton Dickinson, Mountain View, CA). A minimum of 10,000 viable cells were analyzed.
In vivo efficacy studies
Male scid mice were purchased from the National Cancer Institute, Frederick Cancer Research and Development Center, Animal Production Area and housed in an AAALAC-accredited specific pathogen-free facility under the care of the Department of Research Animal Resources, University of Minnesota. Animal research protocols were approved by the University of Minnesota Institutional Animal Care and Use Committee.
In order to test the efficacy of 2219KDEL7mut against metastatic B cell cancer Raji-luc cells were injected intravenously (caudal vein) resulting in systemic metastatic cancer. Mice given Raji-luc cells on day 0 were treated with multiple IP injections of BLT. Mice were imaged in real time and images were captured using Xenogen Ivis imaging system (Xenogen Corporation, Hopkington MA) and analyzed IGOR Pro 4.09a software (WaveMetrics, Inc., Portland, OR). Prior to imaging, mice were anesthetized using isoflorane gas. All mice received 100 μl of a 30 mg/ml D-luciferin aqueous solution (Gold Biotechnology, St. Louis MO) as a substrate for luciferase 10 minutes before imaging. All images represent a 5 minute exposure time and all regions of interest (ROI) are expressed in units of photons/sec/cm2/sr.
Mice were given 4 weekly cycles of IP treatment. A single cycle was 3 QOD IP injections (MWF). Cycles of treatment were begun on day 3, 17, 31, and 45 after Raji-luc inoculation. Thus, mice received a total of 12 injections.
Detection of serum IgG anti-toxin content using ELISA Assay
Our assay to detect IgG anti-toxin antibodies was previously reported (7)
Briefly, immunocompetent normal C57/BL/6 mice (NCI) were immunized with weekly injections of 0.25 ug non-mutated 2219KDEL or mutated 2219KDEL 7 mut. After 5 injections, serum was collected 4 days after the final injection. A standard ELISA assay was used in which recombinant PE38KDEL was adhered to the plate. Test serum from the immunized mice was then added followed by the detection antibody, anti-mouse IgG peroxidase (Sigma). Plates were developed with o-phenylenediamine dihydrochloride (Pierce Biotechnology, Rockford, IL) for 15 minutes at room temperature. The reaction was stopped with the addition of 2.5 M H2SO4. Absorbance was read at 490 nm and the final concentration was determined from a standard curve using highly purified anti-PE38KDEL. All samples and standards were tested in triplicate.
To show neutralization, test serum samples were evaluated for their ability to block the ability of 0.2 nM 2219KDEL to kill Raji cells. BLT was added to test serum and then the mixture added to the cells, and then incubated 72 hours. Thymidine uptake was determined as described above.
Statistical analyses
All statistical analyses of in vivo data were performed using Prism 4 (Graphpad Inc, San Diego CA). Groupwise comparisons of mean data were made by Student’s t-test. Probability (p) values < 0.05 were considered significant.
RESULTS
2219KDEL7mut
Figure 1B shows 2219KDEL7mut construction and a PyMol depiction of x-ray crystallographic structure of PE38KDEL in both front and back (180°) positions. The 8 mutated amino acids are darkened so their surface position on the molecule can be readily visualized. Figure 2A shows the final SDS-PAGE gel analysis of 95 kDa 2219KDEL7mut with a purity of greater than 95% as determined by Coomassie blue staining (photo is grayscale). The procedure was previously reported (4). Molecular weight size of 2219KDEL is estimated at 97.9 kDa from molecular weight standards.
Figure 2.
2219 BLT. A) SDS-PAGE gel analysis. Lane 1 – Bispecific 2219KDEL, Lane 2 – Monospecific 22KDEL, Lane 3 – Monospecific 19KDEL, Lane 4 - PE38, Lane 5 - HD37 monoclonal antibody. The gel was stained using Coomassie blue. Photo is grayscale. B) Raji cells were cultured with the BLT and its monomeric counterparts and proliferation was measured by uptake of tritiated thymidine. Data are percentage of control response where control response is untreated cells. Data are expressed as mean + standard deviation (SD). The mean values of untreated cells were 82,048 ± 13,192 cpm/20,000 cells. C.) To determine the intactness of the anti-CD19 sFv and the anti-CD22 sFv ligands, proliferation studies were performed in which Daudi cells were treated with a constant concentration of 1 nM 2219KDEL 7mut and then blocked with increasing concentrations of HD37 monoclonal antibody, RFB4 monoclonal antibody, or nonreactive control Ly5.2 antibody. Thymidine uptake was then measured. Each line represents the mean of triplicate determinations + standard deviation (SD). Activity was calculated in comparison to the unblocked control and then graphed. Counts for untreated Daudi cells were 111,802 ± 13,786 cpm/20,000 cells. D) To determine the activity of different batches of 2219KDEL in comparison to two different batches of DT2219ARL, proliferation studies were performed in which the various batches were incubated with REH B leukemia cells. Control counts= 65,368 ± 6,621 cpm/20,000 cells.
In vitro Killing of the Raji Cancer Cell Line
Raji, Daudi, and REH were selected as a target cell lines in these studies because flow cytometry studies showed high levels of both CD19 and CD22 expression and the cell lines demonstrated aggressive metastatic growth in scid mice. To determine the ability of BLT to kill Raji, the drugs were tested in proliferation assays and a representative experiment is shown in Figure 2B. Bispecific 2219KDEL was able to kill with an IC50 of 0.04 nM and was 150-fold more effective than CD19KDEL and 7.5-fold more effective than CD22KDEL. Irrelevant control CD3CD3KDEL was minimally inhibitory. Next, proliferation experiments were performed in which increasing amounts of blocking antibody were added to a constant inhibitory concentration of 1 nM 2219KDEL7mut which inhibited Daudi cell proliferation in Figure 2C. Increasing concentrations of RFB4 or HD37 reversed the inhibition of 2219KDEL7mut in a dose-dependent manner. The addition of an irrelevant control antibody anti-Ly5.2 had no effect. Neither antibody blocked 100% of the activity because blocking one ligand would not necessarily fully block the other. In Figure 2C, the killing capability of 2219KDEL and the ability of DT2219ARL were compared in proliferation assays against a third cell line, REH leukemia. This is because DT2219ARL is identical to 2219KDEL but contains DT390 instead of PE and is currently in phase 1 trials (21). When two different batch lots of 2219KDEL were compared to two different batch lots of DT2219ARL, the 2219KDEL was about a log more potent. Again, control Bic3 was minimally inhibitory. Trypan blue viability assays were performed in addition to proliferation assays and as an additional check to verify that drug was indeed killing and not simply inhibiting cell proliferation (not shown).
Together, these findings indicated that bispecific 2219KDEL had superior potency to its monospecific counterparts and killing was selective. Importantly, 2219KDEL was more potent than DT2219ARL and the drug was highly effective against two different B cell lymphoma lines, Raji and Daudi, and one B cell leukemia line REH.
Studies of patient cells
To determine whether 2219KDEL reacted against patient cells, peripheral blood blasts were collected from a B cell ALL patient and then reacted directly with 100 nM FITC labeled BLT. 2219KDEL-FITC showed 84.3 positive cells, whereas control Bic3-FITC showed a background of 19.8% positive cells indicating that patient cells were indeed positive for the drug (not shown).
To study CD22 and CD19 expression on patients with various B cell malignancies, patient cells were stained with either anti-CD22 (RFB4)-FITC or anti-CD19 (HD37-FITC). Table 1 shows that T-ALL and AML cases were mostly negative, while B cell malignancies including B-ALL, B-CLL, and lymphoma were positive. Since peripheral blood B cells are known to be in 6-8% range, our control cases stained appropriately at an average of 6.3% CD19 and 7.4% anti-CD22. Importantly, all B cell cases stained for both markers and would likely benefit from treatment with our BLT. Also, CD19 expression was usually higher than CD22 expression.
Table 1.
CD22 and CD19 Expression on Patient Cells with Various Hematological Malignancies
| Normal Periph. Blood (6 volunteers) | CLL (6 patients) | AML (6 patients) | |||
|---|---|---|---|---|---|
| CD19 | CD22 | CD19 | CD22 | CD19 | CD22 |
| 4.2 | 7.2 | 76.2 | 38.5 | 3.8 | 5.0 |
| 6.6 | 8.7 | 89.1 | 90.9 | 0.6 | 0.4 |
| 7.3 | 7.3 | 66.6 | 58.1 | 0.4 | 1.0 |
| 6.4 | 7.2 | 38.9 | 27.5 | 1.8 | 3.6 |
| 9.2 | 9.3 | 87.7 | 78.7 | 1.6 | 9.9 |
| 4.1 | 4.4 | 80.5 | 75.7 | 0.4 | 0.4 |
| Average | |||||
| 6.3 | 7.4 | 73.2 | 61.6 | 1.4 | 3.4 |
| B-ALL (5 patients) | Lymphoma (12 patients) | T-ALL (2 patients) | |||
|---|---|---|---|---|---|
| CD19 | CD22 | CD19 | CD22 | CD19 | CD22 |
| 27.1 | 20.6 | 16.1 | 13.0 | 3.7 | 4.2 |
| 88.4 | 76.5 | 81.3 | 50.2 | 3.5 | 3.6 |
| 100.0 | 64.2 | 18.4 | 19.0 | ||
| 86.5 | 5.3 | 23.2 | 21.0 | ||
| 93.2 | 97.1 | 23.7 | 23.0 | ||
| 46.4 | 35.8 | ||||
| 98.7 | 83.0 | ||||
| 68.3 | 70.0 | ||||
| 37.4 | 6.9 | ||||
| 89.1 | 13.9 | ||||
| 69.6 | 46.6 | ||||
| 52.5 | 50.3 | ||||
| Average | |||||
| 79.0 | 52.7 | 52.1 | 36.1 | 3.6 | 3.9 |
Cell surface expression of either anti-CD22 or CD19 was determined by standard immunofluorescence with either RFB4-FITC or HD37-FITC. Cells were obtained from C-ALL, B-ALL, B lymphoma, control T-ALL, or control AML patients with Institutional Review Board approval as described in the methods. Data are calculated as percent positive cells relative to a known negative control. Normal enriched peripheral blood cells were obtained from healthy volunteers. Data points were averaged for each group.
“Reduced immunogenicity” 2219KDEL7mut
In order to be able to give multiple treatment to sustain an anti-tumor effect, PE38KDEL was mutated as shown in Figure 1. Evaluation of 2219KDEL7mut must proceed in 3 steps. 1) determining whether mutated 2219KDEL had lost any activity compared to parental 2219KDEL 2) measuring the ability of 2219KDEL7mut compared to non-muted parental 2219KDEL to induce anti-toxin antibodies in immunocompetent mice. 3) determining whether 2219KDEL7mut had the ability to prevent cancer metastasis in vivo. When 3 different mutated batches of 2219KDEL7mut were compared to non-mutated 2219KDEL, Figure 3A shows mutagenesis resulted in no reduction in the in vitro killing of Daudi cells. Normal immunocompetent mice were immunized with weekly doses of 2219KDEL 7mut and blood serum was collected 4 days after each immunization. Using an ELISA assay that measures the amount of anti-PE antibody based on a standard curve of highly purified mouse anti-PE, Figure 3B shows a significant (p<0.05) decline in the level of anti PE-antibody in mice immunized with 2219KDEL7mut compared to mice immunized with parental 2219KDEL (n=5/group). Mutagenesis resulted in a reduction of antibody production of about 80% on day 76 after 9 immunizations. In order to determine whether the anti-toxin antibodies were neutralizing, serum from the 2219KDEL and 2219KDEL7mut immunized mice were incubated with a constant inhibitory concentration of 0.2 nM 2219KDEL in proliferation assays. Figure 3C shows that serum taken from 3 individual mice immunized with 2219KDEL7mut on day 118 did not block 2219KDEL activity. Sera from 2 mice immunized with 2219KDEL that displayed high titers of anti-toxin antibody neutralized 2219KDEL activity. Together, data show that serum from mice immunized with multiple injections of 2219KDEL 7mut has 80% less anti-toxin antibodies than mice immunized with non-mutated parental 2219KDEL and this correlated with a reduction in neutralizing antibodies.
Figure 3.
2219KDEL 7mut has reduced immunogenicity, but not reduced activity. A) To determine the effect of 2219KDEL7mut on Daudi cells, increasing concentrations of 2219KDEL7mut were compared to non-mutated 2219KDEL in a proliferation assay. Counts for untreated Daudi cells were 68,613 ± 4,535 cpm/20,000 cells. Data are presented as % control response. B) To detect anti-toxin antibodies, immuncompetent C57BL/6 mice were immunized with either non-mutated parental EGF4KDEL or mutated EGF4KDEL. Serums from individual mice were analyzed in a modified ELISA measuring ug/ml anti-toxin IgG. Data were represented as the average ug IgG/ml. The two groups significantly differed (p<0.05) C) To determine whether neutralizing antibodies were present, serum from the immunized mice were tested for their ability to neutralize a constant inhibitory concentration of 2219KDEL. Data are depicted as percent control response. SAL=Serum anti-toxin levels. D) To determine whether 2219KDEL7mut was efficacious, scid mice given a lethal injection of Raji-luc cells were treated with 3 courses of 2219KDEL7mut or control CD3CD3KDEL beginning on day 3. Pooled data (experiments 1 and 2) are shown as a Kaplan-Meier plot.
Effects of 2219KDEL7mut in SCID mice with systemic cancer
Injection of the Raji-luc cells intravenously into scid mice results in a systemic tumor that infiltrates all major organs and is reminiscent of human B cell malignancy. The Raji-luc model offers a major advantage since imaging can be performed on individual animals weekly to monitor tumor progression in real time. Malignant progression measured by imaging correlates with histologic progression and mortality in the model.
To determine if 2219KDEL7mut was effective against established systemic cancer, Raji-luc cells were injected iv in mice and ip treatments were started on day 3. The overall pooled survival of all treated mice (experiments 1 and 2) is shown in Figure 3D. The Kaplan-Meier plot shows the long-term results after 4 courses of qod ip treatment with 2219KDEL7mut. The plot shows 7 of 9 animals surviving greater than 225 days post treatment. There were two late unexplained deaths on days 125 and 150 that occurred without weight loss (toxicity or tumor relapse).
Figure 4 shows detailed imaging and aggressiveness of the model. In Figure 4A, 25 days after injection with 106 cells, organs were removed and imaged for tumor presence. High Raji-luc signal levels were detected in the lymph node, ovaries, lung and brain, pancreas, and intestines of mouse 1 (photo 1-3). Low levels were measured in the heart and kidneys (not shown). A tumor with a photon count of 2.6 × 108 photons/sec/cm2/sr is shown in the uterus of a second mouse (photo 4). Thus, we estimate that 2.6 × 108 photons/sec/cm2/sr roughly correlates with a tumor size of 0.75 cm3.
Figure 4.

Efficacy of 2219KDEL7mut in vivo. (A) To determine the metastatic ability of the Raji-luc lymphoma, scid mice were injected iv with Raji-luc. a representative mouse was organ imaged on day 25 following Raji-luc injection on day 0. Two images are shown for each organ. The image on the left is with bioluminescent imaging and on the right, the same organ without bioluminescent imaging. Bioluminescence intensity is shown as a function of photons/sec/sr/cm. B) Bioluminescent imaging is shown for Experiment 1 in which scid mice were given Raji-luc cells iv. Mice M1-M3 were treated IP with 3 courses of 2219KDEL7mut, while mice M4-M6 were untreated. Mice M7-M9 received treatment with the identical dose and schedule, but with anti-T cell Bic3 instead. C) Experiment 2 was done exactly as experiment 1 only using more animals. Mice M10-M15 were treated with 2219KDEL7mut and M16-M20 were not treated. HLP-hind limb paralysis.
In experiment 1 (Figure 4B), tumor progressed quickly since it was detected in all untreated mice by day 10 and all untreated mice developed hind limb paralysis on days 25 through 31. Animals injected with Raji-luc develop this CNS complication with a 100% incidence. All three 2219KDEL7mut-treated mice were completely tumor free on day 166. Animals treated with Bic3 as an irrelevant control drug developed cancer at the same rapid rate as the untreated controls indicating that the activity of 2219KDEL7mut was specific. Experiment 1 was repeated with more mice in experiment 2 (Figure 2C). Mice (n=6/group) were given Raji-luc and then treated with 2219KDEL7mut. All of these mice were long term disease-free survivors, some surviving over 225 days. Untreated controls rapidly developed tumor as before.
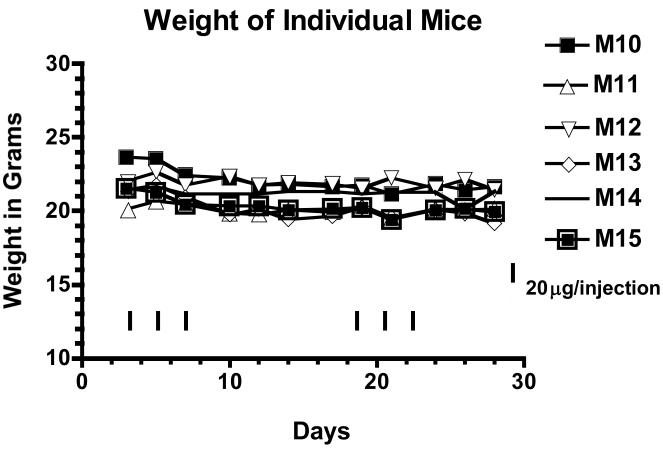
Figure 5 shows that treatment induced minimal weight loss since 2219KDEL7mut-treated mice (M10-M15) lost less than ten percent of their body weight or did not lose any weight at all after the first treatment course. The same was true after the second treatment course.
Figure 5.
Toxicity of 2219KDEL7mut. The individual weights of 2219KDEL7mut treated mice (M10-M15) from experiment 2 were plotted over time. The black arrows indicate the days of treatment with 20ug 2219KDEL7mut.
Together, these data show that the reduced immunogenicity variant 2219KDEL7mut shows a high level of in vivo activity and retained its powerful anti-cancer activity resulting in long-term disease free survivors at dosages that are not toxic even though animals were treated multiple times.
DISCUSSION
If targeted toxins synthesized with bacterial toxins are to be effective for therapy, then a means must be found to reduce their immunogenicity so that multiple treatments can be given without neutralization by patient anti-toxin antibodies. Diphtheria toxin may be the most problematic based on lifetime immunization. For example, in phase III clinical trial of ONTAK (DT389-IL-2), by the end of two courses, 59 of 60 patients had developed anti-toxin antibodies and 32% of the patients had detectable levels of anti-DT antibodies before treatment even had begun (22). The use of pseudomonas toxin has not greatly improved the situation. In a phase 1 trial of anti-mesothelin-PE38 monospecific immunotoxin, 88% developed neutralizing antibodies by day 29 of cycle 1 therapy and were not eligible for retreatment (30). Other approaches for reducing immunogenicity have been investigated including RNAase (31), the use of scFvs (32), alternative toxins such as saporin, (16), and PEGylation (33). After years of testing, none of these have reached the mainstream as viable possibilities for reduced immunogenicity drug.
One logical alternative approach would be the genetic alteration of the toxin gene in order to eliminate peptide regions that interact with T and/or B cells to generate anti-toxin responses. A major concern is that deletion of oligopeptide sequences would alter enzyme conformation enough to inactivate the catalytic active site. Recently, Onda and Pastan reported a potential solution and introduced point mutations in key eptitopic regions (9,10). This approach would only work if immunogenic amino acids could be identified in limited areas and if mutation did not involve excision of peptides that would dramatically alter tertiary structure. A large library of anti-PE (Pseudomonas exotoxin A) monoclonal antibodies was used to map the immunogenic epitopes of PE toxin and seven major epitopes were located on the toxin recognized by B cells (9). Eight amino acids were removed in the 7 regions without compromising toxin activity. The resulting toxin had reduced immune activity and could be used to synthesize immunotoxins with reduced activity (10).
Studies reveal that simultaneously targeting CD22 and CD19 with BLT results in higher levels of killing perhaps due to binding more drug on the cell surface and/or better drug internalization (especially when two highly internalizing markers such as CD22 and CD19 are targeted) (3, 21). The fact that the BLT was more effective than a mixture of its monospecific components indicate the importance of having both ligands on the same single chain molecule. DT2219 is the first BLT that has reached phase 1 clinical trials and has been well-tolerated, but adequate dose levels have not yet been achieved. The synthesis of 2219KDEL7mut, reported herein, combined the BLT discovery with “deimmunized” toxin.
Studies in mice with deimmunized 2219KDEL 7mut showed that immunogenicity was reduced at least 80%, while eliminating the progression of a highly aggressive metastatic human B cell lymphoma in a luciferase reporter gene model that permitted the assessment of systemic tumor development in real time with minimal toxicity. A key question is: Is the mouse an acceptable model for the immunogenicity of PE? Onda and Pastan determined whether treatment with PE immunotoxins induces human antibody responses to the same immunogineic epitopes recognized by mice (34). Sera was taken from 8 patients in phase 1 trials with pancreatic, colon cancer, or mesothelioma treated with two different PE immunotoxins LMB-9 (anti-CD25 PE) (35) or SS1P (anti-mesothelin PE) (8). These patients had developed neutralizing antibodies that prevented further drug administration. Serum samples were analyzed in a published competition assay (9). Most patients with solid tumors produced neutralizing Abs after one cycle of immunotoxin treatment. Competition analysis of paired serum samples showed that before treatment, the sera contained almost no specific Ab to any of the PE38 epitopes. In contrast, the sera obtained after immunotoxin treatment contained anti-PE38 Abs to every topographical epitope recognized the mouse antisera as shown by their ability to inhibit the binding of the corresponding mAbs to each epitope. These results show that human immunotoxin treatment induces human Abs against the same 7 immunogenic epitopes identified by the mouse mAb panel and affirms that the mouse is an acceptable immunogenicity model for humans.
The anti-CD19 and anti-CD22 scFvs of 2219KDEL 7mut are of mouse origin and so they are still immunogenic, although investigators do believe that the majority of the immune response is directed against the bacterial toxin, rather than the scFv (34). This likely relates to the higher homology (about 90%) between mouse and human scFv and the aggressive response of the human immune system against foreign bacterial proteins. Still, in order eliminate any HAMA, we are currently veneering the scFv, thereby converting any mouse aa to human aa that do not compromise the activity of the molecule.
The new “reduced immunogenicity” form of 2219KDEL7mut demonstrated the same in vitro killing ability as non-muted parental 2219KDEL and about a log greater activity than DT2219ARL. This finding is likely attributed to the inclusion of amino acids KDEL at the c-terminus of PE in place of REDLK. The presence of KDEL promotes the specific transport of proteins from the Golgi to the ER. KDEL is recognized within the lumen of the Golgi by its receptor, Erd2p and retrograde transport occurs via Erd2p in Golgi-derived coat protein 1(COPI) vesicles (36,37,38). Recently, investigators showed (with MHC class I as a model) that an additional function of KDEL is to mediate recycling of chaperones escaping from the ER to the cis-Golgi intermediate compartment or the cis-Golgi (39). Thus, terminal KDEL can be viewed as locking the protein into a transport loop favoring cytosolic dislocation and thereby EF-2 target access. Other genetic modifications were employed in the development of 2219KDEL 7mut including VH/VL reversal, inclusion of ARL linkers, and hot spot mutagenesis to enhance affinity (3, 40).
Monospecific HL22, an anti-CD22scFv spliced to truncated PE, has proven strikingly effective against Hairy Cell Leukemia (20) but not against other B cell malignancies. This likely relates to the high level of CD22 expression on patient cells. In our studies, Table 1 examines 23 patients with B cell malignancies and shows at least three patient categories 1) those containing high levels of both CD22 and CD19 blasts, 2) those containing lower levels of both CD22 and CD19 blasts 3) and those containing one marker at a higher level and the other at a lower level. Although this study did not directly measure the expression of CD22 and CD19 on the same cell, the large subgroup of patients with high expression of both markers indicate that a large cohort simultaneously expresses both markers. Targeting with dual ligand BLT could benefit all of these since superior levels of kill are achieved when targeting both markers simultaneously.
Our observation that BLTs are superior is not unique to hematopoietic cancer. The BLT framework also benefits solid tumor targeting. For example, a BLT assembled with scFvs simultaneously targeting EpCAM and Her2/neu had superior activity compared to its monomeric counterparts in a colorectal cancer model (4). Also, BLT constructed with dual cytokines EGF and IL-4, and deimmunized PE showed ability to inhibit the growth of established aggressive systemic breast cancer in mice even after waiting 26 days to begin therapy (7) and was effective against mesothelioma (6).
Eighty percent of the serum IgG anti-toxin antibody level was reduced by immunizing immunocompetent mice with deimmunized drug. Studies also showed in Figure 3C that neutralizing antibodies were reduced. Although we cannot distinguish neutralizing antibodies from non-neutralizing antibodies, it is just as important to reduce non-neutralizing antibodies since they still form drug antigen-anitbody complexes and enhance drug clearance.
In summary, “low immunogenic” BLT were synthesized that have potent anti-B cell cancer effects. These drugs are important alternative cancer therapies. One major advantage of targeted toxins is that they have a unique mechanism of action (protein synthesis inhibition) that differs from the mechanism of conventional chemotherapeutic drugs. A common problem with continually relapsing patients is that they will reach the limits of chemotherapy either because the drugs have become ineffective or because patients reach their toxic thresholds. Dangerously low blood counts are a common reason for discontinuing chemotherapy regimens. We are describing a new alternative drug with reduced immunogenicity and increased potency that can be used in place of chemotherapy. The relevancy of targeted toxins has been rightfully criticized because of their diminished potency, ability to reach tumor, and immunogenicity. However, genetic engineering has been used to address these issues and 2219KDEL7mut stands out in its ability to inhibit the metastatic progression of highly aggressive B cell lymphoma with reduced immunogenicity. Our group has recently reported other BLT that likewise show similar benefits for the treatment of other types of cancer (5-7, 41, 42).
ACKNOWLEDGEMENTS
This work was supported in part by the US Public Health Service Grants RO1-CA36725 and RO1-CA082154 awarded by the NCI and the NIAID, DHHS and Children’s Cancer Research Fund, the Lion’s Children’s Cancer Fund, and the William Lawrence and Blanche Hughes Fund.
Abbreviations
- 2219KDEL7mut
deimmunized pseudomonas exotoxin fused to anti-CD22 scFv and anti-CD19 scFv
- aa
amino acid
- ALL
acute lymphoblastic leukemia
- Ab
antibody
- B-ALL
B cell acute lymphoblastic leukemia
- B-CLL
B cell chronic lymphoblastic leukemia
- Bic3
DT390 fused to two anti-CD3 sFV
- BLT
bispecific ligand-directed toxin
- CD3
cluster determinant 3
- CD19
cluster determinant 19
- CD22
cluster determinant 22
- DT390
truncated diphtheria toxin
- EF-2
elongation factor 2
- ER
endoplasmic reticulum
- FITC
fluorescein isothiocyanate
- HAMA
human anti-mouse antibodies
- HIPAA
Health Insurance Portability and Accountability Act
- HLP
hind limb paralysis
- IC50
concentration of drug required for 50% inhibition
- IP
intraperitoneal
- IT
immunotoxin
- KDEL
amino acid sequence Lys-Asp-Glu-Leu
- mAb
monoclonal antibody
- PE
pseudomonas exotoxin
- photons/sec/cm2/sr
photons per second per square centimeter per steradian
- QOD
every other day (MWF)
- scFv
recombinant single chain VH and VL domain
- SLS
sodium N-lauryl-sarcosine
Footnotes
The authors have no conflicts of interest.
REFERENCES
- 1.List AF. Role of multidrug resistance and its pharmacological modulation in acute myeloid leukemia. Leukemia. 1996;10:937–942. [PubMed] [Google Scholar]
- 2.Kreitman RJ. Recombinant fusion toxins for cancer treatment. Expert Opin Biol Ther. 2002;2:785–91. doi: 10.1517/14712598.2.8.785. [DOI] [PubMed] [Google Scholar]
- 3.Vallera DA, Todhunter DA, Kuroki DW, Shu Y, Sicheneder A, Chen H. A bispecific recombinant immunotoxin, DT2219, targeting human CD19 and CD22 receptors in a mouse xenograft model of B-cell leukemia/lymphoma. Clin Cancer Res. 2005;11:3879–88. doi: 10.1158/1078-0432.CCR-04-2290. [DOI] [PubMed] [Google Scholar]
- 4.Stish BJ, Chen H, Shu Y, Panoskaltsis-Mortari A, Vallera DA. Increasing anticarcinoma activity of an anti-erbB2 recombinant immunotoxin by the addition of an anti-EpCAM sFv. Clin Cancer Res. 2007;13:3058–67. doi: 10.1158/1078-0432.CCR-06-2454. [DOI] [PubMed] [Google Scholar]
- 5.Oh S, Stish BJ, Vickers SM, Buchsbaum BJ, Vallera DA. A new drug delivery method of bispecific ligand-directed toxins that reduces toxicity and promotes efficacy in a model of orthotopic pancreatic cancer. Pancreas. 2010 doi: 10.1097/MPA.0b013e3181cbd908. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stish BJ, Oh S, Chen H, Dudek AZ, Kratzke RA, Vallera DA. Design and modification of EGF4KDEL 7Mut, a novel bispecific ligand-directed toxin, with decreased immunogenicity and potent anti-mesothelioma activity. Br J Cancer. 2009;101:1114–23. doi: 10.1038/sj.bjc.6605297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh S, Stish BJ, Sachdev D, Chen C, Dudek AZ, Vallera DA. A novel “reduced immunogenicity” bispecific targeted toxin simultaneously recognizing human EGF and IL-4 receptors in a mouse model of metastatic breast carcinoma. Clin Cancer Res. 2009;15:6137–47. doi: 10.1158/1078-0432.CCR-09-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassan R, Bullock S, Premkumar A, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian and pancreatic cancers. Clin. Cancer Res. 2007;13:5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 9.Onda M, Nagata S, FitzGerald DJ, et al. Characterization of the B cell epitopes associated with a truncated form of Pseudomonas exotoxin (PE38) used to make immunotoxins for the treatment of cancer patients. J Immunol. 2006;177:8822–34. doi: 10.4049/jimmunol.177.12.8822. [DOI] [PubMed] [Google Scholar]
- 10.Onda M, Beers R, Xiang L, Nagata S, Wang QC, Pastan I. An immunotoxin with greatly reduced immunogenicity by identification and removal of B cell epitopes. Proc Natl Acad Sci USA. 2008;105:11311–6. doi: 10.1073/pnas.0804851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudhary VK, Queen C, Junghans RP, Waldmann TA, FitzGerald DJ, Pastan I. A recombinant immunotoxin consisting of two antibody variable domains fused to Pseudomonas exotoxin. Nature. 1989;339:394–7. doi: 10.1038/339394a0. [DOI] [PubMed] [Google Scholar]
- 12.Kreitman RJ, Pastan I. Accumulation of a recombinant immunotoxin in a tumor in vivo: fewer than 1000 molecules per cell are sufficient for complete responses. Cancer Res. 1998;58:968–75. [PubMed] [Google Scholar]
- 13.Munro S, Pelham HR. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- 14.Goulet AC, Goldmacher VS, Lambert JM, Baron C, Roy DC, Kouassi E. Conjugation of blocked ricin to an anti-CD19 monoclonal antibody increases antibody-induced cell calcium mobilization and CD19 internalization. Blood. 1997;90:2364–75. [PubMed] [Google Scholar]
- 15.Stone MJ, Sausville EA, Fay JW, et al. A phase I study of bolus versus continuous infusion of the anti-CD19 immunotoxin, IgG-HD37-dgA, in patients with B-cell lymphoma. Blood. 1996;88:1188–97. [PubMed] [Google Scholar]
- 16.Flavell DJ, Flavell SU, Boehm DA, et al. Preclinical studies with the anti-CD19-saporin immunotoxin BU12-SAPORIN for the treatment of human-B-cell tumours. Br J Cancer. 1995;72:1373–79. doi: 10.1038/bjc.1995.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghetie MA, Picker LJ, Richardson JA, Tucker K, Uhr JW, Vitetta ES. CD19 inhibits the growth of human B-cell tumor lines in vitro and of Daudi cells in SCID mice by inducing cell cycle arrest. Blood. 1994;83:1329–36. [PubMed] [Google Scholar]
- 18.Uckun FM, Gajl-Peczalska KJ, Kersey JH, Houston LL, Vallera DA. Use of a novel colony assay to evaluate the cytotoxicity of an immunotoxin containing pokeweed antiviral protein against blast progenitor cells freshly obtained from patients with common B-lineage acute lymphoblastic leukemia. J Exp Med. 1986;163:347–68. doi: 10.1084/jem.163.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson KC, Bates MP, Slaughenhoupt BL, Pinkus GS, Schlossman SF, Nadler LM. Expression of human B cell-associated antigens on leukemias and lymphomas: a model of human B cell differentiation. Blood. 1984;63:1424–33. [PubMed] [Google Scholar]
- 20.Kreitman RJ, Squires DR, Stetler-Stevenson M, et al. Phase I trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with B-cell malignancies. J Clin Oncol. 2005;23:6719–29. doi: 10.1200/JCO.2005.11.437. [DOI] [PubMed] [Google Scholar]
- 21.Vallera DA, Chen H, Sicheneder AR, Panoskaltsis-Mortari A, Taras EP. Genetic Alteration of a bispecific ligand directed toxin targeting human CD19 and CD22 receptors resulting in improved efficacy against systemic B cell malignancy. Leuk Res. 2009;33:1233–42. doi: 10.1016/j.leukres.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olsen E, Duvic M, Frankel A, et al. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J Clin Oncol. 2001;19:376–88. doi: 10.1200/JCO.2001.19.2.376. [DOI] [PubMed] [Google Scholar]
- 23.Whitlow M, Bell BA, Feng SL, et al. An improved linker for single-chain Fv with reduced aggregation and enhanced proteolytic stability. Protein Eng. 1993;6:989–95. doi: 10.1093/protein/6.8.989. [DOI] [PubMed] [Google Scholar]
- 24.Vallera DA, Todhunter DA, Kuroki DW, et al. Molecular modification of a recombinant, bivalent anti-human CD3 immunotoxin (Bic3) results in reduced in vivo toxicity in mice. Leuk Res. 2005;29:331–41. doi: 10.1016/j.leukres.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Dorken B, Schwarz E, Feller AC, Hammerling G, Hunstein W. Production of monoclonal antibodies for the diagnosis of minimal infiltration of leukemic cells into the bone marrow. B cell specific antibodies. Verh Dtsch Gs Path. 1983;67:65–69. [Google Scholar]
- 26.Stone MJ, Sausville EA, Fay JW, et al. A phase I study of bolus versus continuous infusion of the anti-CD19 immunotoxin, IgG-HD37-dgA, in patients with B-cell lymphoma. Blood. 1996;88:1188–97. [PubMed] [Google Scholar]
- 27.Morikawa S, Tatsumi E, Baba M, Harada T, Yasuhira K. Two E-rosette-forming lymphoid cell lines. Int J Cancer. 1978;21:166–70. doi: 10.1002/ijc.2910210207. [DOI] [PubMed] [Google Scholar]
- 28.Pulvertaft RJV. Cytology of Burkitt’s Tumor. Lancet. 1964;1:238–40. doi: 10.1016/s0140-6736(64)92345-1. [DOI] [PubMed] [Google Scholar]
- 29.Klein E, Klein G, Nadkarni JS, Nadkarni JJ, Wigzell H, Clifford P. Surface IgM-kappa specificity on a Burkitt lymphoma cell in vivo and in derived culture lines. Cancer Res. 1968;28:1300–10. [PubMed] [Google Scholar]
- 30.Hassan R, Bullock S, Premkumar A, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–9. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 31.Schirrmann T, Krauss J, Arndt MA, Rybak SM, Dübel S. Targeted therapeutic RNases (ImmunoRNases) Expert Opin Biol Ther. 2009;9:79–95. doi: 10.1517/14712590802631862. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Cheung LH, Hittelman WN, Rosenblum MG. Targeted delivery of human proapoptotic enzymes to tumor cells: In vitro studies describing a novel class of recombinant highly cytotoxic agents. Mol Cancer Ther. 2003;2:1341–50. [PubMed] [Google Scholar]
- 33.Tsutsumi Y, Onda M, Nagata S, Lee B, Kreitman RJ, Pastan I. Sitespecific chemical modification with polyethylene glycol of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) improves antitumor activity and reduces 34 animal toxicity and immunogenicity. Proc Natl Acad Sci USA. 2000;97:8548–8553. doi: 10.1073/pnas.140210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagata S, Pastan I. Removal of B cell epitopes as a practical approach for reducing the immunogenicity of foreign protein-based therapeutics. Adv Drug Deliv Rev. 2009;61:977–85. doi: 10.1016/j.addr.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powell DJ, Jr, Felipe-Silva A, Merino MJ, et al. Administration of a CD25-directed immunotoxin, LMB-2, to patients with metastatic melanoma induces a selective partial reduction in regulatory T cells in vivo. J Immunol. 2007;179:4919–28. doi: 10.4049/jimmunol.179.7.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sönnichsen B, Watson R, Clausen H, Misteli T, Warren G. Sorting of COPI-coated vesicles under interphase and mitotic conditions. J Cell Biol. 1996;134:1411–25. doi: 10.1083/jcb.134.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orci L, Stamnes M, Ravazzola M, et al. Bidirectional transport by distinct populations of COPI-coated vesicles. Cell. 1997 Jul 25;90(2):335–49. doi: 10.1016/s0092-8674(00)80341-4. [DOI] [PubMed] [Google Scholar]
- 38.Majoul I, Sohn K, Wieland FT, et al. KDEL receptor (Erd2p)-mediated retrograde transport of the cholera toxin A subunit from the Golgi involves COPI, p23, and the COOH terminus of Erd2p. J Cell Biol. 1998;143:601–12. doi: 10.1083/jcb.143.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howe C, Garstka M, Al-Balushi M, et al. Calreticulin-dependent recycling in the early secretory pathway mediates optimal peptide loading of MHC class I molecules. EMBO J. 2009 Dec 2;28:3730–44. doi: 10.1038/emboj.2009.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salvatore G, Beers R, Margulies I, Kreitman RJ, Pastan I. Improved cytotoxic activity toward cell lines and fresh leukemia cells of a mutant anti-CD22 immunotoxin obtained by antibody phage display. Clin Cancer Res. 2002;8:995–1002. [PubMed] [Google Scholar]
- 41.Stish BJ, Chen H, Shu Y, Panoskaltsis-Mortari A, Vallera DA. A bispecific recombinant cytotoxin (DTEGF13) targeting human IL-13 and EGF receptors in a mouse xenograft model of prostate cancer. Clin Cancer Res. 2007;13:6486–93. doi: 10.1158/1078-0432.CCR-07-0938. [DOI] [PubMed] [Google Scholar]
- 42.Oh S, Ohlfest JR, Todhunter DA, et al. Intracranial elimination of human glioblastoma brain tumors in nude rats using the bispecific ligand-directed toxin, DTEGF13 and convection enhanced delivery. J Neurooncol. 2009;95:331–42. doi: 10.1007/s11060-009-9932-2. [DOI] [PubMed] [Google Scholar]