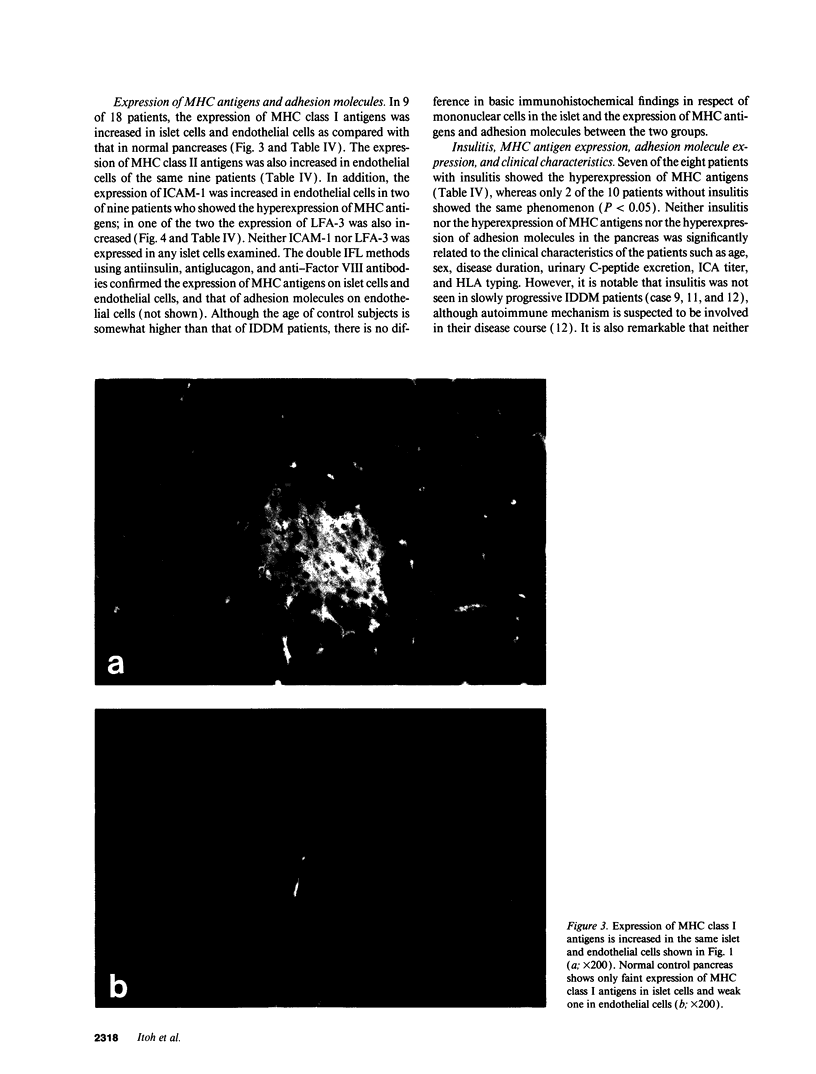
Abstract
We examined pancreas biopsy specimens from 18 newly diagnosed insulin-dependent diabetes mellitus (IDDM) patients to elucidate the mechanism underlying beta cell destruction. Pancreas islets were seen in all patients and insulitis in eight patients. Infiltrating mononuclear cells consisted of CD4+T, CD8+T, B lymphocytes, and macrophages. Among them, CD8+T lymphocytes were predominant and macrophages followed. The expression of MHC class I antigens was increased in islet and endothelial cells in nine patients. MHC class II expression was increased in endothelial cells of the same patients. The expression of intercellular adhesion molecule-1 was increased in endothelial cells in two of the nine patients with MHC hyperexpression; in one of them, lymphocyte function-associated antigen-3 expression was also increased. Out of the eight patients with insulitis, seven showed MHC class I hyper-expression, whereas 2 of the 10 patients without insulitis showed the phenomenon (P < 0.05). The relation between insulitis and the hyperexpression of adhesion molecules was not evident. In conclusion, we revealed the close relation between CD8+T lymphocyte-predominant insulitis and MHC class I hyperexpression in islet cells. This suggests that infiltrating CD8+T lymphocytes recognize islet autoantigens in association with increased MHC class I molecules and act as major effector cells in autoimmune response against islet cells in IDDM pancreases. The role of adhesion molecules in the pathogenesis of IDDM still remains to be elucidated.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedossa P., Bendelac A., Bach J. F., Carnaud C. Syngeneic T cell transfer of diabetes into NOD newborn mice: in situ studies of the autoimmune steps leading to insulin-producing cell destruction. Eur J Immunol. 1989 Oct;19(10):1947–1951. doi: 10.1002/eji.1830191028. [DOI] [PubMed] [Google Scholar]
- Bendelac A., Carnaud C., Boitard C., Bach J. F. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates. Requirement for both L3T4+ and Lyt-2+ T cells. J Exp Med. 1987 Oct 1;166(4):823–832. doi: 10.1084/jem.166.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottazzo G. F., Dean B. M., McNally J. M., MacKay E. H., Swift P. G., Gamble D. R. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med. 1985 Aug 8;313(6):353–360. doi: 10.1056/NEJM198508083130604. [DOI] [PubMed] [Google Scholar]
- Campbell I. L., Cutri A., Wilkinson D., Boyd A. W., Harrison L. C. Intercellular adhesion molecule 1 is induced on isolated endocrine islet cells by cytokines but not by reovirus infection. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4282–4286. doi: 10.1073/pnas.86.11.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson S. W., Shultz L. D., Leiter E. H. Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice. Relative contributions of CD4+ and CD8+ T-cells from diabetic versus prediabetic NOD.NON-Thy-1a donors. Diabetes. 1993 Jan;42(1):44–55. doi: 10.2337/diab.42.1.44. [DOI] [PubMed] [Google Scholar]
- Foulis A. K., Farquharson M. A. Aberrant expression of HLA-DR antigens by insulin-containing beta-cells in recent-onset type I diabetes mellitus. Diabetes. 1986 Nov;35(11):1215–1224. doi: 10.2337/diab.35.11.1215. [DOI] [PubMed] [Google Scholar]
- Foulis A. K., Farquharson M. A., Hardman R. Aberrant expression of class II major histocompatibility complex molecules by B cells and hyperexpression of class I major histocompatibility complex molecules by insulin containing islets in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1987 May;30(5):333–343. doi: 10.1007/BF00299027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulis A. K., Farquharson M. A., Meager A. Immunoreactive alpha-interferon in insulin-secreting beta cells in type 1 diabetes mellitus. Lancet. 1987 Dec 19;2(8573):1423–1427. doi: 10.1016/s0140-6736(87)91128-7. [DOI] [PubMed] [Google Scholar]
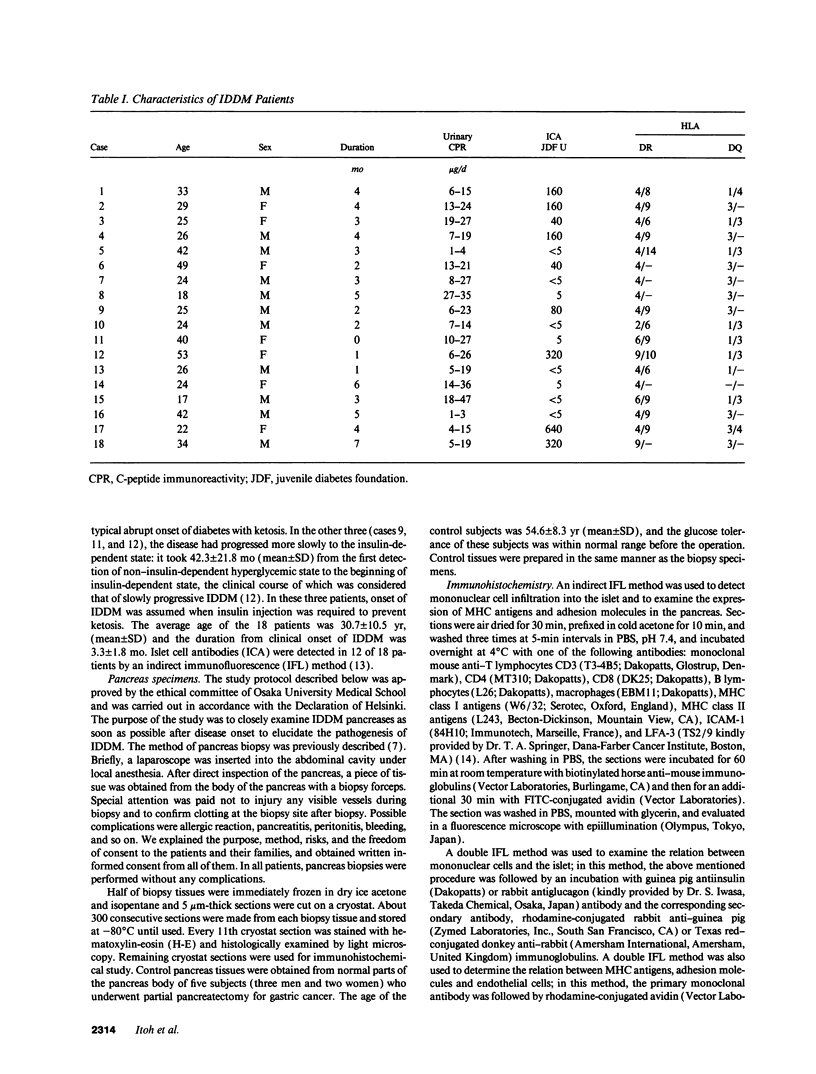
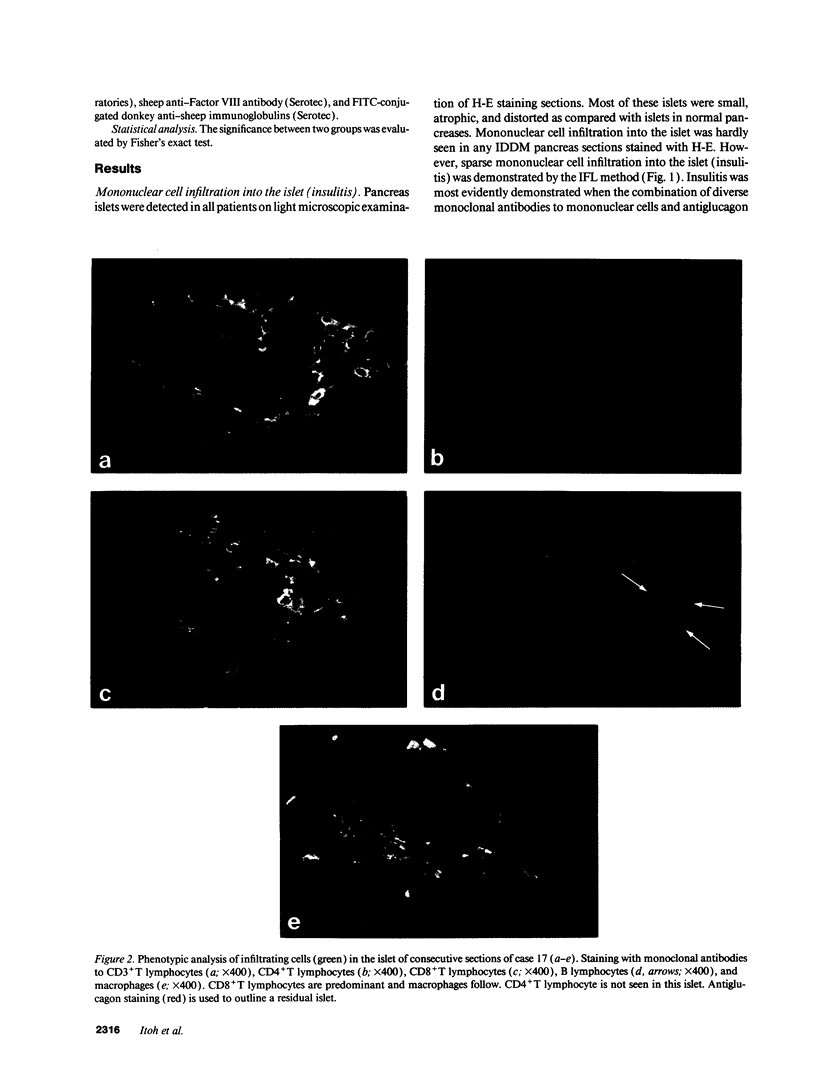
- Foulis A. K., Liddle C. N., Farquharson M. A., Richmond J. A., Weir R. S. The histopathology of the pancreas in type 1 (insulin-dependent) diabetes mellitus: a 25-year review of deaths in patients under 20 years of age in the United Kingdom. Diabetologia. 1986 May;29(5):267–274. doi: 10.1007/BF00452061. [DOI] [PubMed] [Google Scholar]
- Foulis A. K., McGill M., Farquharson M. A. Insulitis in type 1 (insulin-dependent) diabetes mellitus in man--macrophages, lymphocytes, and interferon-gamma containing cells. J Pathol. 1991 Oct;165(2):97–103. doi: 10.1002/path.1711650203. [DOI] [PubMed] [Google Scholar]
- Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965 Oct;14(10):619–633. doi: 10.2337/diab.14.10.619. [DOI] [PubMed] [Google Scholar]
- Hale L. P., Martin M. E., McCollum D. E., Nunley J. A., Springer T. A., Singer K. H., Haynes B. F. Immunohistologic analysis of the distribution of cell adhesion molecules within the inflammatory synovial microenvironment. Arthritis Rheum. 1989 Jan;32(1):22–30. doi: 10.1002/anr.1780320105. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Miyazaki A., Miyagawa J., Tamura S., Inada M., Yamada K., Shinji Y., Katsura H., Yamagata K., Itoh N. Examination of islets in the pancreas biopsy specimens from newly diagnosed type 1 (insulin-dependent) diabetic patients. Diabetologia. 1990 Feb;33(2):105–111. doi: 10.1007/BF00401048. [DOI] [PubMed] [Google Scholar]
- Hayakawa M., Yokono K., Nagata M., Hatamori N., Ogawa W., Miki A., Mizoguti H., Baba S. Morphological analysis of selective destruction of pancreatic beta-cells by cytotoxic T lymphocytes in NOD mice. Diabetes. 1991 Sep;40(9):1210–1217. doi: 10.2337/diab.40.9.1210. [DOI] [PubMed] [Google Scholar]
- Hutchings P., Rosen H., O'Reilly L., Simpson E., Gordon S., Cooke A. Transfer of diabetes in mice prevented by blockade of adhesion-promoting receptor on macrophages. Nature. 1990 Dec 13;348(6302):639–642. doi: 10.1038/348639a0. [DOI] [PubMed] [Google Scholar]
- Hänninen A., Jalkanen S., Salmi M., Toikkanen S., Nikolakaros G., Simell O. Macrophages, T cell receptor usage, and endothelial cell activation in the pancreas at the onset of insulin-dependent diabetes mellitus. J Clin Invest. 1992 Nov;90(5):1901–1910. doi: 10.1172/JCI116067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N., Hanafusa T., Katsura H., Yamamoto K., Takeda A., Kurahashi A., Nabika T., Miyazaki A., Miyagawa J., Kono N. Two types of autoantibodies to adrenal medullary cells in type 1 (insulin-dependent) diabetic patients: prevalence, properties and implications. J Autoimmun. 1991 Oct;4(5):807–818. doi: 10.1016/0896-8411(91)90175-c. [DOI] [PubMed] [Google Scholar]
- Itoh N., Hanafusa T., Miyagawa J., Tamura S., Inada M., Kawata S., Kono N., Tarui S. Transthyretin (prealbumin) in the pancreas and sera of newly diagnosed type I (insulin-dependent) diabetic patients. J Clin Endocrinol Metab. 1992 Jun;74(6):1372–1377. doi: 10.1210/jcem.74.6.1592883. [DOI] [PubMed] [Google Scholar]
- Junker K., Egeberg J., Kromann H., Nerup J. An autopsy study of the islets of Langerhans in acute-onset juvenile diabetes mellitus. Acta Pathol Microbiol Scand A. 1977 Sep;85(5):699–706. doi: 10.1111/j.1699-0463.1977.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Kay T. W., Campbell I. L., Oxbrow L., Harrison L. C. Overexpression of class I major histocompatibility complex accompanies insulitis in the non-obese diabetic mouse and is prevented by anti-interferon-gamma antibody. Diabetologia. 1991 Nov;34(11):779–785. doi: 10.1007/BF00408350. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Itoh T., Kosaka K., Sato K., Tsuji K. Time course of islet cell antibodies and beta-cell function in non-insulin-dependent stage of type I diabetes. Diabetes. 1987 Apr;36(4):510–517. doi: 10.2337/diab.36.4.510. [DOI] [PubMed] [Google Scholar]
- Lampeter E. R., Kishimoto T. K., Rothlein R., Mainolfi E. A., Bertrams J., Kolb H., Martin S. Elevated levels of circulating adhesion molecules in IDDM patients and in subjects at risk for IDDM. Diabetes. 1992 Dec;41(12):1668–1671. doi: 10.2337/diab.41.12.1668. [DOI] [PubMed] [Google Scholar]
- Lee K. U., Amano K., Yoon J. W. Evidence for initial involvement of macrophage in development of insulitis in NOD mice. Diabetes. 1988 Jul;37(7):989–991. doi: 10.2337/diab.37.7.989. [DOI] [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Shaw S. The CD2-LFA-3 and LFA-1-ICAM pathways: relevance to T-cell recognition. Immunol Today. 1989 Dec;10(12):417–422. doi: 10.1016/0167-5699(89)90039-X. [DOI] [PubMed] [Google Scholar]
- Miller B. J., Appel M. C., O'Neil J. J., Wicker L. S. Both the Lyt-2+ and L3T4+ T cell subsets are required for the transfer of diabetes in nonobese diabetic mice. J Immunol. 1988 Jan 1;140(1):52–58. [PubMed] [Google Scholar]
- Oschilewski U., Kiesel U., Kolb H. Administration of silica prevents diabetes in BB-rats. Diabetes. 1985 Feb;34(2):197–199. doi: 10.2337/diab.34.2.197. [DOI] [PubMed] [Google Scholar]
- Pujol-Borrell R., Todd I., Doshi M., Gray D., Feldmann M., Bottazzo G. F. Differential expression and regulation of MHC products in the endocrine and exocrine cells of the human pancreas. Clin Exp Immunol. 1986 Jul;65(1):128–139. [PMC free article] [PubMed] [Google Scholar]
- Santamaria P., Nakhleh R. E., Sutherland D. E., Barbosa J. J. Characterization of T lymphocytes infiltrating human pancreas allograft affected by isletitis and recurrent diabetes. Diabetes. 1992 Jan;41(1):53–61. doi: 10.2337/diab.41.1.53. [DOI] [PubMed] [Google Scholar]
- Shaw S., Luce G. E., Quinones R., Gress R. E., Springer T. A., Sanders M. E. Two antigen-independent adhesion pathways used by human cytotoxic T-cell clones. Nature. 1986 Sep 18;323(6085):262–264. doi: 10.1038/323262a0. [DOI] [PubMed] [Google Scholar]
- Sibley R. K., Sutherland D. E., Goetz F., Michael A. F. Recurrent diabetes mellitus in the pancreas iso- and allograft. A light and electron microscopic and immunohistochemical analysis of four cases. Lab Invest. 1985 Aug;53(2):132–144. [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Vives M., Soldevila G., Alcalde L., Lorenzo C., Somoza N., Pujol-Borrell R. Adhesion molecules in human islet beta-cells. De novo induction of ICAM-1 but not LFA-3. Diabetes. 1991 Nov;40(11):1382–1390. doi: 10.2337/diab.40.11.1382. [DOI] [PubMed] [Google Scholar]
- Wang Y., Pontesilli O., Gill R. G., La Rosa F. G., Lafferty K. J. The role of CD4+ and CD8+ T cells in the destruction of islet grafts by spontaneously diabetic mice. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):527–531. doi: 10.1073/pnas.88.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K., Nakajima H., Hanafusa T., Noguchi T., Miyazaki A., Miyagawa J., Sada M., Amemiya H., Tanaka T., Kono N. Aspartic acid at position 57 of DQ beta chain does not protect against type 1 (insulin-dependent) diabetes mellitus in Japanese subjects. Diabetologia. 1989 Oct;32(10):762–764. doi: 10.1007/BF00274539. [DOI] [PubMed] [Google Scholar]
- Zheng R. Q., Abney E. R., Grubeck-Loebenstein B., Dayan C., Maini R. N., Feldmann M. Expression of intercellular adhesion molecule-1 and lymphocyte function-associated antigen-3 on human thyroid epithelial cells in Graves' and Hashimoto's diseases. J Autoimmun. 1990 Dec;3(6):727–736. doi: 10.1016/s0896-8411(05)80039-3. [DOI] [PubMed] [Google Scholar]