Abstract
Modafinil has been shown to promote wakefulness and some studies suggest the drug can improve cognitive function. Because of many similarities, the mechanism of action may be comparable to classical psychostimulants, although the exact mechanisms of modafinil’s actions in wakefulness and cognitive enhancement are unknown. The current study aims to further examine the effects of modafinil as a cognitive enhancer on hippocampus-dependent memory in mice. A high dose of modafinil (75 mg/kg, i.p.) given before training improved acquisition on a Morris water maze. When given only before testing, modafinil did not affect water maze performance. We also examined modafinil (0.075 – 75 mg/kg) on Pavlovian fear conditioning. A low dose of pre-training modafinil (0.75 mg/kg) enhanced memory of contextual fear conditioning (tested off-drug one week later) while a high dose (75 mg/kg) disrupted memory. Pre-training modafinil did not affect cued conditioning at any dose tested, and immediate post-training modafinil had no effect on either cued or contextual fear. These results suggest that modafinil’s effects of memory are more selective than amphetamine or cocaine, and specific to hippocampus-dependent memory.
Keywords: modafinil, memory, hippocampus, fear conditioning, Morris water maze
Modafinil (marketed as Provigil® in the U.S.) is a novel wake-promoting stimulant with low abuse potential used to treat excessive sleepiness and narcolepsy (Bastuji and Jouvet, 1988). Clinical testing in humans has found positive results while testing its effects on depression (Kaufman et al., 2002), schizophrenia (Turner et al., 2004), shift work sleep disorder (Roth and Roehrs, 1996), obstructive sleep apnea syndrome (Pack et al., 2001), Parkinson’s disease (Nieves and Lang, 2002), and attention deficit hyperactivity disorder (Taylor and Russo, 2000). It is currently approved by the U.S. Food and Drug Administration (FDA) for treatment of narcolepsy, shift work sleep disorder, and obstructive sleep apnea/hypopnea syndrome. The drug is also widely prescribed off-label to enhance alertness, attention, or memory for dementia, attention deficit hyperactivity disorder, excessive daytime sleepiness, and depression (O’Connor, 2004); an illicit market exists for academic doping as well (Garreau, 2006). However, research has yet to determine if these latter effects are due solely to its wake-promoting ability or to a specific ability as a cognitive enhancer, or nootropic (Giurgea, 1982).
Recent studies indicate that modafinil has some cognitive enhancing abilities in rodents performing a variety of learning and memory tasks. Beracochea et al. (2001) found that chronic high doses of modafinil (64 mg/kg, but not 8 mg/kg or 32 mg/kg) produced a delay-dependent increase in spontaneous alternation rates on a T-maze, indicating enhanced working memory. This enhancement was only present when intertrial intervals were extended from 5s to 60s or 180s, making the task much more difficult for controls. Also on a T-maze, modafinil given chronically or acutely (64 mg/kg, but not 32 mg/kg) produced a faster adaptation to a win-stay strategy in a serial spatial discrimination reversal task while not affecting a win-shift strategy (Beracochea et al., 2002, 2003). Since the win-shift strategy was not impaired, this suggests that overall cognitive ability is increased, rather than just a selective inability to shift responses. In addition, control subjects performed very well on the win-shift task, but very poorly on win-stay; thus, a lack of enhancement on the win-shift task may have been due to ceiling effects or interactions between the drug and the complexity of the task.
In rats, modafinil (55 mg/kg and 100 mg/kg, but not 30 mg/kg) increased choice accuracy on a delayed nonmatching to position swim task by improving the rate of learning, indicating an enhancement of cognitive processing (Ward et al., 2004). Control animals had difficulty learning this task, which further suggests an interaction between the drug effects and task complexity. Recently, Morgan et al. (2007) reported that modafinil (64 mg/kg) enhanced choice accuracy on a 3-choice sustained attention task, but only when attention load was increased by adding a delay in stimulus onset. No effect on visual discrimination was found. Finally, modafinil (32 mg/kg, 64 mg/kg, and 128 mg/kg) did not increase choice accuracy on a five-choice serial reaction time test in rats and the two high doses increased premature responding, indicating stronger impulsivity (Waters et al., 2005). This failure to find an enhancement of choice accuracy may be due to the high accuracy of controls, but even when task difficulty was increased by weakened stimulus duration and intensity, the drug did not facilitate performance. These findings indicate that modafinil does not improve visual sensitivity, but they do not rule out the drug as a cognitive enhancer. The attention load in this study may not have been large enough to see any subtle enhancement of working memory (Morgan, 2007).
Modafinil’s ability to rescue cognitive impairments in sleep-deprived humans is well established (Pigeau et al., 1995) but many recent studies have focused on possible cognitive enhancing effects in healthy, non-sleep deprived individuals. Turner et al. (2003) found that in healthy volunteers, modafinil (100mg or 200mg, p.o.; equivalent to about 1 – 3 mg/kg) selectively enhanced performance on cognitive tasks including digit span, visual pattern recognition memory, spatial planning, and stop-signal reaction time. Ironically, a number of cognitive tasks also showed a slowing in response latency. Modafinil (200mg) also facilitated performance on both a delayed matching task and a numeric manipulation task, indicating that the drug facilitates maintenance and manipulation of information in working memory (Müller et al., 2004). As seen with rodents, increasing the difficulty of the set asks produced stronger enhancement of cognitive processes. Randall et al. (2003, 2005) offered conflicting evidence when modafinil (100mg or 200mg) caused few differences in the performance of healthy volunteers on an extensive battery of cognitive tests. Only digit span and visual pattern recognition memory showed enhancement with modafinil, while numerous working memory and attention tasks were unaffected. Recent evidence suggests an interaction between the drug and IQ, which may limit the cognitive enhancing effects of the drug in subjects with high IQ scores (Randall, Shneerson, & File, 2005).
While modafinil may generally be classified as a psychostimulant, evidence suggests it may act via a neural pathway distinct from classical psychostimulants, such as amphetamine or cocaine. C-fos and 2-deoxyglucose autoradiography studies in cats and rats, respectively, demonstrate that modafinil selectively increases activity in the anterior hypothalamic nucleus while classical psychostimulants cause broad activation and generally work through the caudate nucleus and prefrontal cortex (Engber et al., 1998; Lin et al., 1996). Modafinil, like amphetamine and cocaine, relies on dopamine transporters (Wisor et al., 2001), but unlike classical psychostimulants, this drug does not significantly increase dopamine levels in the nucleus accumbens (Ferraro, 1997), which likely accounts for its low abuse potential (Deroche-Gamonet et al., 2002). Modafinil appears to amplify the release of glutamate and serotonin while inhibiting the release of GABA (Ferraro et al., 1998, 1999, 2000). This may be achieved by blocking dopamine and/or norepinephrine transporters (Madras, 2006; Wisor, 2005). In this manner, modafinil may promote wakefulness through inhibition of the ventrolateral preoptic nucleus of the hypothalamus, a region known to promote sleep (Gallopin, 2004; Sherin, 1996). This theory, however, remains controversial (Saper, 2004).
The current study explored the cognitive enhancing effects of modafinil on two popular memory tasks in mice, the Morris water maze and Pavlovian fear conditioning (freezing). As modafinil appears to be selective in its cognitive enhancing effects, this study tested three different types of memory (context fear memory, cued fear memory, and spatial memory) to further extract subtle effects of the drug.
The Morris water maze task requires an animal to repeatedly find a hidden platform in order to escape a pool of opaque water (Morris, 1984). Animals are placed in random starting locations and must use distal spatial cues in order to find the platform. This test of spatial memory relies heavily on the hippocampus and surrounding structures, even after long delays between the training and the lesion (Clark et al., 2005). Thus, experiment 1 tested the effects of pre-training modafinil on spatial memory using a Morris water maze. Experiment 2 examined the effects of modafinil on Pavlovian fear conditioning. Fear conditioning relies on the association between an initially neutral conditioned stimulus (CS) and an aversive unconditioned stimulus (US). After a single pairing, the CS alone will elicit a response similar that elicited by the US. When fearful, mice display an innate tendency to exhibit freezing behavior, defined as a lack of movement other than which to breathe. Thus, after a tone (CS) is paired with a shock (US) in a distinct context, mice will freeze in response to presentation of either the tone or contextual stimuli (Fanselow, 1980; Anagnostaras et al., 2000). Context fear, unlike cued (tone) fear, is dependent on the hippocampus, while both are dependent on the amygdala (Anagnostaras, Gale, & Fanselow, 2001; Gale et al., 2004).
In experiment 1, we began by examining the effects of a high dose of modafinil on water maze learning, which produced substantially enhanced learning. In experiment 2, we examined Pavlovian fear conditioning in order to examine hippocampus-dependent and independent learning. We began by giving injections of a high dose of modafinil either before or after training to assess its effects on memory formation and consolidation. If modafinil delivered before training affected memory of the task, this would suggest the drug facilitates the formation of memory. However, if modafinil delivered after training had enhanced memory of the task, the drug could be involved in memory consolidation. As reported below, post-training injections had no effect on memory formation and were thus discontinued in subsequent studies. A high dose of modafinil, pre-training, however produced a substantial deficit in context conditioning. Recent evidence from our laboratory (Wood et al., 2007; Wood & Anagnostaras, 2008) indicates that the classical psychostimulants cocaine and amphetamine both enhance Pavlovian conditioned fear at very low doses and disrupt it at high doses; those effects were not specific to hippocampus-dependent learning. Thus, we predicted modafinil would have similar effects, which could be specific to hippocampus-dependent memory. Therefore, in experiment 3, we set out to establish a dose-response curve for modafinil using Pavlovian fear conditioning. The results are discussed in terms of modafinils’ ability to enhance or disrupt memory.
Methods and Materials
Subjects
Experiments were conducted using hybrid C57B6x129T2SvEms/J (129B6, stock from The Jackson Laboratory, West Sacramento, CA) hybrid mice that were at least 8 weeks old before testing. Mice were weaned 3 weeks after birth and group-housed (2–5 mice per cage) with unrestricted access to food and water under a 14:10 light/dark cycle. All animal care and experimental procedures were approved by the UCSD IACUC and in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals.
Experiment 1 – The Effect of Pre-training Modafinil on Morris Water Maze Learning
Mice were placed into a water maze (made opaque with white tempera paint) and used distal cues (i.e., posters, etc.) to locate a stationary square platform hidden 1 cm below water. The water maze was made of a circular tub (height = 74 cm, diameter = 122 cm) and the water was heated to 23.5°C using a built in heater. Distal cues were arranged throughout the room and included a door, a computer, and several posters. The platform, 1 cm below the pool surface, was 12 cm square and covered with a plastic mesh to provide a textured surface for the mice to grip. The water was colored with white tempera paint so the platform would not be visible. During training, a mouse was lowered into the pool from one of four randomly assigned starting locations; the trial lasted until the mouse found the platform where it remained for 5 seconds. If the mouse did not find the platform in 60 seconds, it was placed on the platform and remained there for 20 seconds in order to ensure exposure to the reinforcement and distal cues. Probe tests consisted of 60 second trials with the platform removed. Mice were dried in a towel after completing the final trial each day.
Each training day consisted of three consecutive trials administered on days 1–4 and 6–8. In addition, a probe test (given 15 minutes after training when on the same day) was administered on days 5, 7, and 8. Location was tracked using an automated video tracking system connected to an overhead video camera (Actimetrics Inc., Evanston, IL). Probe trials were scored by computer using Water maze software, measuring percent of time in target quadrant (TQ).
The modafinil group (n = 10) received modafinil (Cephalon Inc., Frazer, PA), 75 mg/kg i.p, 15 minutes before each of the first seven days of training and saline on the final day while the saline group (n =10) received saline (0.9% sodium chloride, 10 ml/kg) before each of the first seven days of training and modafinil on the final day. Modafinil was suspended 7.5 mg/ml in sterile water with 10% Tween 80.
Experiment 2 – The Effect of Pre- and Post-Training Modafinil on Fear Conditioning
Conditioning context
Four mice were tested concurrently, in individual conditioning chambers housed in a windowless room. Each chamber (32 cm wide, 25 cm high, 25 cm deep) was located within a sound attenuating chamber (63.5 cm wide, 35.5 cm high, 76 cm deep; Med-Associates Inc., Georgia, VT) and equipped with a speaker in the side wall and a stainless steel grid floor (36 rods, each rod 2-mm diameter, 8-mm center to center; Med-Associates Inc., Georgia, VT) and stainless steel drop-pan. During each trial chambers were scented with 7% isopropyl alcohol to provide a background odor and background noise (65-dB) was provided by internal fans. Each sound attenuating chamber was equipped with an overhead LED light source providing white and near infrared light, and an IEEE 1394 progressive scan video camera with a visible light filter (VID-CAM-MONO-2A; Med-Associates Inc., Georgia, VT) connected to a computer and video equipment in an adjacent room. Each chamber was connected to a solid-state scrambler, providing AC constant current shock, and an audio stimulus generator, controlled via an interface connected to a Windows computer running Video Freeze (Med-Associates Inc., Georgia, VT), a novel program designed for the automated assessment of freezing and activity. In results that will be published more fully elsewhere, computer and human scored data had a correlation of 0.971 and a fit of computer = −0.007 + 0.974 × human (for more detail on this calculation see, for e.g., Anagnostaras et al., 2000)
Alternate context
For testing tone fear the conditioning context was modified along several dimensions. White acrylic sheets were placed over the grid floor to provide a different sensory experience and a black plastic, triangular tent translucent only to near infrared light was placed inside each box, with each side of the triangle measuring 23cm. Only near infrared light was used creating a completely dark environment visible only to the video camera. Between tests, the chambers were cleaned and scented with a 5% white vinegar solution.
During each phase, freezing was measured by computer. During fear conditioning training, mice were given an i.p. injection of modafinil or saline and 15 min later placed into one of four identical chambers. After 2 minutes of baseline activity, a 30 second tone (2.8-kHz, 85dB/A-scale) was presented and coterminated with a scrambled footshock (2 seconds, 0.75mA, AC constant current) delivered through the floor of the cages. The training trial continued for 2.5 additional minutes before an additional five minute test of immediate memory (post-shock freezing) in the same chamber. Mice then received a second injection of modafinil or saline (see groups below) and were returned to their home cages. One week later, mice were returned to the training chambers for an assessment of context fear, during which no injection or shock was given. Freezing was monitored for 5 minutes and mice were then returned to their home cages. Twenty-four hours later, mice were given at one test; they were placed in the alternative context described above. Baseline activity was assessed for 2 minutes, after which the training tone was presented for 3 minutes and then the mice were returned to their home cages.
Mice were randomly assigned to one of three groups indicating the type of injection given before and after training, respectively: Modafinil/Saline (n=10), Saline/Modafinil (n=10), or Saline/Saline (n=10). Each injection was 75 mg/kg modafinil, i.p., or saline (0.9%, 10 ml/kg).
Experiment 3 – Dose Response Curve with Pre-training Modafinil
Mice were given the same training as in experiment 2, although a 0.5 mA shock was used after pilot data that suggested it would be easier to observe learning enhancements at lower levels of fear. Given that post-training modafinil at a high dose had no effect on fear conditioning, we only examined the full dose-effect curve for modafinil given pre-training.
Mice were randomly assigned to one of five groups indicating the amount of modafinil given prior to training: 0 mg/kg (saline control, n = 16), 0.075 (n = 13), 0.75 (n = 13), 7.5 (n = 12), or 75 mg/kg (n = 12), to form a full dose-effect curve. In pilot experiments, we found a high rate of lethality above 100 mg/kg so higher doses were not explored. Each dose of modafinil was suspended in sterile water with 10% Tween 80 and administered at 10 ml/kg i.p.
Results
Experiment 1 – Water maze
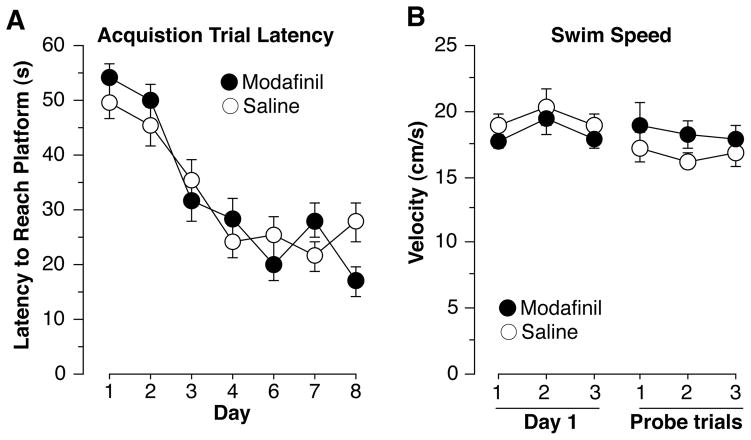
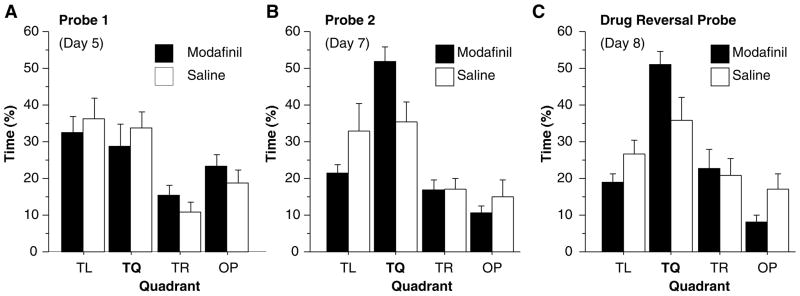
Figure 1 depicts the latency (fig 1A) to reach the platform for each of the training trials and the average velocity (fig 1B) for each group during training trials on day 1 and each probe test. Data were entered into a multivariate analysis of variance (MANOVA) and the level of significance was set at α < 0.05. Post hoc comparisons were done with Fisher’s protected least significant difference (PLSD). No group differences were found during acquisition for latency [F(1,18) < 0.1, n.s.] or velocity [F(1,18) = 3.23, n.s.]. Figure 2 depicts the time spent in each quadrant during the first (2A), second (2B), and third probe tests (2C). Since mice often become disinterested and wander once they discover the platform is not there, only the first 30 seconds were analyzed. To assess learning of the task, paired comparisons between the target quadrant (TQ) and opposite quadrant (OP) were used. During probe 1, time spent in each quadrant did not differ between Modafinil mice and Saline mice [F(1,18)=0.425, n.s.] and neither group spent significantly more time in TQ than OP [Modafinil: F(1,9) = 0.34, n.s., Saline: F(1,9) = 4.78, n.s.] indicating that neither group had learned the task. During probe 2, Modafinil mice spent more time in TQ than Saline mice [F(1,18) = 5.33, p<0.05]. Modafinil mice spent more time in TQ than OP [F(1,9) = 50.84, p < 0.001] while Saline mice did not [F(1,9) = 5.02, n.s.] indicating that Modafinil mice learned the location of the platform while Saline mice did not. When treatments were reversed in probe 3, Modafinil mice (that received saline) continued to spend more time in TQ than Saline mice (that received modafinil) although this result was out of the range of significance [F(1,18) = 4.09, p=0.058]. Modafinil mice spent more time in TQ than OP [F(1,9) = 68.72, p<0.001] while Saline mice did not [F(1,9) = 3.28, n.s.]. These results indicate that Modafinil mice learned the task by the seventh day of training while Saline mice did not, given the relatively small amount of training. Also, Modafinil mice retained this knowledge of the task when tested off drug and Saline mice remained unaware of the location of the platform when tested on drug.
Figure 1.
(A) Trial latency for each day of training. Trial sended when the subject reached the platform. No differences were found in acquisition trial latency. (B) Velocity of the first day of training and each of the three probe trials. No differences were found in velocity on the acquisition trials or the probe trials. Each point represents the mean ± S.E.M.
Figure 2.
(A) Percent time spent in each quadrant during probe 1 performed on day 5. Neither modafinil nor saline mice spent more time in the target quadrant (TQ) than other quadrants (TL: target left; TR: target right; OP: opposite). (B) Percent time spent in each quadrant during probe 2 performed on day 7. Modafinil mice learned the task, spending more time in TQ than OP, while Saline mice did not. (C) Percent time spent in each quadrant during probe 3 performed on day 8. Modafinil mice received saline prior to training and testing while Saline mice received modafinil. Despite the drug reversal, Modafinil mice continued to perform much better than Saline mice. Each point represents the mean + S.E.M.
Experiment 2 - Fear Conditioning with Pre- and Post-training Modafinil
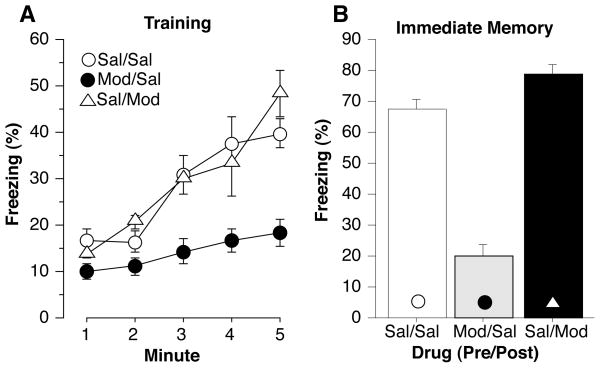
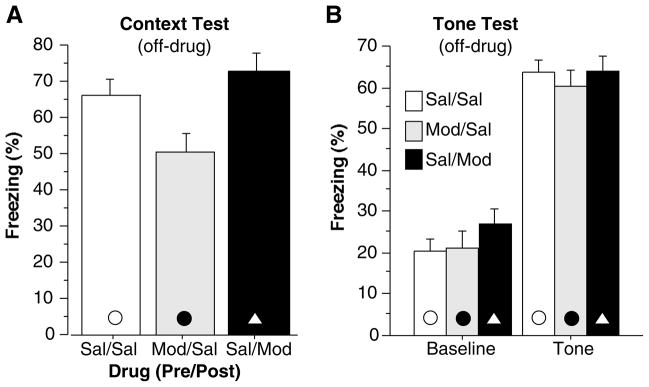
Percent freezing during training and the immediate memory test is illustrated in Figure 3. Group differences were observed during training [F(2,39) = 22.16, p<0.05] and the immediate memory test [F(2,39)=70.77, p<0.05]. When compared to Sal/Sal controls, mice that received modafinil prior to training (Mod/Sal) showed a strong deficit in freezing during training [F(1,30) = 30.65, p<0.001] and the immediate memory test [F(1,30) = 82.52, p<0.001] perhaps caused by the locomotor stimulatory effects of the drug or an immediate memory defect. Group differences were also observed during the off-drug context test one week later [Figure 4a, F(2,39) = 5.33, p<0.05]. Mod/Sal mice showed a strong deficit in context memory compared to Sal/Sal controls [F(1,30) = 5.35, p<0.05]. This difference cannot be attributed to the stimulatory effects of the drug as this test was performed off drug, and modafinil did not produce an increase in activity at any dose (see, for example, Fig 1b and 6a). During the tone test (Figure 4b) no group differences were observed in baseline freezing [F(2,39) = 0.76, n.s.] or tone memory [F(2,39) = 0.29, n.s.].
Figure 3.
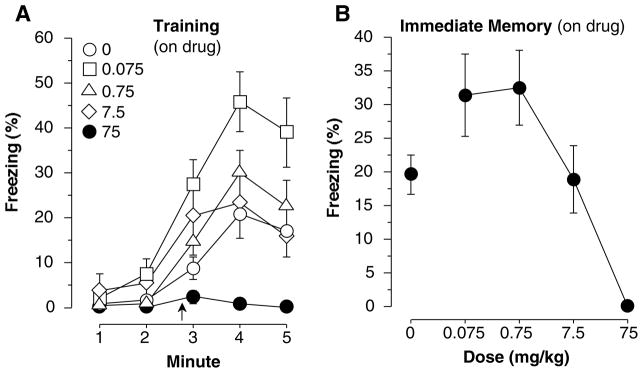
Percent time spent freezing during (A) training and (B) the immediate memory test. The shock was presented 2 minutes and 28 seconds into training and lasted for 2 seconds. Modafinil administered before training (Mod/Sal) caused mice to freeze less than controls during both training and the immediate memory test. Each point represents the mean ± S.E.M.
Figure 4.
Percent time spent freezing during the (A) context test and (B) tone test. Context test consisted of five minutes in training chambers with no tone or shock. Tone test consisted of five minutes in a novel chamber with a two minute baseline followed by a three minute tone presentation. Modafinil administered before training (Mod/Sal) disrupted contextual fear memory but did not affect cued fear memory. Modafinil administered after training (Sal/Mod) did not affect contextual fear memory or cued fear memory. Each point represents the mean + S.E.M.
Figure 6.
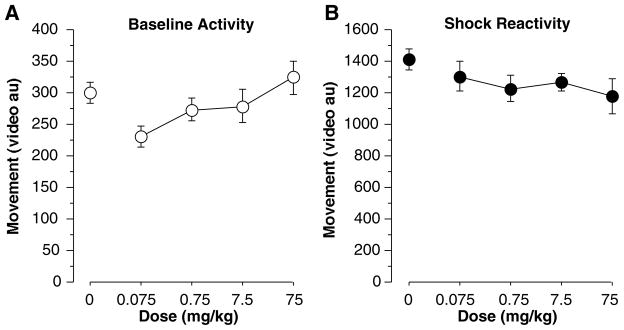
(A) Average movement, measured in arbitrary units (au), over the first two minutes of training before any tone or shock. Mice that received 0.075 mg/kg modafinil were significantly less active than controls. (B) Average movement (au) during the two second shock. No group differences were found indicating that each group had the same reaction to the shock. Each point represents the mean ± S.E.M.
Experiment 3 – Dose Response Curve with Pre-training Modafinil
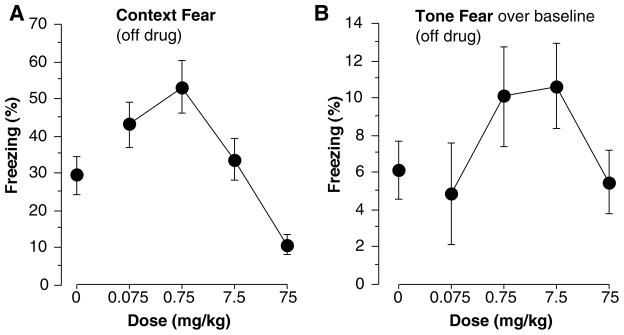
Percent freezing differed among groups during training [Figure 5a, F(4,61) = 7.88, p<0.001]. Mice that received 0.075 mg/kg modafinil froze significantly more than saline controls (i.e., 0 mg/kg; Fisher’s PLSD, p<0.05) and mice that received 75 mg/kg again showed a strong deficit in freezing (p<0.05). No other doses differed significantly from saline controls. Differences were also found during the immediate memory test [F(4,61) =8.10, p<0.001]. Mice that received 0.75 mg/kg showed enhanced immediate memory (Figure 5b, p<0.05) while mice that received 75 mg/kg mice froze significantly less than controls (p<0.05). As stated previously, this decrease in freezing may be caused by the stimulatory effects of the drug or an immediate memory defect, although we did not detect any increase in activity from any dose of modafinil (Figs 1a, 6b). Baseline activity (measured in arbitrary units) during the first two minutes of training (Figure 6a) showed group differences [F(4,61) = 2.63, p<0.05]. Post-hoc analysis showed that activity was reduced in mice that received 0.075 mg/kg compared to saline controls (p<0.05). No other doses produced significant changes in activity. Figure 6b illustrates that during the 2-second shock no differences in activity were found [F(4,61) = 1.26, n.s.] indicating that all groups had a similar reaction to the shock. Group differences were again found during the context test, done off-drug one week later [F(4,61) = 7.76, p<0.001]. Compared to controls, contextual memory (Figure 7a) was enhanced in mice that received 0.75 mg/kg (p<0.05) and disrupted in those that received 75 mg/kg (p<0.05). No other groups differed significantly from controls. During the tone test no differences were found during the two minute baseline [F(4,61) = 1.52, n.s.] or the tone-on portion of the test [F(4,61) = 1.40, n.s.] (data not depicted). In this experiment, a moderately high baseline was encountered, and less tone-elicited freezing, so we further examined tone freezing by subtracting the baseline freezing from tone-elicited freezing. This was likely due to the fact that we reduced shock intensity from Exp 2 (0.75 to 0.5 mA) because pilot data showed cleaner effects on context freezing. However, no significant differences were found after subtracting the baseline from the tone-on portion of the test [Figure 7b, F(4,61) = 1.45, n.s.]. Taken together with the results of experiment 2, where much more robust freezing was encountered, there is little evidence that modafinil affected tone conditioning at any dose. However, mice given saline exhibited 6.1±1.5% freezing while those given 0.75 and 7.5 mg/kg exhibited 10.1±2.7% and 10.6±2.3% freezing, respectively. This suggests that we may not have had enough power to detect a potential enhancement of tone fear conditioning at those doses (Figure 7b).
Figure 5.
(A) Percent time spent freezing in each minute of training. The shock was administered 2 minutes and 28 seconds into training. (B) Percent time spent freezing during the immediate memory test performed immediately after training. Mice that received 0.75 mg/kg modafinil froze more than controls while mice that received 75 mg/kg froze less than controls. Each point represents the mean ± S.E.M.
Figure 7.
(A) Percent time spent freezing during the context test. Mice that received 0.75 mg/kg modafinil showed an enhancement while those that received 75 mg/kg showed a disruption of contextual fear. (B) Percent time spent freezing during the two minute baseline was subtracted from the three minute tone to get a measure of cued fear memory. No group differences were found. Each point represents the mean ± S.E.M.
Discussion
The current study examined the effects of modafinil on three types of memory (spatial memory, context fear memory, and cued fear memory) and found specific enhancements of hippocampus-dependent spatial memory and contextual fear memory, but found no effect on hippocampus-independent cued fear memory. In contrast, the classical stimulants amphetamine and cocaine produced similar enhancements and deficits that were not specific to contextual versus cued memory (Wood et al., 2007; Wood & Anagnostaras, 2008). These data suggest that modafinil has similar effects on memory as the classical psychostimulants but acts more specifically on hippocampus-dependent memory.
A high dose of pre-training modafinil (75 mg/kg) enhanced performance on Morris water maze learning. After six days of training, the modafinil group successfully learned the task while controls did not. Interestingly, the effect was not state-dependent as the group trained on modafinil continued to outperform controls during the reversal trial. This indicates that modafinil facilitated the acquisition of spatial memory, rather than retrieval or performance (Fig 2).
Using a standard fear conditioning protocol, we found that a large dose of pre-training modafinil (75 mg/kg) disrupted contextual fear memory but spared cued fear memory. We also found that the same dose of modafinil delivered post-training did not affect context or cued fear memory. This suggests that modafinil does not affect consolidation, and thus, any effects are likely due to changes in memory formation. Finally, we found that a very low dose of pre-training modafinil (0.75 mg/kg) enhanced contextual fear memory and we replicated the finding that a large dose disrupted it. We found no effect of modafinil at any dose on cued fear memory.
These results suggest that modafinil works selectively on hippocampus-dependent memory. The animal literature thus far has focused on relatively high doses of the drug, because most of the effects have only been detected with large doses. We are the first to report a selective enhancement of memory at very low, clinically relevant, doses of modafinil. The dose that enhanced contextual fear memory is roughly equivalent to the dose used in human clinical patients (typically 100–200mg; about 1 – 3 mg/kg). This enhancement may be unique to contextual fear conditioning, but it warrants testing on other tasks, and thus, future studies investigating the cognitive effects of modafinil should include similar, more clinically relevant doses. Although many differences in human and mouse drug disposition may exist, given the little knowledge we have, we feel that a straight mg/kg conversion is the most conservative approach (Wood & Anagnostaras, 2008). Certainly higher doses used in other animal studies (e.g., 75 mg/kg, or about 60, 100 mg modafinil tablets) are not likely to be clinically relevant.
The Morris water maze task is a well-established spatial memory task in rodents (Morris, 1984). However, to ensure the drug was active during training, the typical protocol was modified to ensure all training and probe trials occurred shortly after drug administration. Therefore, trials were administered in a massed fashion, rather than distributed over a few hours. This may have made it more difficult for controls, and possibly made it more susceptible to the enhancing effects of modafinil. The fixed-location hidden platform version of the maze, as we used here, is not generally thought to involve working memory and by definition is a reference memory task (c.f., Steele & Morris, 1999). Additionally, probes 2 and 3 were administered 15 minutes after the end of a training day, while no training occurred on the day before probe 1. This may account for some of the differences between the results on the probe trials.
Throughout the animal literature modafinil’s effects appear to be related to task demands (Morgan, 2007). High doses of the drug have enhanced performance on a T-maze (Beracochea, 2001, 2002, 2003), delayed non-match to position swim task (Ward, 2004), 3-choice sustained attention task (Morgan, 2007), and now the Morris water maze. These tasks are difficult for mice and require extensive training, and in some studies the effects were not seen until the attention or working memory demands of the tasks were increased. Thus, high doses of modafinil may only enhance performance only on tasks that involve considerable difficulty, working memory, or attention.
Contextual fear memory is characterized by a two-step associative process whereby the animal must first form a memory of the context (thought to occur in the hippocampus) and then an association between a shock and the context must be formed (thought to occur in the amygdala; see Anagnostaras et al., 2001; Wiltgen et al., 2006). A low dose of pre-training modafinil enhanced contextual fear memory without affecting tone memory, suggesting that the drug was able to strengthen contextual memories. This task is relatively simple, and generally not thought to involve working memory (Anagnostaras et al., 2003).
Given that the shock is very arousing and the attentional demands necessary for the task are limited, it is surprising that a drug known for increasing attention and arousal would increase contextual fear memory. It may be that at low doses, modafinil selectively enhances associative ability while at high doses, it may benefit tasks that require increased attention and arousal or working memory. Most studies have been done with cognitive tasks have shown dose-dependent increases with the highest doses being the most efficacious. Alternatively, the drug may enhance attention processes which manifest themselves in a similar way. This alternative is supported by the disruption of contextual fear memory at a high dose suggesting an inverted U shaped curve, a common characteristic of attention. Consistent with this, chronically stressed mice showed decreased performance on a T-maze spontaneous alternation task when given a large dose (32 mg/kg) of modafinil (Pierard, 2006). Increases in attention from both stress and the drug may interact to overwhelm the animal and decrease performance. Interestingly, under stressful conditions, the lowest drug dose tested (8 mg/kg) showed the highest level of performance. In light of the current study, even lower doses of modafinil may have further facilitated T-maze performance in chronically stressed mice.
Another interesting finding presented here is the unexpected dissociation between Morris water maze learning and contextual fear conditioning, two hippocampus-dependent tasks. While a high dose of modafinil enhanced Morris water maze learning, it disrupted contextual fear conditioning. This seems to indicate that modafinil may act in different ways to produce enhancements (or deficits) on these tasks. Further study is required to understand these differences, but would begin with site-directed administration of modafinil in suspect learning and memory structures, such as the hippocampus and amygdala.
A number of alternative explanations must be considered to account for the selective enhancement and disruption of contextual fear conditioning. For instance, it is possible that modafinil may dose-dependently disrupt nociception. However, we found no evidence that modafinil altered shock sensitivity (Fig 6b). Moreover, psychostimulants (Markham, et al., 2006), can produce anxiogenic states that might increase fear. This is also unlikely as modafinil did not affect tone fear memory suggesting it did not promiscuously increase fear. Moreover, in clinical trials, the incidence of anxiety in patients treated with modafinil was around 5% and similar to placebo-treated controls suggesting the drug does not produce much anxiety overall (Cephalon, 2004).
Another possible explanation is that when learning occurs on-drug, the subject would perform better when tested on-drug. State-dependent learning could theoretically account for the deficit in contextual fear conditioning at high doses, but cannot explain the enhancement at the low dose. This theory is further undermined by the enhancement and deficit in the immediate memory test performed on the drug, which is similar to the effects seen off drug. Also, state dependent learning would be unlikely to affect contextual fear memory without having an effect on tone fear memory, which is what we observed. Finally, when directly tested on the Morris water maze we found no evidence of state-dependent effects of modafinil (Figure 2c).
Psychostimulants can produce locomotor activity which could in theory disrupt freezing when the animals are on drug (Wood et al., 2007; Wood & Anagnostaras, 2008). This is unlikely in the present case as we found no increase in activity at any dose of the drug (Figs 1b, 6a). Moreover any undetected hyperactivity on training is unlikely to be responsible for the deficit in contextual fear memory since this test occurred off-drug one week later. In fact, only the group that exhibited any change in activity received 0.075 mg/kg of modafinil, and that dose produced no effects in any of the memory tests. Thus, the dose-effect curves for activity and the effects on learning were so markedly different they suggest the drug’s locomotor affects and mnemonic effects are unrelated.
Wood et al. (2007) showed that a low dose of acute pre-training cocaine enhanced contextual fear memory and cued fear memory. A high dose of cocaine disrupted both forms of memory. The present study suggests that modafinil works through a similar, but more selective, mechanism as classical psychostimulants. Thus modafinil is very valuable as a cognitive performance enhancer because classical psychostimulants have often appeared to be cognitive enhancers, but their side effects and abuse potential have limit clinical use as nootropics. There are no reports of modafinil addiction, rather modafinil appears useful in treating cocaine, amphetamine, and nicotine addiction (Brower, 2006; Ling et al., 2006; Sofuoglu et al., 2008). Overall, the data suggests that modafinil is a better choice as a cognitive enhancer than classical psychostimulants for humans. In addition, the combination of its wake-promoting and cognitive enhancing effects makes it an ideal candidate for improving cognition in professions that often suffer from a lack of sleep, such as shift-workers or military personnel.
Acknowledgments
We thank Dr. Jennifer Sage and Denise Cai for helpful comments on an earlier version of this manuscript. We also thank Jeesun Kim, Joseph Cheong, and Stephanie Buss for excellent technical assistance. This work was supported by grant DA020041 from the National Institute on Drug Abuse and a Hellman Fellowship (SGA). The authors did not receive funding from the makers of modafinil.
References
- Anagnostaras SG, Gale GD, Fanselow MS. The hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Josselyn SA, Frankland PW, Silva AJ. Computer-assisted behavioral assessment of Pavlovian fear conditioning in mice. Learning and Memory. 2000;7:58–72. doi: 10.1101/lm.7.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastuji H, Jouvet M. Successful treatment of idiopathic hypersomnia and narcolepsy with modafinil. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1988;12:695–700. doi: 10.1016/0278-5846(88)90014-0. [DOI] [PubMed] [Google Scholar]
- Beracochea D, Cagnard B, Celerier A, le Merrer J, Pere M, Pierard C. First evidence of a delay-dependent working memory-enhancing effect of modafinil in mice. Learning and Memory. 2001;12:375–378. doi: 10.1097/00001756-200102120-00038. [DOI] [PubMed] [Google Scholar]
- Beracochea D, Celerier A, Borde N, Valleau M, Peres M, Pierard C. Improvement of learning processes following chronic systemic administration of modafinil in mice. Pharmacology, Biochemistry and Behavior. 2002;73:723–728. doi: 10.1016/s0091-3057(02)00877-8. [DOI] [PubMed] [Google Scholar]
- Beracochea D, Celerier A, Peres M, Pierard C. Enhancement of learning processes following an acute modafinil injection in mice. Pharmacology, Biochemistry and Behavior. 2003;76:473–479. doi: 10.1016/j.pbb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Brower V. Loosening addiction’s deadly grip. Recent research paints a picture of addiction as a progressive, chronic neurological disease that wreaks havoc with brain chemistry. European Molecular Biology Organization. 2006;7:140–142. doi: 10.1038/sj.embor.7400635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cephalon, FDA Approved Labeling Text for NDA20-717/S-005 & S-008. Provigil® (modafinil) Tablets [C-IV], Approved-23-JAN-2004.
- Clarke RE, Broadbent NJ, Squire LR. Hippocampus and remote spatial memory in rats. Hippocampus. 2005;15:260–272. doi: 10.1002/hipo.20056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Darnaudery M, Bruins-Slot L, Piat F, Le Moal M, Piazza PV. Study of the addictive potential of modafinil in naïve and cocaine-experienced rats. Psychopharmacology. 2002;161:387–395. doi: 10.1007/s00213-002-1080-8. [DOI] [PubMed] [Google Scholar]
- Engber TM, Dennis SA, Jones BE, Miller MS, Contreras PC. Brain regional substrates for the actions of the novel wake-promoting agent modafinil in the rat: comparison with amphetamine. Neuroscience. 1998;87:905–911. doi: 10.1016/s0306-4522(98)00015-3. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlovian Journal of Biological Sciences. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, O’Connor WT, Tanganelli S, Rambert F, Fuxe K. Modafinil: an antinarcoleptic drug with a different neurochemical profile to d-amphetamine and dopamine uptake blockers. Biological Psychiatry. 1997;42:1181–1183. doi: 10.1016/s0006-3223(97)00353-3. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, O’Connor WT, Tangenelli S, Rambert F, Fuxe K. The effects of modafinil on striatal, pallidal and nigral GABA and glutamate release in the conscious rat: evidence for a preferential inhibition of striato-pallidal GABA transmission. Neuroscience Letters. 1998;253:135–138. doi: 10.1016/s0304-3940(98)00629-6. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, Tangenelli S, O’Connor WT, Perez dela Mora M, Mendez-Franco J, et al. The vigilance-promoting drug modafinil increases extracellular glutamate levels in the medial preoptic area and the posterior hypothalamus of the conscious rat: prevention by local GABA-A receptor blockade. Neuropsychopharmacology. 1999;20:346–356. doi: 10.1016/S0893-133X(98)00085-2. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Fuxe K, Tanganelli S, Fernandez M, Rambert FA, Antonelli T. Amplification of cortical serotonin release: a further neurochemical action of the vigilance-promoting drug modafinil. Neuropharmacology. 2000;39:1974–1983. doi: 10.1016/s0028-3908(00)00019-8. [DOI] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, et al. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. Journal of Neuroscience. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallopin T, Luppi PH, Rambert FA, Frydman A, Fort P. Effect of the wake-promoting agent modafinil on sleep-promoting neurons from the ventrolateral preoptic nucleus: an in vitro pharmacologic study. Sleep. 2004;27:19–25. [PubMed] [Google Scholar]
- Garreau J. A Dose of Genius: ‘Smart Pills’ Are on The Rise. But Is Taking Them Wise? Washington Post. 2006 June 11; [Google Scholar]
- Giurgea C. The nootropic concept and it’s prospective implications. Drug Development Research. 1982;2:441–446. [Google Scholar]
- Kaufman KR, Menza MA, Fitzsimmons A. Modafinil monotherapy for depression. European Psychiatry. 2002;17:167–169. doi: 10.1016/s0924-9338(02)00646-6. [DOI] [PubMed] [Google Scholar]
- Lin JS, Hou Y, Jouvet M. Potential brain neuronal targets for amphetamine-, methylphenidate-, and modafinil-induced wakefulness, evidenced by c-fos immunocytochemistry in the cat. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:14128–14133. doi: 10.1073/pnas.93.24.14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Rawson R, Shoptaw S, Ling W. Management of methamphetamine abuse and dependence. Current Psychiatry Reports. 2006;8:345–354. doi: 10.1007/s11920-006-0035-x. [DOI] [PubMed] [Google Scholar]
- Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L, et al. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. Journal of Pharmacology and Experimental Therapeutics. 2006;319:561–569. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- Morgan RE, Crowley JM, Smith RH, LaRoche RB, Dopheide MM. Modafinil improves attention, inhibitory control, and reaction time in healthy, middle-aged rats. Pharmacology, Biochemistry, and Behavior. 2007;86:531–541. doi: 10.1016/j.pbb.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. Journal of Neuroscience Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Müller U, Steffenhagen N, Regenthal R, Bublak P. Effects of modafinil on working memory processes in humans. Psychopharmacology. 2004;177:161–169. doi: 10.1007/s00213-004-1926-3. [DOI] [PubMed] [Google Scholar]
- Nieves AV, Lang AE. Treatment of excessive daytime sleepiness in patients with Parkinson’s disease with modafinil. Clinical Neuropharmacology. 2002;25:111–114. doi: 10.1097/00002826-200203000-00010. [DOI] [PubMed] [Google Scholar]
- O’Connor A. Wakefulness finds a powerful ally. New York Times. 2004 June 29; [Google Scholar]
- Pack AI, Black JE, Schwartz JR, Matheson JK. Modafinil as adjunct therapy for daytime sleepiness in obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine. 2001;164:1675–1681. doi: 10.1164/ajrccm.164.9.2103032. [DOI] [PubMed] [Google Scholar]
- Pierard C, Liscia P, Valleau M, Drouet I, Chauveau F, Huart B, et al. Modafinil-induced modulation of working memory and plasma corticosterone in chronically-stressed mice. Pharmacology, Biochemistry, and Behavior. 2006;83:1–8. doi: 10.1016/j.pbb.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Pigeau R, Naitoh P, Buguet A, McCann C, Baranski J, Taylor M, et al. Modafinil, d-amphetamine and placebo during 64 hours of sustained mental work: I. Effects on mood, fatigue, cognitive performance and body temperature. Journal of Sleep Research. 1995;4:212–228. doi: 10.1111/j.1365-2869.1995.tb00172.x. [DOI] [PubMed] [Google Scholar]
- Randall DC, Shneerson JM, File SE. Cognitive effects of modafinil in student volunteers may depend on IQ. Pharmacology, Biochemistry and Behavior. 2005;82:133–139. doi: 10.1016/j.pbb.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Randall DC, Shneerson JM, Plaha KK, File SE. Modafinil affects mood, but not cognitive function, in healthy young volunteers. Human Psychopharmacology. 2003;18:163–173. doi: 10.1002/hup.456. [DOI] [PubMed] [Google Scholar]
- Roth T, Roehrs TA. Etiologies and sequelae of excessive daytime sleepiness. Clinical Therapeutics. 1996;18:562–576. doi: 10.1016/s0149-2918(96)80207-4. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE. Modafinil: a drug in search of a mechanism. Sleep. 2004;27:11–12. [PubMed] [Google Scholar]
- Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Waters AJ, Mooney M. Modafinil and nicotine interactions in abstinent smokers. Human Psychopharmacology. 2008;23:21–30. doi: 10.1002/hup.878. [DOI] [PubMed] [Google Scholar]
- Steele RJ, Morris RGM. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus. 1999;9 (2):118–136. doi: 10.1002/(SICI)1098-1063(1999)9:2<118::AID-HIPO4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Taylor FB, Russo J. Efficacy of modafinil compared to dextroamphetamine for the treatment of attention deficit hyperactivity disorder in adults. Journal of Child and Adolescent Psychopharmacology. 2000;10:311–320. doi: 10.1089/cap.2000.10.311. [DOI] [PubMed] [Google Scholar]
- Turner DC, Clark L, Pomarol-Clotet E, McKenna P, Robbins TW, Sahakian BJ. Modafinil improves cognition and attentional set shifting in patients with chronic schizophrenia. Neuropsychopharmacology. 2004;29:1363–1373. doi: 10.1038/sj.npp.1300457. [DOI] [PubMed] [Google Scholar]
- Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology. 2003;165:260–269. doi: 10.1007/s00213-002-1250-8. [DOI] [PubMed] [Google Scholar]
- Ward CP, Harsh JR, York KM, Stewart KL, McCoy JG. Modafinil facilitates performance on a delayed nonmatching to position swim task in rats. Pharmacology, Biochemistry and Behavior. 2004;78:735–741. doi: 10.1016/j.pbb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Waters KA, Burnham KE, O’Connor D, Dawson GR, Dias R. Assessment of modafinil on attentional processes in a five-choice serial reaction time test in the rat. Journal of Psychopharmacology. 2005;19:149–158. doi: 10.1177/0269881105048995. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. Journal of Neuroscience. 2006;26:5484–91. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisor JP, Eriksson KS. Dopaminergic-adrenergic interactions in the wake promoting mechanism of modafinil. Neuroscience. 2005;132:1027–1034. doi: 10.1016/j.neuroscience.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. The Journal of Neuroscience. 2001;21:1787–1794. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SC, Fay J, Sage JR, Anagnostaras SG. Cocaine and Pavlovian fear conditioning: dose-effect analysis. Behavioral Brain Research. 2007;176:244–250. doi: 10.1016/j.bbr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SC, Anagnostaras SG. Memory and psychostimulants: modulation of Pavlovian fear conditioning by amphetamine in C57BL/6 mice. Psychopharmacology. 2008 doi: 10.1007/s00213-008-1185-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]