Abstract
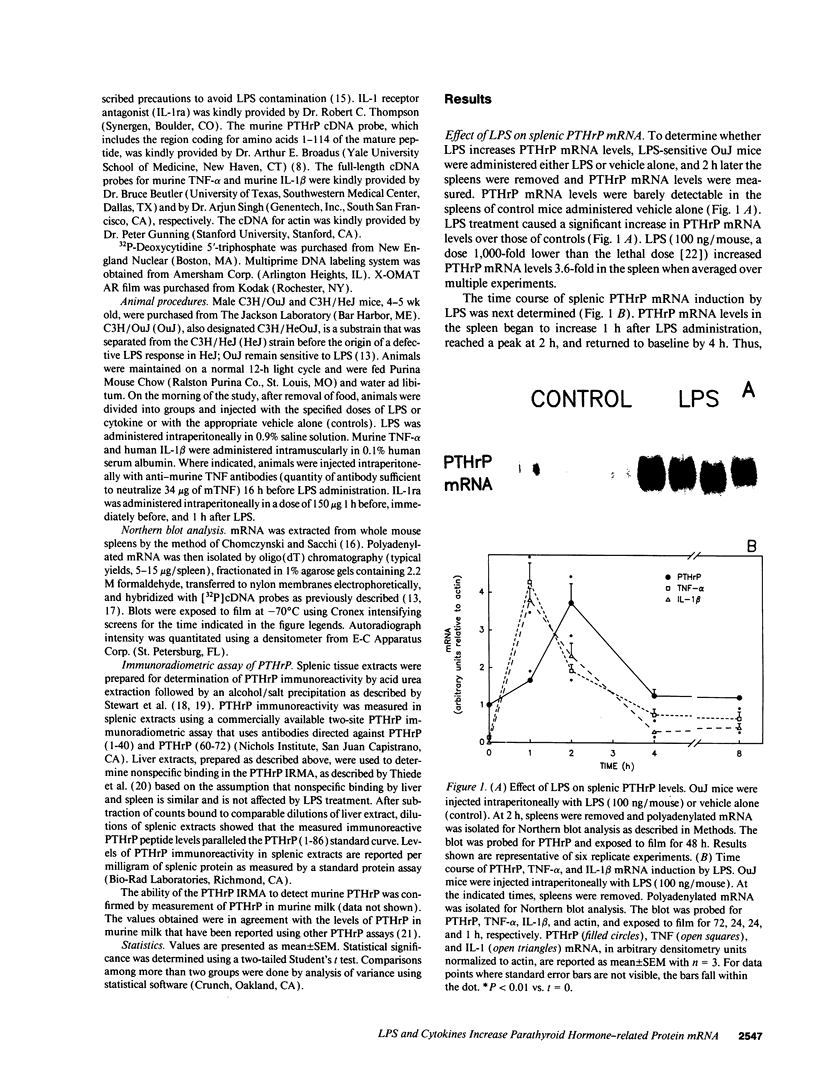
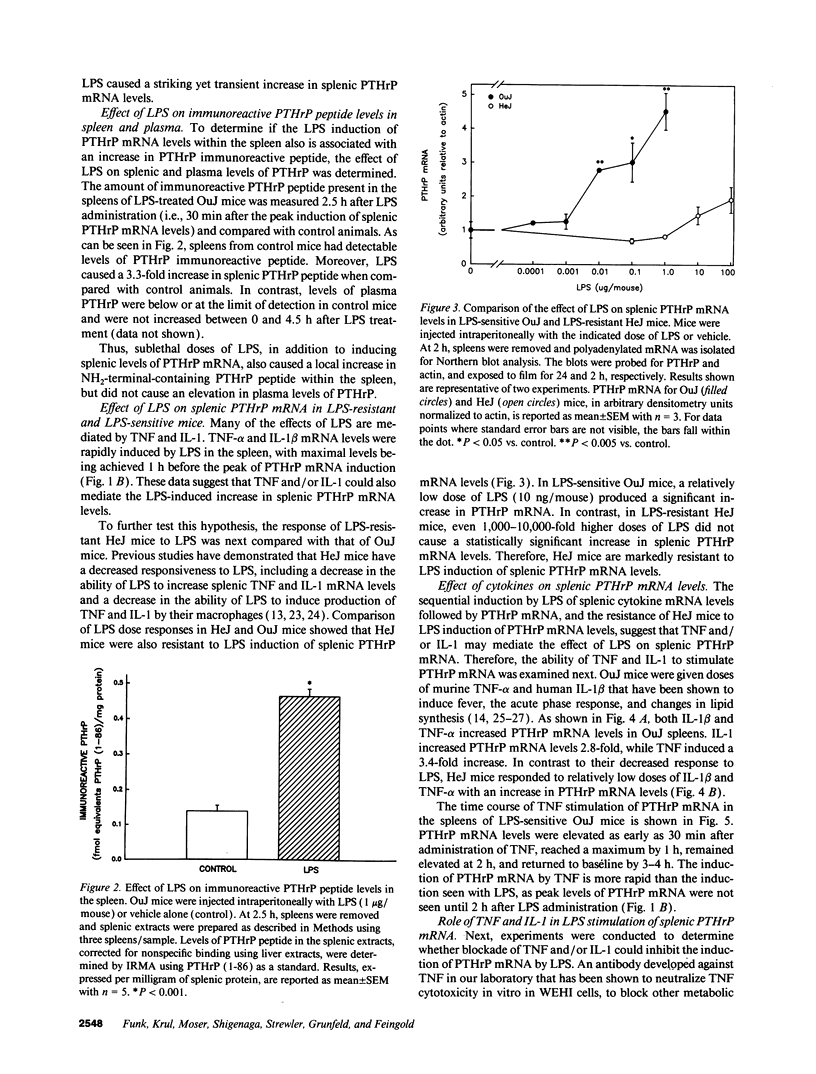
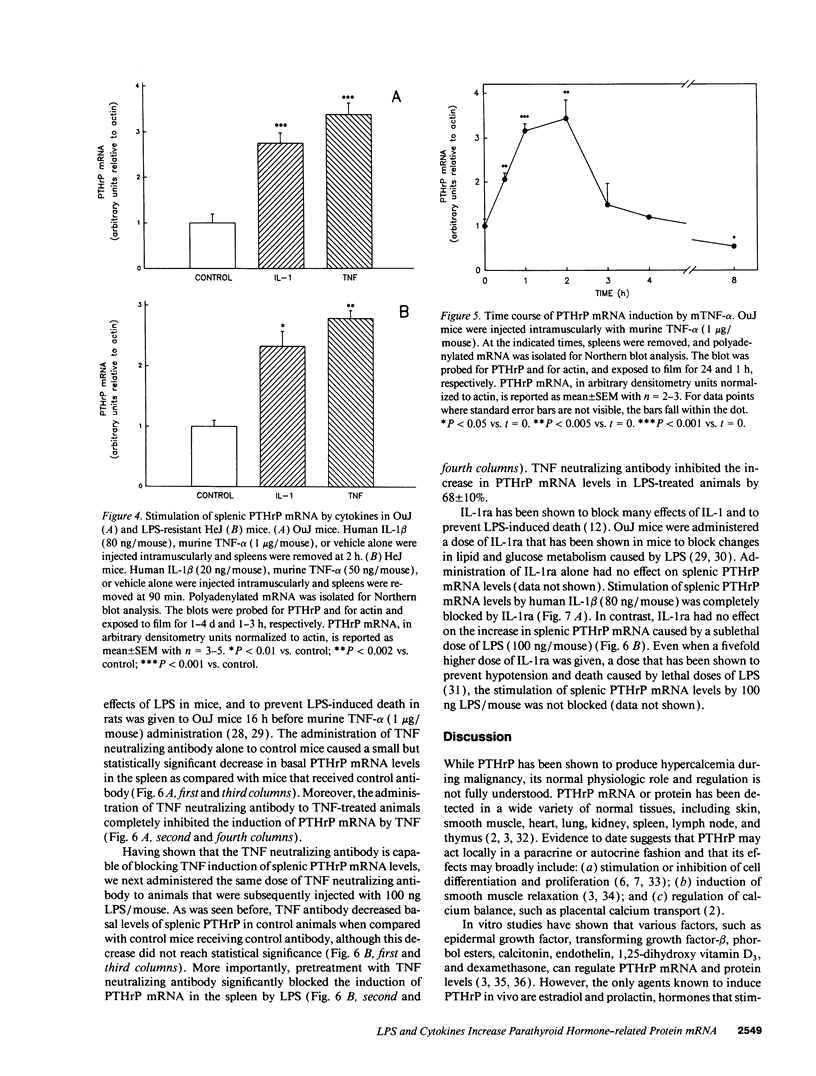
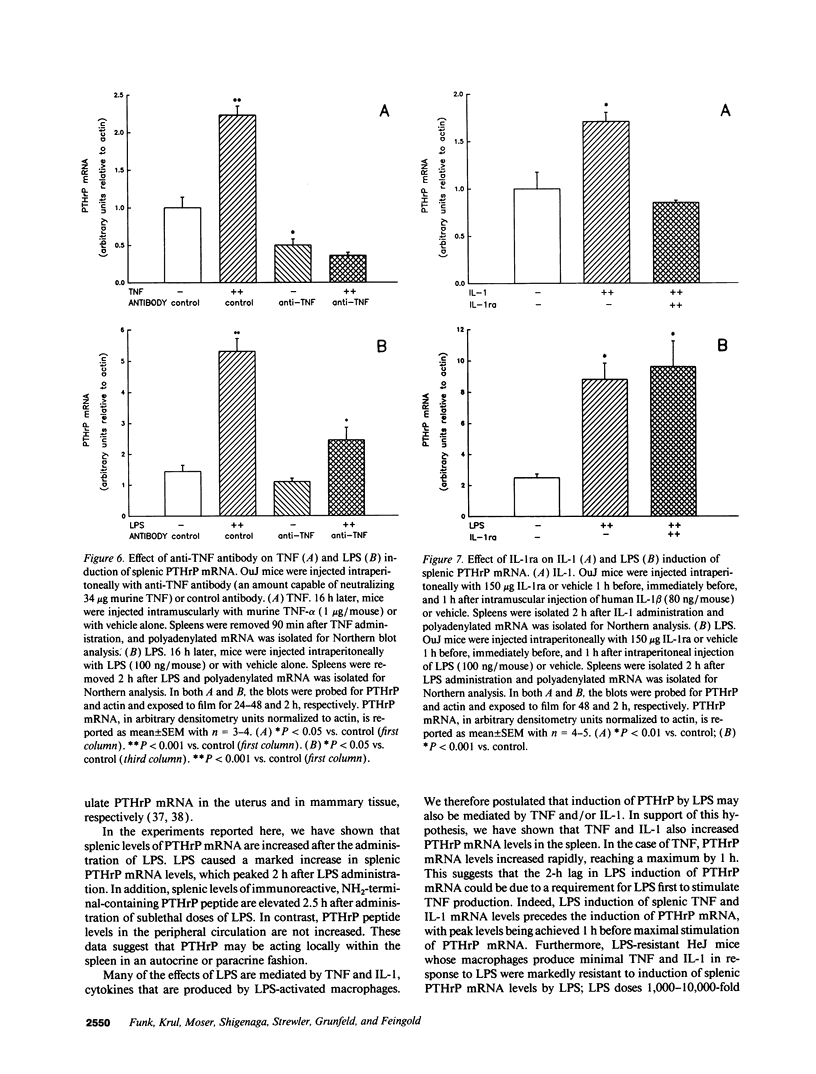
Parathyroid hormone-related protein (PTHrP) causes hypercalcemia in malignancy. However, the role and regulation of PTHrP in normal physiology is just beginning to be explored. PTHrP is found in the spleen and has several other features common to cytokines. Since endotoxin (LPS) causes many of its effects indirectly by inducing cytokines, studies were undertaken to determine whether LPS might also induce splenic PTHrP expression. LPS (100 ng/mouse) increased splenic PTHrP mRNA levels 3.6-fold in C3H/OuJ mice. This effect was maximal at 2 h and returned to baseline by 4 h. PTHrP peptide levels also increased 3.3-fold in splenic extracts in response to LPS (1 microgram/mouse). Murine TNF-alpha and human IL-1 beta, cytokines that mediate many of the effects of LPS, also increased splenic PTHrP mRNA levels. LPS-resistant C3H/HeJ mice, which produce minimal amounts of TNF and IL-1 in response to LPS, were resistant to LPS induction of splenic PTHrP mRNA, while TNF-alpha and IL-1 beta readily increased PTHrP mRNA levels in C3H/HeJ mice. Anti-TNF antibody blocked LPS induction of splenic PTHrP mRNA in C3H/OuJ mice by 68%, indicating that TNF is a mediator of the LPS induction of PTHrP levels. In contrast, an IL-1 receptor antagonist (IL-1ra) was ineffective. The increase in PTHrP in the spleen during the immune response suggests that PTHrP may play an important role in immune modulation, perhaps by mediating changes in lymphocyte proliferation and/or function.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi N., Yamaguchi K., Miyake Y., Honda S., Nagasaki K., Akiyama Y., Adachi I., Abe K. Parathyroid hormone-related protein is a possible autocrine growth inhibitor for lymphocytes. Biochem Biophys Res Commun. 1990 Feb 14;166(3):1088–1094. doi: 10.1016/0006-291x(90)90978-v. [DOI] [PubMed] [Google Scholar]
- Adi S., Pollock A. S., Shigenaga J. K., Moser A. H., Feingold K. R., Grunfeld C. Role for monokines in the metabolic effects of endotoxin. Interferon-gamma restores responsiveness of C3H/HeJ mice in vivo. J Clin Invest. 1992 May;89(5):1603–1609. doi: 10.1172/JCI115755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Bialasiewicz A. A., Jüppner H., Diehl V., Hesch R. D. Binding of bovine parathyroid hormone to surface receptors of cultured B-lymphocytes. Biochim Biophys Acta. 1979 May 16;584(3):467–478. doi: 10.1016/0304-4165(79)90120-x. [DOI] [PubMed] [Google Scholar]
- Budayr A. A., Nissenson R. A., Klein R. F., Pun K. K., Clark O. H., Diep D., Arnaud C. D., Strewler G. J. Increased serum levels of a parathyroid hormone-like protein in malignancy-associated hypercalcemia. Ann Intern Med. 1989 Nov 15;111(10):807–812. doi: 10.7326/0003-4819-111-10-807. [DOI] [PubMed] [Google Scholar]
- Burtis W. J., Brady T. G., Orloff J. J., Ersbak J. B., Warrell R. P., Jr, Olson B. R., Wu T. L., Mitnick M. E., Broadus A. E., Stewart A. F. Immunochemical characterization of circulating parathyroid hormone-related protein in patients with humoral hypercalcemia of cancer. N Engl J Med. 1990 Apr 19;322(16):1106–1112. doi: 10.1056/NEJM199004193221603. [DOI] [PubMed] [Google Scholar]
- Chan S. D., Strewler G. J., King K. L., Nissenson R. A. Expression of a parathyroid hormone-like protein and its receptor during differentiation of embryonal carcinoma cells. Mol Endocrinol. 1990 Apr;4(4):638–646. doi: 10.1210/mend-4-4-638. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Mier J. W., Bernheim H. A., LoPreste G., Lynn D. L., Love R. N., Webb A. C., Auron P. E., Reuben R. C. Multiple biological activities of human recombinant interleukin 1. J Clin Invest. 1986 Jun;77(6):1734–1739. doi: 10.1172/JCI112495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Doherty G. M., Lange J. R., Langstein H. N., Alexander H. R., Buresh C. M., Norton J. A. Evidence for IFN-gamma as a mediator of the lethality of endotoxin and tumor necrosis factor-alpha. J Immunol. 1992 Sep 1;149(5):1666–1670. [PubMed] [Google Scholar]
- Feingold K. R., Staprans I., Memon R. A., Moser A. H., Shigenaga J. K., Doerrler W., Dinarello C. A., Grunfeld C. Endotoxin rapidly induces changes in lipid metabolism that produce hypertriglyceridemia: low doses stimulate hepatic triglyceride production while high doses inhibit clearance. J Lipid Res. 1992 Dec;33(12):1765–1776. [PubMed] [Google Scholar]
- Fong Y. M., Marano M. A., Moldawer L. L., Wei H., Calvano S. E., Kenney J. S., Allison A. C., Cerami A., Shires G. T., Lowry S. F. The acute splanchnic and peripheral tissue metabolic response to endotoxin in humans. J Clin Invest. 1990 Jun;85(6):1896–1904. doi: 10.1172/JCI114651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong Y., Tracey K. J., Moldawer L. L., Hesse D. G., Manogue K. B., Kenney J. S., Lee A. T., Kuo G. C., Allison A. C., Lowry S. F. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1 beta and interleukin 6 appearance during lethal bacteremia. J Exp Med. 1989 Nov 1;170(5):1627–1633. doi: 10.1084/jem.170.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris H. W., Grunfeld C., Feingold K. R., Rapp J. H. Human very low density lipoproteins and chylomicrons can protect against endotoxin-induced death in mice. J Clin Invest. 1990 Sep;86(3):696–702. doi: 10.1172/JCI114765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T., Kupfer J., Enomoto H., Sharifi B., Giannella-Neto D., Forrester J. S., Singer F. R., Goltzman D., Hendy G. N., Pirola C. Abundant expression of parathyroid hormone-related protein in primary rat aortic smooth muscle cells accompanies serum-induced proliferation. J Clin Invest. 1991 Dec;88(6):1841–1847. doi: 10.1172/JCI115505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide S. L., Inaba K., Steinman R. M. Interleukin 1 enhances T-dependent immune responses by amplifying the function of dendritic cells. J Exp Med. 1987 Feb 1;165(2):515–530. doi: 10.1084/jem.165.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer S., Reynolds F. H., Jr, Castillo M., Valenzuela D. M., Thorikay M., Sorvillo J. M. Immunological identification and distribution of parathyroid hormone-like protein polypeptides in normal and malignant tissues. Endocrinology. 1991 Apr;128(4):1927–1937. doi: 10.1210/endo-128-4-1927. [DOI] [PubMed] [Google Scholar]
- Mangin M., Ikeda K., Broadus A. E. Structure of the mouse gene encoding parathyroid hormone-related peptide. Gene. 1990 Nov 15;95(2):195–202. doi: 10.1016/0378-1119(90)90362-u. [DOI] [PubMed] [Google Scholar]
- Martin T. J., Moseley J. M., Gillespie M. T. Parathyroid hormone-related protein: biochemistry and molecular biology. Crit Rev Biochem Mol Biol. 1991;26(3-4):377–395. doi: 10.3109/10409239109114073. [DOI] [PubMed] [Google Scholar]
- McCauley L. K., Rosol T. J., Merryman J. I., Capen C. C. Parathyroid hormone-related protein binding to human T-cell lymphotropic virus type I-infected lymphocytes. Endocrinology. 1992 Jan;130(1):300–306. doi: 10.1210/endo.130.1.1309334. [DOI] [PubMed] [Google Scholar]
- Memon R. A., Feingold K. R., Moser A. H., Doerrler W., Adi S., Dinarello C. A., Grunfeld C. Differential effects of interleukin-1 and tumor necrosis factor on ketogenesis. Am J Physiol. 1992 Aug;263(2 Pt 1):E301–E309. doi: 10.1152/ajpendo.1992.263.2.E301. [DOI] [PubMed] [Google Scholar]
- Mortensen R. F., Shapiro J., Lin B. F., Douches S., Neta R. Interaction of recombinant IL-1 and recombinant tumor necrosis factor in the induction of mouse acute phase proteins. J Immunol. 1988 Apr 1;140(7):2260–2266. [PubMed] [Google Scholar]
- Ohlsson K., Björk P., Bergenfeldt M., Hageman R., Thompson R. C. Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature. 1990 Dec 6;348(6301):550–552. doi: 10.1038/348550a0. [DOI] [PubMed] [Google Scholar]
- Perry H. M., 3rd, Chappel J. C., Bellorin-Font E., Tamao J., Martin K. J., Teitelbaum S. L. Parathyroid hormone receptors in circulating human mononuclear leukocytes. J Biol Chem. 1984 May 10;259(9):5531–5535. [PubMed] [Google Scholar]
- Rodan S. B., Wesolowski G., Ianacone J., Thiede M. A., Rodan G. A. Production of parathyroid hormone-like peptide in a human osteosarcoma cell line: stimulation by phorbol esters and epidermal growth factor. J Endocrinol. 1989 Jul;122(1):219–227. doi: 10.1677/joe.0.1220219. [DOI] [PubMed] [Google Scholar]
- Rosenstreich D. L., Vogel S. N., Jacques A. R., Wahl L. M., Oppenheim J. J. Macrophage sensitivity to endotoxin: genetic control by a single codominant gene. J Immunol. 1978 Nov;121(5):1664–1670. [PubMed] [Google Scholar]
- Selvanayagam P., Graves K., Cooper C., Rajaraman S. Expression of the parathyroid hormone-related peptide gene in rat tissues. Lab Invest. 1991 May;64(5):713–717. [PubMed] [Google Scholar]
- Stewart A. F., Broadus A. E. Clinical review 16: Parathyroid hormone-related proteins: coming of age in the 1990s. J Clin Endocrinol Metab. 1990 Dec;71(6):1410–1414. doi: 10.1210/jcem-71-6-1410. [DOI] [PubMed] [Google Scholar]
- Stewart A. F., Insogna K. L., Burtis W. J., Aminiafshar A., Wu T., Weir E. C., Broadus A. E. Frequency and partial characterization of adenylate cyclase-stimulating activity in tumors associated with humoral hypercalcemia of malignancy. J Bone Miner Res. 1986 Jun;1(3):267–276. doi: 10.1002/jbmr.5650010305. [DOI] [PubMed] [Google Scholar]
- Stewart A. F., Insogna K. L., Goltzman D., Broadus A. E. Identification of adenylate cyclase-stimulating activity and cytochemical glucose-6-phosphate dehydrogenase-stimulating activity in extracts of tumors from patients with humoral hypercalcemia of malignancy. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1454–1458. doi: 10.1073/pnas.80.5.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strewler G. J., Nissenson R. A. Peptide mediators of hypercalcemia in malignancy. Annu Rev Med. 1990;41:35–44. doi: 10.1146/annurev.me.41.020190.000343. [DOI] [PubMed] [Google Scholar]
- Thiede M. A., Daifotis A. G., Weir E. C., Brines M. L., Burtis W. J., Ikeda K., Dreyer B. E., Garfield R. E., Broadus A. E. Intrauterine occupancy controls expression of the parathyroid hormone-related peptide gene in preterm rat myometrium. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6969–6973. doi: 10.1073/pnas.87.18.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiede M. A., Harm S. C., Hasson D. M., Gardner R. M. In vivo regulation of parathyroid hormone-related peptide messenger ribonucleic acid in the rat uterus by 17 beta-estradiol. Endocrinology. 1991 May;128(5):2317–2323. doi: 10.1210/endo-128-5-2317. [DOI] [PubMed] [Google Scholar]
- Thiede M. A. The mRNA encoding a parathyroid hormone-like peptide is produced in mammary tissue in response to elevations in serum prolactin. Mol Endocrinol. 1989 Sep;3(9):1443–1447. doi: 10.1210/mend-3-9-1443. [DOI] [PubMed] [Google Scholar]
- Thurston A. W., Cole J. A., Hillman L. S., Im J. H., Thorne P. K., Krause W. J., Jones J. R., Eber S. L., Forte L. R. Purification and properties of parathyroid hormone-related peptide isolated from milk. Endocrinology. 1990 Feb;126(2):1183–1190. doi: 10.1210/endo-126-2-1183. [DOI] [PubMed] [Google Scholar]
- Vogel S. N., Henricson B. E., Neta R. Roles of interleukin-1 and tumor necrosis factor in lipopolysaccharide-induced hypoglycemia. Infect Immun. 1991 Jul;59(7):2494–2498. doi: 10.1128/iai.59.7.2494-2498.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood L. C., Jackson S. M., Elias P. M., Grunfeld C., Feingold K. R. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest. 1992 Aug;90(2):482–487. doi: 10.1172/JCI115884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto I., Potts J. T., Jr, Segre G. V. Circulating bovine lymphocytes contain receptors for parathyroid hormone. J Clin Invest. 1983 Feb;71(2):404–407. doi: 10.1172/JCI110784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Harm S. C., Grasser W. A., Thiede M. A. Parathyroid hormone-related protein in the rat urinary bladder: a smooth muscle relaxant produced locally in response to mechanical stretch. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5326–5330. doi: 10.1073/pnas.89.12.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]



