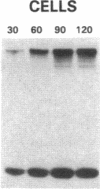
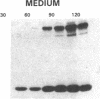
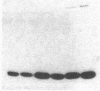
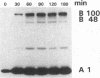
Abstract
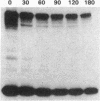
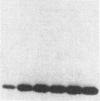
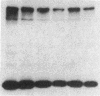
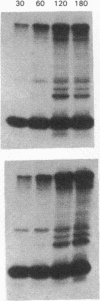
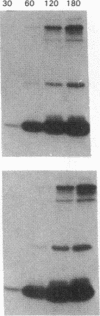
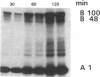
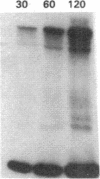
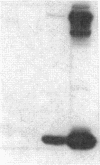
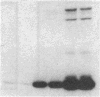
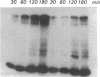
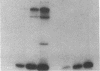
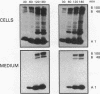
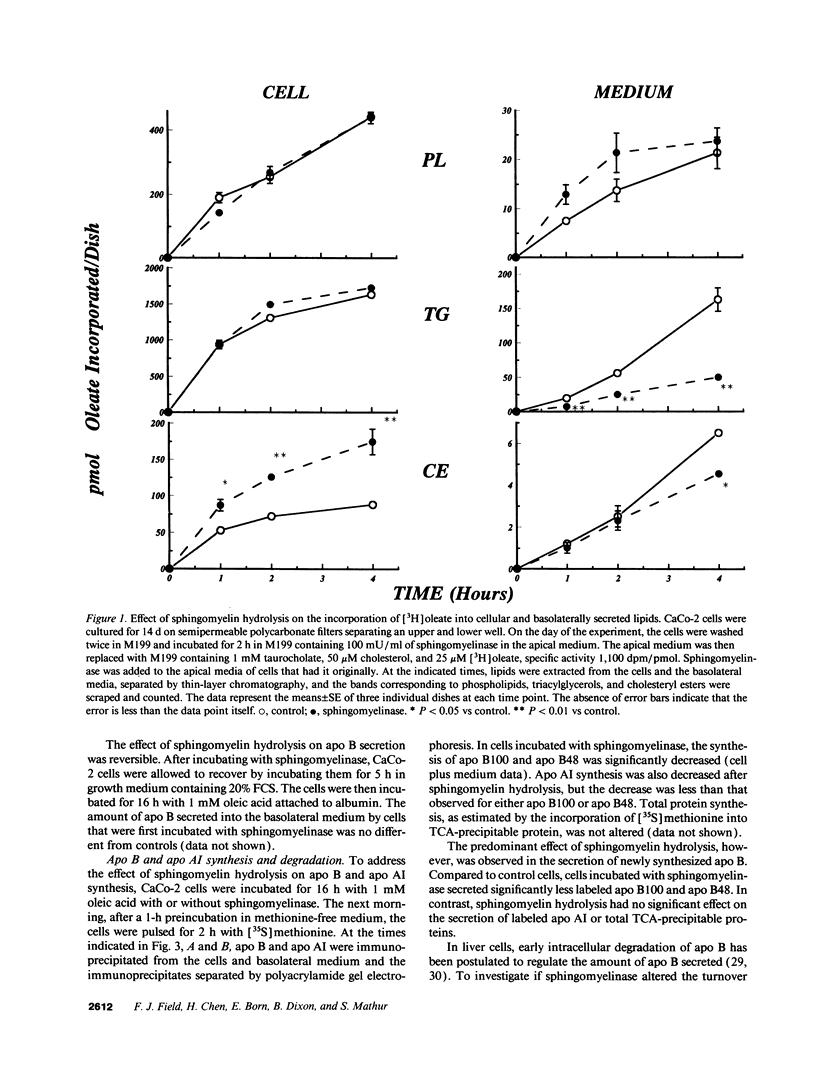
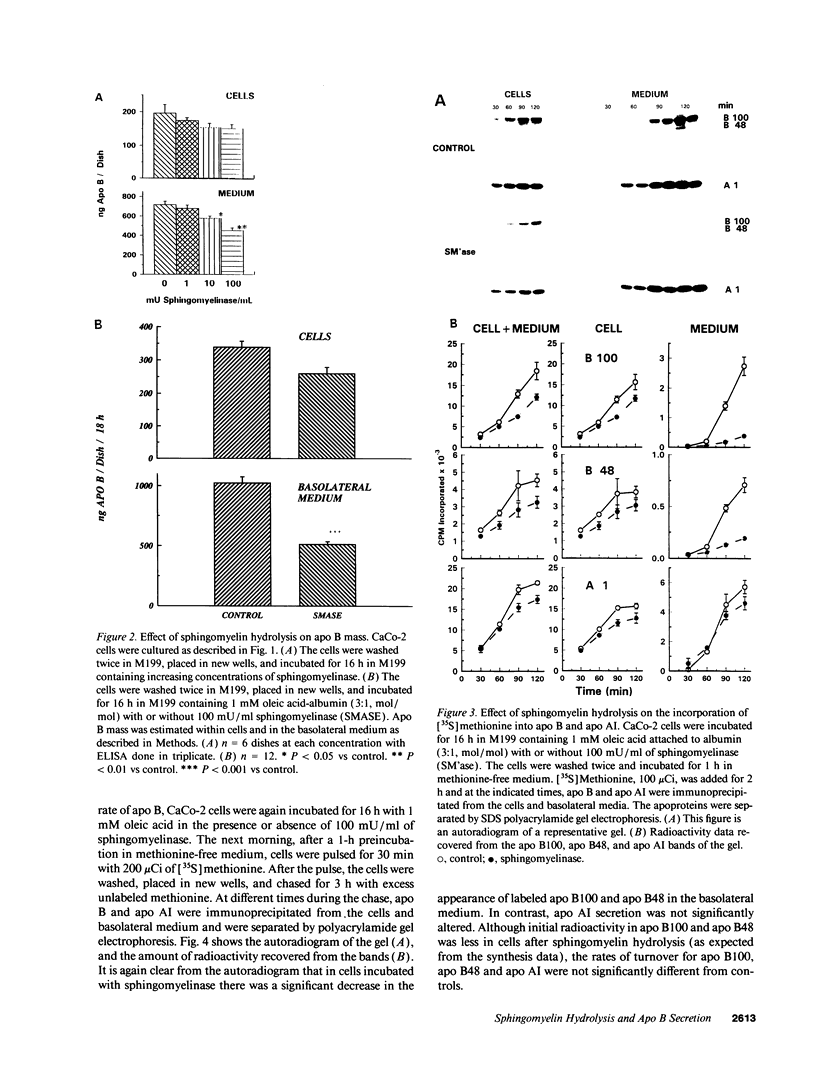
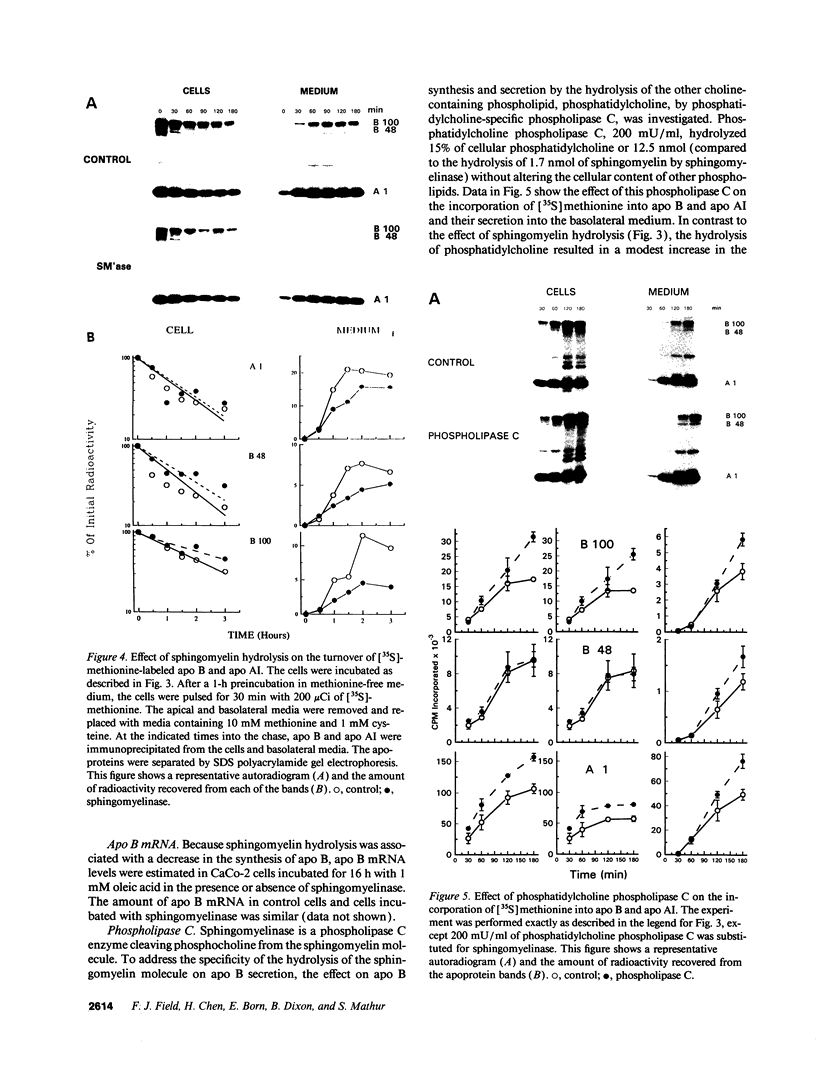
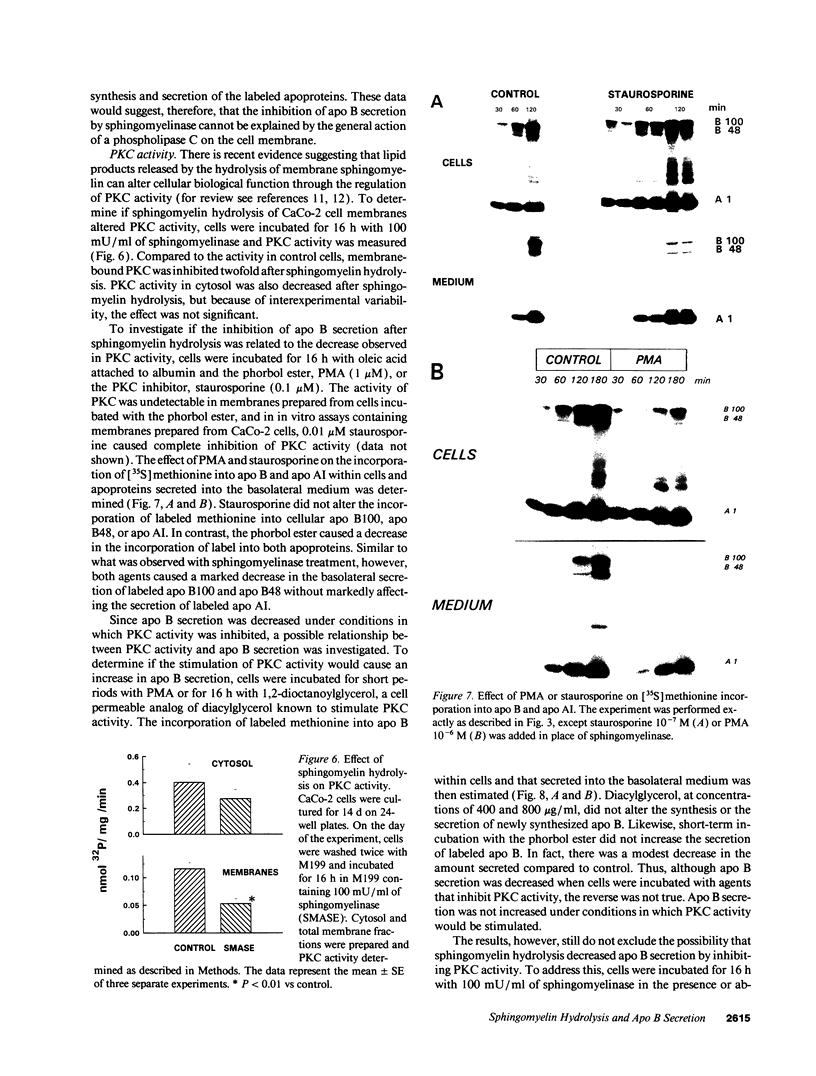
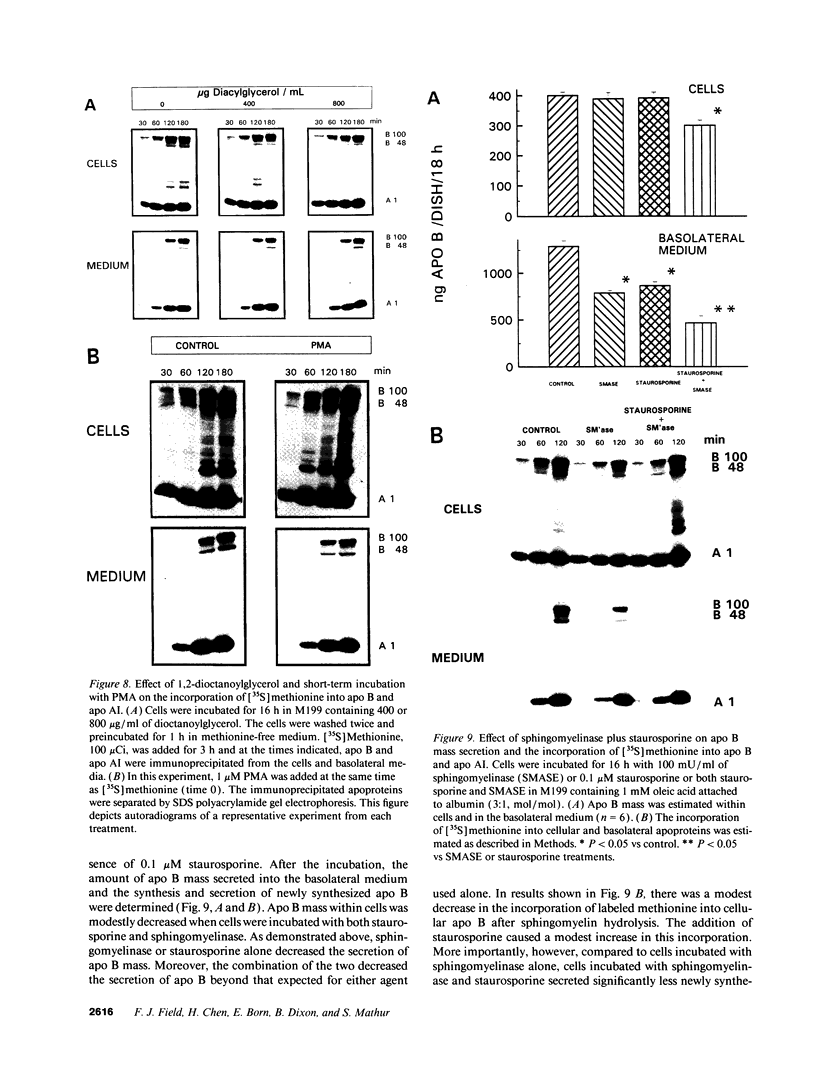
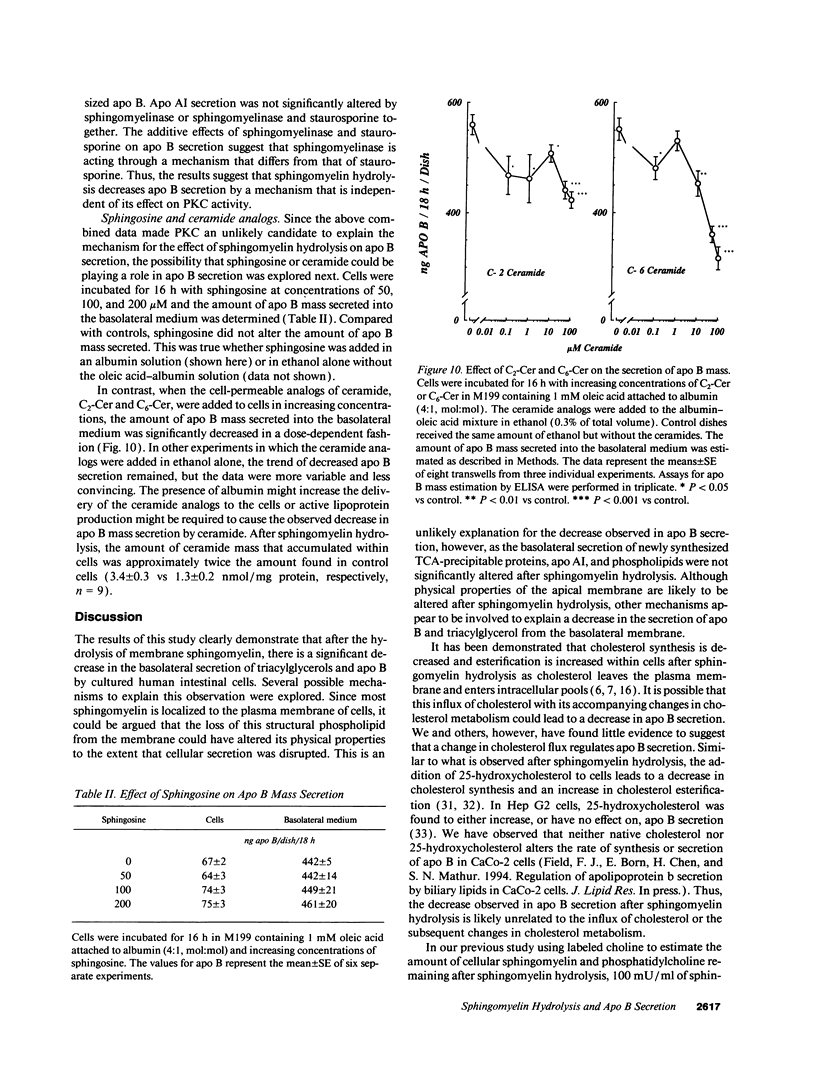
The effect of sphingomyelin hydrolysis on triacylglycerol-rich lipoprotein secretion was examined in the human intestinal cell line, CaCo-2. Addition of sphingomyelinase decreased sphingomyelin and phosphatidylethanolamine by 60 and 20%, respectively. Sphingomyelin hydrolysis decreased the basolateral secretion of triacylglycerol mass, newly synthesized triacylglycerol, and apo B mass. Pulse-chase experiments with [35S]methionine demonstrated a decrease in apo B synthesis and a marked decrease in apo B100 and apo B48 secretion without altering apo A1 secretion. Sphingomyelin hydrolysis did not change apo B mRNA levels nor apo B turnover. Phosphatidylcholine-specific phospholipase C did not decrease apo B synthesis or its basolateral secretion. Membrane protein kinase C (PKC) activity was decreased twofold after sphingomyelin hydrolysis. The PKC inhibitor staurosporine decreased apo B mass and newly synthesized apo B secretion. Sphingomyelinase and staurosporine together caused an additional decrease in apo B secretion suggesting that sphingomyelin hydrolysis decreased apo B secretion independently of its effect on PKC activity. Moreover, conditions that increase PKC activity did not increase apo B secretion. Cell-permeable analogs of ceramide decreased immunoreactive apo B secretion. Sphingosine was without effect. The hydrolysis of membrane sphingomyelin by intestinal or pancreatic neutral sphingomyelinase may lead to the accumulation of cellular ceramide, which, in turn, could inhibit triacylglycerol-rich lipoprotein secretion.
Full text
PDF




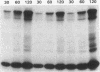
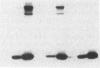
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chalvardjian A., Rudnicki E. Determination of lipid phosphorus in the nanomolar range. Anal Biochem. 1970 Jul;36(1):225–226. doi: 10.1016/0003-2697(70)90352-0. [DOI] [PubMed] [Google Scholar]
- Chen H., Born E., Mathur S. N., Johlin F. C., Jr, Field F. J. Sphingomyelin content of intestinal cell membranes regulates cholesterol absorption. Evidence for pancreatic and intestinal cell sphingomyelinase activity. Biochem J. 1992 Sep 15;286(Pt 3):771–777. doi: 10.1042/bj2860771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dashti N., Smith E. A., Alaupovic P. Increased production of apolipoprotein B and its lipoproteins by oleic acid in Caco-2 cells. J Lipid Res. 1990 Jan;31(1):113–123. [PubMed] [Google Scholar]
- Dashti N. The effect of low density lipoproteins, cholesterol, and 25-hydroxycholesterol on apolipoprotein B gene expression in HepG2 cells. J Biol Chem. 1992 Apr 5;267(10):7160–7169. [PubMed] [Google Scholar]
- Dixon B. S., Breckon R., Burke C., Anderson R. J. Phorbol esters inhibit adenylate cyclase activity in cultured collecting tubular cells. Am J Physiol. 1988 Jan;254(1 Pt 1):C183–C191. doi: 10.1152/ajpcell.1988.254.1.C183. [DOI] [PubMed] [Google Scholar]
- Dixon J. L., Furukawa S., Ginsberg H. N. Oleate stimulates secretion of apolipoprotein B-containing lipoproteins from Hep G2 cells by inhibiting early intracellular degradation of apolipoprotein B. J Biol Chem. 1991 Mar 15;266(8):5080–5086. [PubMed] [Google Scholar]
- Dobrowsky R. T., Hannun Y. A. Ceramide stimulates a cytosolic protein phosphatase. J Biol Chem. 1992 Mar 15;267(8):5048–5051. [PubMed] [Google Scholar]
- Doucet J. P., Murphy B. J., Tuana B. S. Modification of a discontinuous and highly porous sodium dodecyl sulfate-polyacrylamide gel system for minigel electrophoresis. Anal Biochem. 1990 Nov 1;190(2):209–211. doi: 10.1016/0003-2697(90)90182-9. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Field F. J., Albright E., Mathur S. Inhibition of acylcoenzyme A: cholesterol acyltransferase activity by PD128O42: effect on cholesterol metabolism and secretion in CaCo-2 cells. Lipids. 1991 Jan;26(1):1–8. doi: 10.1007/BF02544016. [DOI] [PubMed] [Google Scholar]
- Field F. J., Mathur S. N. Regulation of acyl CoA:cholesterol acyltransferase by 25-hydroxycholesterol in rabbit intestinal microsomes and absorptive cells. J Lipid Res. 1983 Aug;24(8):1049–1059. [PubMed] [Google Scholar]
- Goldkorn T., Dressler K. A., Muindi J., Radin N. S., Mendelsohn J., Menaldino D., Liotta D., Kolesnick R. N. Ceramide stimulates epidermal growth factor receptor phosphorylation in A431 human epidermoid carcinoma cells. Evidence that ceramide may mediate sphingosine action. J Biol Chem. 1991 Aug 25;266(24):16092–16097. [PubMed] [Google Scholar]
- Gupta A. K., Rudney H. Plasma membrane sphingomyelin and the regulation of HMG-CoA reductase activity and cholesterol biosynthesis in cell cultures. J Lipid Res. 1991 Jan;32(1):125–136. [PubMed] [Google Scholar]
- Hampton R. Y., Morand O. H. Sphingomyelin synthase and PKC activation. Science. 1989 Nov 24;246(4933):1050–1050. doi: 10.1126/science.2555921. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A., Bell R. M. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 1989 Jan 27;243(4890):500–507. doi: 10.1126/science.2643164. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Merrill A. H., Jr, Bell R. M. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J Biol Chem. 1986 Sep 25;261(27):12604–12609. [PubMed] [Google Scholar]
- Hostetler K. Y., Zenner B. D., Morris H. P. Phospholipid content of mitochondrial and microsomal membranes from Morris hepatomas of varying growth rates. Cancer Res. 1979 Aug;39(8):2978–2983. [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W. Regulation of sterol synthesis in cultured cells by oxygenated derivatives of cholesterol. J Cell Physiol. 1975 Apr;85(2 Pt 2 Suppl 1):415–424. doi: 10.1002/jcp.1040850408. [DOI] [PubMed] [Google Scholar]
- Kolesnick R. N., Clegg S. 1,2-Diacylglycerols, but not phorbol esters, activate a potential inhibitory pathway for protein kinase C in GH3 pituitary cells. Evidence for involvement of a sphingomyelinase. J Biol Chem. 1988 May 15;263(14):6534–6537. [PubMed] [Google Scholar]
- Kolesnick R. N. Sphingomyelin and derivatives as cellular signals. Prog Lipid Res. 1991;30(1):1–38. doi: 10.1016/0163-7827(91)90005-p. [DOI] [PubMed] [Google Scholar]
- Koval M., Pagano R. E. Intracellular transport and metabolism of sphingomyelin. Biochim Biophys Acta. 1991 Mar 12;1082(2):113–125. doi: 10.1016/0005-2760(91)90184-j. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lange Y., D'Alessandro J. S., Small D. M. The affinity of cholesterol for phosphatidylcholine and sphingomyelin. Biochim Biophys Acta. 1979 Oct 5;556(3):388–398. doi: 10.1016/0005-2736(79)90127-5. [DOI] [PubMed] [Google Scholar]
- Lange Y., Swaisgood M. H., Ramos B. V., Steck T. L. Plasma membranes contain half the phospholipid and 90% of the cholesterol and sphingomyelin in cultured human fibroblasts. J Biol Chem. 1989 Mar 5;264(7):3786–3793. [PubMed] [Google Scholar]
- Liscum L., Faust J. R. Low density lipoprotein (LDL)-mediated suppression of cholesterol synthesis and LDL uptake is defective in Niemann-Pick type C fibroblasts. J Biol Chem. 1987 Dec 15;262(35):17002–17008. [PubMed] [Google Scholar]
- Moberly J. B., Cole T. G., Alpers D. H., Schonfeld G. Oleic acid stimulation of apolipoprotein B secretion from HepG2 and Caco-2 cells occurs post-transcriptionally. Biochim Biophys Acta. 1990 Jan 16;1042(1):70–80. doi: 10.1016/0005-2760(90)90058-6. [DOI] [PubMed] [Google Scholar]
- Murthy S., Albright E., Mathur S. N., Davidson N. O., Field F. J. Apolipoprotein B mRNA abundance is decreased by eicosapentaenoic acid in CaCo-2 cells. Effect on the synthesis and secretion of apolipoprotein B. Arterioscler Thromb. 1992 Jun;12(6):691–700. doi: 10.1161/01.atv.12.6.691. [DOI] [PubMed] [Google Scholar]
- Murthy S., Albright E., Mathur S. N., Field F. J. Effect of eicosapentaenoic acid on triacylglycerol transport in CaCo-2 cells. Biochim Biophys Acta. 1990 Jul 16;1045(2):147–155. doi: 10.1016/0005-2760(90)90144-m. [DOI] [PubMed] [Google Scholar]
- Okazaki T., Bielawska A., Bell R. M., Hannun Y. A. Role of ceramide as a lipid mediator of 1 alpha,25-dihydroxyvitamin D3-induced HL-60 cell differentiation. J Biol Chem. 1990 Sep 15;265(26):15823–15831. [PubMed] [Google Scholar]
- Olivera A., Buckley N. E., Spiegel S. Sphingomyelinase and cell-permeable ceramide analogs stimulate cellular proliferation in quiescent Swiss 3T3 fibroblasts. J Biol Chem. 1992 Dec 25;267(36):26121–26127. [PubMed] [Google Scholar]
- Pagano R. E., Martin O. C., Kang H. C., Haugland R. P. A novel fluorescent ceramide analogue for studying membrane traffic in animal cells: accumulation at the Golgi apparatus results in altered spectral properties of the sphingolipid precursor. J Cell Biol. 1991 Jun;113(6):1267–1279. doi: 10.1083/jcb.113.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentchev P. G., Kruth H. S., Comly M. E., Butler J. D., Vanier M. T., Wenger D. A., Patel S. Type C Niemann-Pick disease. A parallel loss of regulatory responses in both the uptake and esterification of low density lipoprotein-derived cholesterol in cultured fibroblasts. J Biol Chem. 1986 Dec 15;261(35):16775–16780. [PubMed] [Google Scholar]
- Preiss J. E., Loomis C. R., Bell R. M., Niedel J. E. Quantitative measurement of sn-1,2-diacylglycerols. Methods Enzymol. 1987;141:294–300. doi: 10.1016/0076-6879(87)41077-x. [DOI] [PubMed] [Google Scholar]
- Rosenwald A. G., Pagano R. E. Inhibition of glycoprotein traffic through the secretory pathway by ceramide. J Biol Chem. 1993 Mar 5;268(7):4577–4579. [PubMed] [Google Scholar]
- Sidhu H. S., Rastogi S. A., Byers D. M., Cook H. W., Palmer F. B., Spence M. W. Cultured fibroblasts from patients with Niemann-Pick disease type C and type D exhibit distinct defects in cholesterol esterification. Biochim Biophys Acta. 1992 Feb 20;1124(1):29–35. doi: 10.1016/0005-2760(92)90122-c. [DOI] [PubMed] [Google Scholar]
- Slotte J. P., Bierman E. L. Depletion of plasma-membrane sphingomyelin rapidly alters the distribution of cholesterol between plasma membranes and intracellular cholesterol pools in cultured fibroblasts. Biochem J. 1988 Mar 15;250(3):653–658. doi: 10.1042/bj2500653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks J. D., Sparks C. E. Insulin modulation of hepatic synthesis and secretion of apolipoprotein B by rat hepatocytes. J Biol Chem. 1990 May 25;265(15):8854–8862. [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Williams R. D., Sgoutas D. S., Zaatari G. S. Enzymology of long-chain base synthesis by aorta: induction of serine palmitoyltransferase activity in rabbit aorta during atherogenesis. J Lipid Res. 1986 Jul;27(7):763–770. [PubMed] [Google Scholar]
- Yao Z. M., Vance D. E. Head group specificity in the requirement of phosphatidylcholine biosynthesis for very low density lipoprotein secretion from cultured hepatocytes. J Biol Chem. 1989 Jul 5;264(19):11373–11380. [PubMed] [Google Scholar]
- Yao Z. M., Vance D. E. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J Biol Chem. 1988 Feb 25;263(6):2998–3004. [PubMed] [Google Scholar]
- Yechiel E., Henis Y. I., Barenholz Y. Aging of rat heart fibroblasts: relationship between lipid composition, membrane organization and biological properties. Biochim Biophys Acta. 1986 Jul 10;859(1):95–104. doi: 10.1016/0005-2736(86)90322-6. [DOI] [PubMed] [Google Scholar]