Abstract
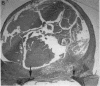
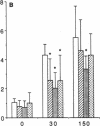
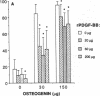
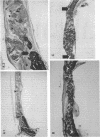
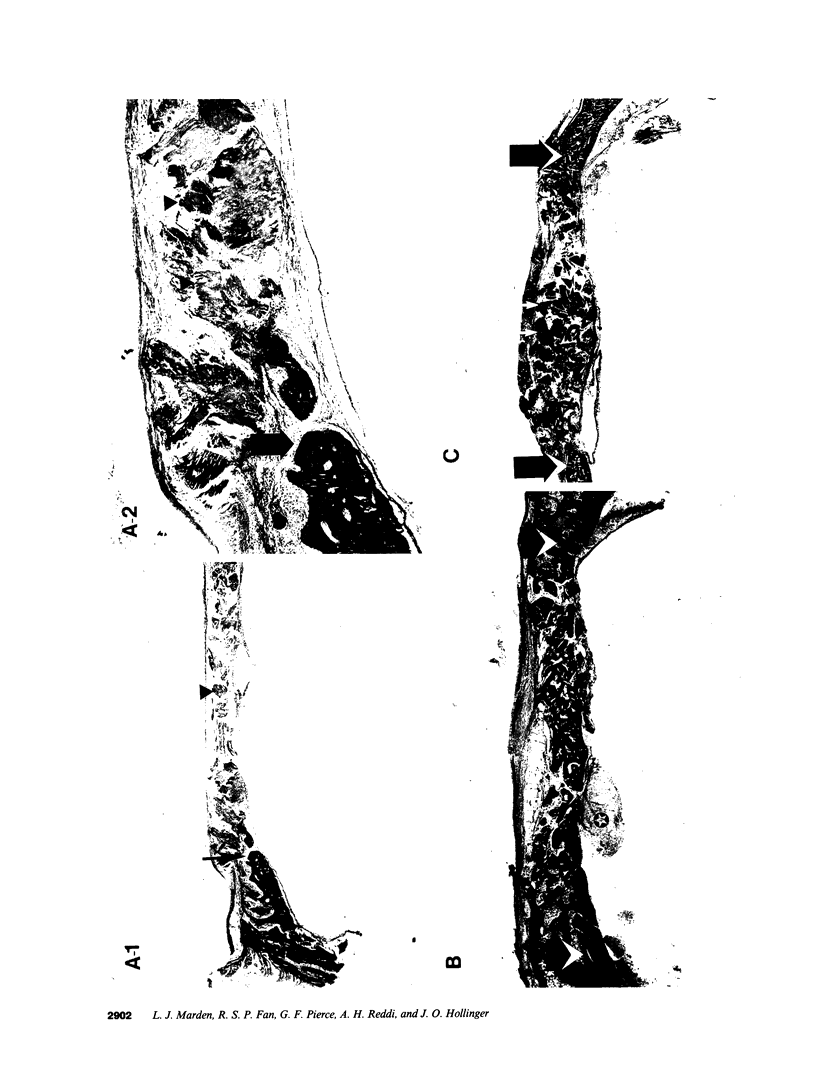
Platelet-derived growth factor (PDGF) is a potent moderator of soft tissue repair through induction of the inflammatory phase of repair and subsequent enhanced collagen deposition. We examined the effect of recombinant BB homodimer PDGF (rPDGF-BB) applied to rat craniotomy defects, treated with and without bovine osteogenin (OG), to see if bone regeneration would be stimulated. Implants containing 0, 20, 60, or 200 micrograms rPDGF-BB, reconstituted with insoluble rat collagenous bone matrix containing 0, 30, or 150 micrograms OG, were placed into 8-mm craniotomies. After 11 d, 21 of the 144 rats presented subcutaneous masses superior to the defect sites. The masses, comprised of serosanguinous fluid encapsulated by fibrous connective tissue, were larger and occurred more frequently in rats treated with 200 micrograms rPDGF-BB, and were absent in rats not treated with rPDGF-BB. The masses underwent resorption within 28 d after surgery. OG (2-256 micrograms) caused a dose-dependent increase in radiopacity and a marked regeneration of calcified tissue in a dose-dependent fashion within defect sites. However, OG-induced bone regeneration was inhibited 17-53% in the presence of rPDGF-BB. These results suggest that rPDGF-BB inhibited OG-induced bone regeneration and stimulated a soft tissue repair wound phenotype and response.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Canalis E. Effect of platelet-derived growth factor on DNA and protein synthesis in cultured rat calvaria. Metabolism. 1981 Oct;30(10):970–975. doi: 10.1016/0026-0495(81)90094-9. [DOI] [PubMed] [Google Scholar]
- Centrella M., McCarthy T. L., Kusmik W. F., Canalis E. Isoform-specific regulation of platelet-derived growth factor activity and binding in osteoblast-enriched cultures from fetal rat bone. J Clin Invest. 1992 Apr;89(4):1076–1084. doi: 10.1172/JCI115687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centrella M., McCarthy T. L., Kusmik W. F., Canalis E. Relative binding and biochemical effects of heterodimeric and homodimeric isoforms of platelet-derived growth factor in osteoblast-enriched cultures from fetal rat bone. J Cell Physiol. 1991 Jun;147(3):420–426. doi: 10.1002/jcp.1041470306. [DOI] [PubMed] [Google Scholar]
- Cohen A. M., Soderberg C., Thomason A. Plasma clearance and tissue distribution of recombinant human platelet-derived growth factor (B-chain homodimer) in rats. J Surg Res. 1990 Nov;49(5):447–452. doi: 10.1016/0022-4804(90)90194-7. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Huang J. S., Griffin G. L. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J Clin Invest. 1982 Apr;69(4):1046–1049. doi: 10.1172/JCI110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppley B. L., Doucet M., Connolly D. T., Feder J. Enhancement of angiogenesis by bFGF in mandibular bone graft healing in the rabbit. J Oral Maxillofac Surg. 1988 May;46(5):391–398. doi: 10.1016/0278-2391(88)90223-6. [DOI] [PubMed] [Google Scholar]
- Graves D. T., Valentin-Opran A., Delgado R., Valente A. J., Mundy G., Piche J. The potential role of platelet-derived growth factor as an autocrine or paracrine factor for human bone cells. Connect Tissue Res. 1989;23(2-3):209–218. doi: 10.3109/03008208909002419. [DOI] [PubMed] [Google Scholar]
- Greenhalgh D. G., Sprugel K. H., Murray M. J., Ross R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol. 1990 Jun;136(6):1235–1246. [PMC free article] [PubMed] [Google Scholar]
- Grotendorst G. R. Alteration of the chemotactic response of NIH/3T3 cells to PDGF by growth factors, transformation, and tumor promoters. Cell. 1984 Feb;36(2):279–285. doi: 10.1016/0092-8674(84)90221-6. [DOI] [PubMed] [Google Scholar]
- Grotendorst G. R., Martin G. R., Pencev D., Sodek J., Harvey A. K. Stimulation of granulation tissue formation by platelet-derived growth factor in normal and diabetic rats. J Clin Invest. 1985 Dec;76(6):2323–2329. doi: 10.1172/JCI112243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollinger J. O., Kleinschmidt J. C. The critical size defect as an experimental model to test bone repair materials. J Craniofac Surg. 1990 Jan;1(1):60–68. doi: 10.1097/00001665-199001000-00011. [DOI] [PubMed] [Google Scholar]
- Hollinger J. O., Schmitz J. P., Mark D. E., Seyfer A. E. Osseous wound healing with xenogeneic bone implants with a biodegradable carrier. Surgery. 1990 Jan;107(1):50–54. [PubMed] [Google Scholar]
- Howes R., Bowness J. M., Grotendorst G. R., Martin G. R., Reddi A. H. Platelet-derived growth factor enhances demineralized bone matrix-induced cartilage and bone formation. Calcif Tissue Int. 1988 Jan;42(1):34–38. doi: 10.1007/BF02555836. [DOI] [PubMed] [Google Scholar]
- Joyce M. E., Roberts A. B., Sporn M. B., Bolander M. E. Transforming growth factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol. 1990 Jun;110(6):2195–2207. doi: 10.1083/jcb.110.6.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten F. P., Cunningham N. S., Ma S., Muthukumaran N., Hammonds R. G., Nevins W. B., Woods W. I., Reddi A. H. Purification and partial amino acid sequence of osteogenin, a protein initiating bone differentiation. J Biol Chem. 1989 Aug 15;264(23):13377–13380. [PubMed] [Google Scholar]
- Marden L. J., Reddi A. H., Hollinger J. O. Growth and differentiation factors: role in bone induction and potential application in craniofacial surgery. J Craniofac Surg. 1990 Jul;1(3):154–160. [PubMed] [Google Scholar]
- Mark D. E., Hollinger J. O., Hastings C., Jr, Chen G., Marden L. J., Reddi A. H. Repair of calvarial nonunions by osteogenin, a bone-inductive protein. Plast Reconstr Surg. 1990 Oct;86(4):623–632. [PubMed] [Google Scholar]
- Mustoe T. A., Pierce G. F., Morishima C., Deuel T. F. Growth factor-induced acceleration of tissue repair through direct and inductive activities in a rabbit dermal ulcer model. J Clin Invest. 1991 Feb;87(2):694–703. doi: 10.1172/JCI115048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaran N., Ma S., Reddi A. H. Dose-dependence of and threshold for optimal bone induction by collagenous bone matrix and osteogenin-enriched fraction. Coll Relat Res. 1988 Sep;8(5):433–441. doi: 10.1016/s0174-173x(88)80016-5. [DOI] [PubMed] [Google Scholar]
- Noda M., Camilliere J. J. In vivo stimulation of bone formation by transforming growth factor-beta. Endocrinology. 1989 Jun;124(6):2991–2994. doi: 10.1210/endo-124-6-2991. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Oechsner M., Naumann A., Gronwald R. G., Minne H. W., Ziegler R. Stimulation of bone matrix apposition in vitro by local growth factors: a comparison between insulin-like growth factor I, platelet-derived growth factor, and transforming growth factor beta. Endocrinology. 1990 Jul;127(1):69–75. doi: 10.1210/endo-127-1-69. [DOI] [PubMed] [Google Scholar]
- Piché J. E., Graves D. T. Study of the growth factor requirements of human bone-derived cells: a comparison with human fibroblasts. Bone. 1989;10(2):131–138. doi: 10.1016/8756-3282(89)90011-2. [DOI] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Altrock B. W., Deuel T. F., Thomason A. Role of platelet-derived growth factor in wound healing. J Cell Biochem. 1991 Apr;45(4):319–326. doi: 10.1002/jcb.240450403. [DOI] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Lingelbach J., Masakowski V. R., Gramates P., Deuel T. F. Transforming growth factor beta reverses the glucocorticoid-induced wound-healing deficit in rats: possible regulation in macrophages by platelet-derived growth factor. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2229–2233. doi: 10.1073/pnas.86.7.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Lingelbach J., Masakowski V. R., Griffin G. L., Senior R. M., Deuel T. F. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J Cell Biol. 1989 Jul;109(1):429–440. doi: 10.1083/jcb.109.1.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Senior R. M., Reed J., Griffin G. L., Thomason A., Deuel T. F. In vivo incisional wound healing augmented by platelet-derived growth factor and recombinant c-sis gene homodimeric proteins. J Exp Med. 1988 Mar 1;167(3):974–987. doi: 10.1084/jem.167.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Tarpley J. E., Yanagihara D., Mustoe T. A., Fox G. M., Thomason A. Platelet-derived growth factor (BB homodimer), transforming growth factor-beta 1, and basic fibroblast growth factor in dermal wound healing. Neovessel and matrix formation and cessation of repair. Am J Pathol. 1992 Jun;140(6):1375–1388. [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Vande Berg J., Rudolph R., Tarpley J., Mustoe T. A. Platelet-derived growth factor-BB and transforming growth factor beta 1 selectively modulate glycosaminoglycans, collagen, and myofibroblasts in excisional wounds. Am J Pathol. 1991 Mar;138(3):629–646. [PMC free article] [PubMed] [Google Scholar]
- Russell S. M., Spencer E. M. Local injections of human or rat growth hormone or of purified human somatomedin-C stimulate unilateral tibial epiphyseal growth in hypophysectomized rats. Endocrinology. 1985 Jun;116(6):2563–2567. doi: 10.1210/endo-116-6-2563. [DOI] [PubMed] [Google Scholar]
- Sampath T. K., Reddi A. H. Dissociative extraction and reconstitution of extracellular matrix components involved in local bone differentiation. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7599–7603. doi: 10.1073/pnas.78.12.7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath T. K., Reddi A. H. Homology of bone-inductive proteins from human, monkey, bovine, and rat extracellular matrix. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6591–6595. doi: 10.1073/pnas.80.21.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlechter N. L., Russell S. M., Spencer E. M., Nicoll C. S. Evidence suggesting that the direct growth-promoting effect of growth hormone on cartilage in vivo is mediated by local production of somatomedin. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7932–7934. doi: 10.1073/pnas.83.20.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J. P., Schwartz Z., Hollinger J. O., Boyan B. D. Characterization of rat calvarial nonunion defects. Acta Anat (Basel) 1990;138(3):185–192. doi: 10.1159/000146937. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Huang J. S., Walz D. A., Deuel T. F. Chemotactic activity of platelet alpha granule proteins for fibroblasts. J Cell Biol. 1983 Feb;96(2):382–385. doi: 10.1083/jcb.96.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Singh J. P., Lillquist J. S., Goon D. S., Stiles C. D. Growth factors adherent to cell substrate are mitogenically active in situ. Nature. 1982 Mar 11;296(5853):154–156. doi: 10.1038/296154a0. [DOI] [PubMed] [Google Scholar]
- Sprugel K. H., McPherson J. M., Clowes A. W., Ross R. Effects of growth factors in vivo. I. Cell ingrowth into porous subcutaneous chambers. Am J Pathol. 1987 Dec;129(3):601–613. [PMC free article] [PubMed] [Google Scholar]
- Vukicevic S., Luyten F. P., Reddi A. H. Osteogenin inhibits proliferation and stimulates differentiation in mouse osteoblast-like cells (MC3T3-E1). Biochem Biophys Res Commun. 1990 Jan 30;166(2):750–756. doi: 10.1016/0006-291x(90)90873-l. [DOI] [PubMed] [Google Scholar]
- Vukicevic S., Luyten F. P., Reddi A. H. Stimulation of the expression of osteogenic and chondrogenic phenotypes in vitro by osteogenin. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8793–8797. doi: 10.1073/pnas.86.22.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. T. Signal transduction by the platelet-derived growth factor receptor. Science. 1989 Mar 24;243(4898):1564–1570. doi: 10.1126/science.2538922. [DOI] [PubMed] [Google Scholar]