Abstract
Classical zinc-dependent histone deacetylases (HDACs) catalyse the removal of acetyl groups from histone tails and also from many non-histone proteins, including the transcription factor FOXP3, a key regulator of the development and function of regulatory T cells. Many HDAC inhibitors are in cancer clinical trials, but a subset of HDAC inhibitors has important anti-inflammatory or immunosuppressive effects that might be of therapeutic benefit in immuno-inflammatory disorders or post-transplantation. At least some of these effects result from the ability of HDAC inhibitors to enhance the production and suppressive functions of FOXP3+ regulatory T cells. Understanding which HDACs contribute to the regulation of the functions of regulatory T cells may further stimulate the development of new class- or subclass-specific HDAC inhibitors with applications beyond oncology.
Control of autoimmunity and other undesired immune responses through manipulation of endogenous regulatory mechanisms has long been a dream of physicians and scientists. Recent insights into the role of epigenetics in the regulation of gene expression and cell function suggest new therapeutic opportunities, including regulation of chromatin remodelling and gene transcription by inhibition of histone deacetylases (HDACs) and DNA methyltransferases1-4. Moreover, although deacetylation of ε-acetyl-lysine residues in the amino-terminal tail of core histones is a key function of several HDACs, HDACs also deacetylate many non-histone proteins1-5, including the forkhead transcription factor FOXP3, which is important in the development and regulation of regulatory T cells (Tregs)6,7. Protein acetylation can affect DNA binding, either positively or negatively, protein–protein interactions and enhance protein stability4. Acetylation can promote the activation, nuclear translocation and DNA binding of transcription factors such as STAT3, NF-κB and RUNX1, and thereby promote expression of multiple genes, including pro-inflammatory cytokines and other mediators of inflammation and immunity.
The evidence of the anti-inflammatory effects of HDAC inhibitors (HDACIs) has been accruing for many years, but has not led to their development for immuno-inflammatory disorders by pharmaceutical companies. Despite their potency, existing HDACI drugs have toxicities or other limitations that have largely restricted their development to the potential treatment of patients with malignancies. However, this assessment is changing as new insights into the roles of individual HDAC enzymes are emerging and new cellular targets are identified.
The 18 HDACs are classified structurally into class I (HDAC1, HDAC2, HDAC3, HDAC8), class IIa (HDAC4, HDAC5, HDAC7, HDAC9), class IIb (HDAC6, HDAC10), class III (SIRT1-7) and class IV (HDAC11) groups8,9. Class III HDACs or sirtuins act by a nicotinamide-dependent mechanism and are structurally and functionally distinct from class I, II and IV HDAC metalloenzymes. Activation of SIRT1, using resveratrol or newer analogues10, has antioxidant effects that might be therapeutically useful in metabolic, neurological and cardiac diseases11. However, little is yet known about the involvement of sirtuins in immune responses12. Similarly, there is only one study on HDAC11, the sole class IV member, showing that it inhibits expression of interleukin (IL)-10 by dendritic cells in vitro13. Hence, this Review focuses on the classical zinc-dependent class I and II HDACs.
As detailed below, class I HDACs are expressed in all cells and are essential for cell differentiation by contributing to a closed chromatin state and suppression of gene transcription; for example, a cell can become a lymphocyte or a myocyte by turning off genes that promote neuronal or endothelial differentiation, and class I HDACs have a major role in this suppression. Class II HDACs have more limited cellular expression and often control regulatory processes in a gradual or more subtle manner than their class I counterparts.
Much of the biology of HDACs has been unravelled through the use of HDACIs. Surprisingly, given the key roles of class I HDACs in normal cells, these compounds have not proven overwhelmingly toxic. Rather, as the field has learnt to optimize their potency, pharmacokinetics and, increasingly, delineate their precise specificities, roles for selected HDACIs in the control of inflammatory and immune processes have begun to be explored. These studies have shown potent in vivo effects of HDACIs on the differentiation and activation of dendritic cells, T cells and other components of the immune response (for recent reviews, see REFS 9,14-16).
Over the past decade, the recognition and characterization of Tregs that express FOXP3 in maintaining host homeostasis has captured the attention of many investigators. FOXP3+ Tregs play a key part in limiting autoimmunity and maintaining peripheral tolerance, and mutations of FOXP3 lead to lethal autoimmunity in humans and mice17-21. From a therapeutic perspective, some groups are testing expansion of small numbers of Tregs before adoptive transfer back into an individual. However, repeated stimulation of expanded Tregs has been found to lead to loss of FOXP3 expression, especially when using Tregs generated in vitro22,23. An alternative approach has arisen from recent insights into the epigenetic regulation of FOXP3 (REFS 6,7,24,25). Therapeutic manipulation of FOXP3 acetylation using HDACIs can promote the development and suppressive functions of FOXP3+ Tregs, with beneficial consequences in models of transplant rejection, colitis and arthritis7,26-28.
After summarizing the relevant background on HDAC complexes and HDACIs, this Review will discuss the role of histone acetylation in inflammation and autoimmunity, and consider the therapeutic potential of HDACIs as anti-inflammatory agents, including their use to promote FOXP3 acetylation and Treg-mediated immunosuppression. The ongoing research at the intersections of epigenetics, pharmacology and Treg biology may lead to new therapies for immuno-inflammatory disorders and transplant rejection.
HDAC complexes
Deacetylation of ε-acetyl-lysine residues in the N-terminal tail of core histones is a key function of class I HDACs, although new data indicate that they may also deacetylate 1,750 or more non-histone proteins5. HDACs do not have direct DNA binding activity, but function through interactions with other proteins in large molecular complexes.
Class I HDAC complexes
Class I HDAC proteins are present within intranuclear SIN3 or NURD complexes29 and are ubiquitously expressed30. HDAC1 and HDAC2 in the SIN3 complex provide deacetylase activity, whereas retinoblastoma-associated proteins stabilize interactions with histones and the SIN3 and SAP30 proteins promote interactions with other molecules. The NURD complex has the same core components as the SIN3 complex, but has a different set of interacting proteins: namely the ATP-dependent chromatin remodelling protein Mi2 (also known as CHD4); one or more metastasis-associated proteins, for example, MTA2; and the methyl-binding domain protein 3 MBD3 (REF. 29). NURD complexes promote histone deacetylation, like SIN3 complexes, but also regulate ATP-dependent chromatin remodelling, and act more globally through their recruitment to the tails of histone 3 and to sites of CpG-methylated DNA29.
HDAC3 is a transcriptional repressor of a specific set of genes involved in nuclear receptor signalling and functions as part of a complex that contains the silencing mediator of retinoid and thyroid hormone receptor (SMRT) and the nuclear hormone receptor corepressor (NCoR), and other proteins31,32. SMRT and NCoR repress transcription factors such as NF-κB and activator protein 1 (AP1; also known as JUN), and also bind to class II HDACs31,32.
Class IIa HDAC complexes
In contrast to class I HDACs, expression of class IIa HDACs (HDAC4, HDAC5, HDAC7 and HDAC9) varies with cell differentiation and by tissue; expression is notably high in brain, muscle and T lymphocytes9. Class IIa HDACs shuttle between the nucleus and cytoplasm as a result of activating stimuli, and are thought to largely lack deacetylase activity as determined using conventional substrates, although this assessment may change as alternative substrates are identified33.
The HDAC domains of HDAC4, HDAC5, HDAC7 and HDAC9 can bind the SMRT/NCoR complex and associated HDAC3 (REFS 31,32), and their N-terminal domains bind to the transcriptional repressor BCL6 (REF. 34), which can also recruit SMRT/NCoR. Thus, much of the deacetylase activity associated with class IIa HDACs is attributed to HDAC3 (REFS 31-33). Additional repressor activity can arise from the binding of C-terminal-binding protein 1 (CTBP1)35 and hetero-chromatin protein 1 (HP1; also known as DeF1)36 to the N-terminal regions of class IIa HDACs. DeF1 binds methylated H3K9 and recruits the histone lysine methyl-transferase SUV39H1, so that deacetylation of target genes can lead to the development of the repressive chromatin mark of methylated H3K9 and further gene silencing36. This ability of class IIa HDACs to repress genes independently of intrinsic deacetylase activity is exemplified by the HDAC9 isoform, myocyte enhancing factor 2 (MeF2)-interacting transcription repressor MITR, which lacks a catalytic domain but still represses MeF2 (REF. 37).
Class IIa HDACs have conserved N-terminal serines that are phosphorylated by enzymes such as Ca2+/calmodulin-dependent kinase (CaMK), protein kinase D, salt-inducible kinase and MAP/microtubule affinity-regulating kinase38,39. Upon phosphorylation, class IIa HDACs bind to export proteins such as 14-3-3 and move to the cytoplasm40, allowing derepression of target genes including MeF2, which binds class IIa HDACs through a highly conserved 17-amino acid N-terminal motif41. once free of class IIa HDAC inhibition, MeF can be acetylated by the histone acetyl transferase (HAT) p300 and activate transcription, or can undergo sumoylation to maintain repression of target genes42,43. In the cytosol, phosphorylated class IIa HDACs are dephosphorylated by protein phosphatase 2A and recycled to the nucleus44.
Class IIb HDAC complexes
Both class IIb HDACs — HDAC6 and HDAC10 — are distinct from the other HDAC classes in that they have two catalytic domains and can be detected within the nucleus and the cytoplasm20,45. Little is known about the function of HDAC10, but HDAC6 has been found to deacetylate proteins within the cytoplasm and the nucleus, and to also have deacetylase-independent functions46.
HDAC inhibitors
The first HDACIs were identified by their anticancer effects during drug screens, before the existence of HDACs was known47. Even today, crystallographic structures have only been reported for the catalytic domains of HDAC4 (REF. 48), HDAC7 (REF. 49) and HDAC8 (REFS 50,51). The ‘classical’ HDACIs acting on zinc-dependent HDACs (HDAC1-11) include short-chain fatty acids, such as sodium butyrate and valproic acid (VPA); hydroxamic acids, such as trichostatin A (TsA) and suberoylanilide hydroxamic acid (SAHA); benzamides, such as MS275; and cyclic tetrapeptides such as trapoxin and depsipeptide2,52-54. SAHA, also known as vorinostat (Zolinza; Merck) and approved as a monotherapy for cutaneous T cell lymphoma, is the only FDA-approved HDACI, although the approval of depsipeptide (also known as romidepsin) has been recommended for the same indication.
Agents with HDACI activity as well as other actions, such as butyrate and VPA, have long been used clinically in non-oncologic contexts. For example, butyrate is used as a therapy for inflammatory bowel disease, although whether its benefits are due to inhibition of HDAC activity is controversial55. Likewise, no data are available as to whether prolonged treatment of epileptic patients with VPA also protects against co-morbid immuno-inflammatory diseases through the inhibition of HDACs56.
Classical HDACIs are being tested in oncology trials due to their abilities to promote tumour cell-cycle arrest, differentiation and apoptosis57,58. These drugs have proven relatively non-toxic towards neighbouring normal cells, despite inducing hyperacetylation in both tumour and normal cells59. Given the paucity of knowledge of the functions of individual HDACs, an empirical one-size-fits-all strategy for the development of HDACs has largely prevailed. However, so-called ‘pan-HDACIs’ such as the hydroxamic acid compounds (for example, TsA, SAHA, M344, Scriptaid) may actually only block the HDAC activity of class I and class IIb HDACs, as class IIa HDACs largely lack detectable deacetylase activity9,60. By contrast, class I-selective HDACIs (for example, MS275 (REF. 61), 4-phenylimidazole62 and MC1293 (REF. 63)), class II-selective HDACIs (such as MC1568 and MC1575 (REF. 64)) and HDAC isoform-specific inhibitors (for example, selective for HDAC4 (REF. 65), HDAC6 (REF. 66) or HDAC8 (REF. 67)) have been identified. Development of class I-specific HDACIs may be particularly relevant in cancer15, but as will be discussed, anti-inflammatory and Treg-dependent mechanisms often involve targeting class II HDACs.
Classical HDACIs act by chelating or displacing the Zn2+ present in the catalytic site, and their specificities arise from interactions with residues in the cap region that is adjacent to the catalytic site47-49. However, additional mechanisms of action of individual HDACIs are increasingly being recognized. For example, the short-chain fatty acid HDACI compounds VPA and butyrate, but not pan-HDACIs, selectively promote the proteasomal degradation and depletion of HDAC2 by induction of E2 and E3 ubiquitin ligases68. Likewise, two structurally distinct pan-HDACIs — SAHA and depsipeptide — promote the selective downregulation of HDAC7 gene transcription but by yet unknown mechanisms69. Lastly, selective downregulation of class IIa HDACs occurs in response to exposure to pan-HDACIs or class II-specific HDACIs, but not class I-specific HDACIs70. The downregulation of class IIa HDACs results from sumoylation and proteasomal degradation rather than inhibition of transcription, suggesting that pan-HDACIs can not only inhibit the action of class I HDACs by occupying their active sites, without altering protein expression, but that they may also decrease class II HDAC expression by promoting proteasomal degradation.
Given the poor catalytic activity of class IIa HDACs against standard substrates (acetylated lysine residues), various groups are investigating whether small-molecule inhibitors of protein–protein interactions may also function as atypical HDACIs. For example, HDAC4 and HDAC5 form hetero-oligomers, allowing CAMKII to promote the export of HDAC5 despite it lacking a CAMKII docking site, and pointing to the potential to maintain repression of MEF2 or other target genes by disrupting the interaction of HDAC4 and HDAC5 (REF. 71). Alternatively, disrupting the interaction of the MEF2-binding motif of class IIa HDACs with MEF2 might allow selective inhibition of some or all of the MEF2-dependent functions of class IIa HDACs41. Searches for inhibitors of such protein–protein interactions are typically more challenging to set up and run than standard high-throughput screens for enzyme inhibitors, but may prove a fertile area for future development as rationales for their use are established.
Anti-inflammatory effects of HDACIs
Inflammatory responses involve the orchestrated function of a host of bodily defences mobilized in response to injury and intended to result in healing and repair. However, as a result of defects in this response, failure of coordination or inability to clear the inflammatory stimulus, excessive and damaging inflammatory responses may develop and persist long after their normal utility has passed. Similarly, immune responses might be excessive or directed against self-antigens, leading to the development of autoimmune diseases.
The role of cytokines and other mediators in the development and persistence of inflammatory diseases is well established. More recently, recognition of a requirement for chromatin remodelling to promote transcription factor binding and activation of cytokine and other genes has led researchers to consider HDACI use as a new approach to treat immuno-inflammatory disorders.
Histone acetylation and inflammatory gene expression
Eukaryotic DNA is tightly wrapped around an octamer of histone proteins and various post-translational modifications of the N-terminal tails of these histones, including methylation, acetylation, phosphorylation and ubiq-uitination, control the accessibility of transcriptional machinery to the DNA and hence gene expression. Lysine acetylation as a result of HAT activity or inhibition of HDAC function leads to recruitment of transcription factors, bromodomain-containing proteins that promote chromatin remodelling and activation of transcription, and RNA polymerase II72. The coordinated role of HATs and HDACs in regulating gene expression on a genome-wide scale was recently demonstrated45. However, as noted below, HDACI use is typically associated with repression of pro-inflammatory cytokine expression, which seems to conflict with this paradigm.
Resolution of this paradox probably involves several points. First, effective gene transcription is correlated with rapid acetylation and deacetylation rather than with sustained acetylation45,73, and HDACI use may upset this balance. Second, HDACI-induced chromatin remodelling may result in the recruitment of as many repressors as activators, or may lead to expression of pro-apoptotic molecules and induction of cytotoxicity74. Third, HDACIs affect the function of many non-histone proteins5, including FOXP3 (REF. 7) and potentially other inhibitory pathways. Indeed, phylogenetic analysis showed that, at least in bacteria, all four HDAC classes preceded the evolution of histone proteins, emphasizing the likely importance of non-histone proteins as targets of HDACIs75. Fourth, HDACIs have effects beyond promoting acetylation; for example, HDACIs can promote proteasomal degradation68-70 or impair AKT activation by releasing protein phosphatase 1 from HDAC binding76. Until more insights are available, a need for cell-type and context-dependent analysis when assessing effects of HDACI use is paramount.
Microarray studies
Despite there being many clinical trials of HDACIs in oncology underway, little information is available concerning the effects of HDACIs on normal cells. In a pioneering study74, microarray and additional analyses of primary murine CD4+ T cells undergoing activation induced by CD3/CD28 monoclonal antibodies showed that 5 nM concentrations of the pan-HDACI TsA induced mitochondria-dependent apoptosis of ~50% of cells and reversible G1 cell cycle arrest in the remainder. Whereas T cell production of IL-2 and the expression of many cell surface molecules were downregulated by TsA, microarray studies showed time- and stimulus-dependent effects of TsA on T cell gene expression. Surprisingly, TsA only modulated 2% of genes, and equal numbers of genes were downregulated as were upregulated74.
A subsequent analysis of the effects of another hydroxamate, LAQ824, on Toll-like receptor 4 (TLR4)-dependent activation of macrophages also showed that pan-HDACI therapy up- or down-regulated expression of only about 5% of genes77. Interestingly, therapy with LAQ824 prevented development of TH1 but not TH2 T cell responses as a result of genes expressed by pan-HDACI-treated antigen-presenting cells. Together, these studies indicate that inhibition of HDAC activity alone is insufficient to drive broad gene expression, and point to the need for a careful understanding of the roles of multiple interacting pathways affecting chromatin remodelling and gene expression, before the results of HDACI therapy can confidently be predicted at the cellular level.
In vitro and in vivo immune cell studies
Many studies have documented multiple effects of HDACIs on cells of the innate and adaptive immune systems. However, these studies have typically used a ‘black box’ approach, whereby effects of the HDACIs in vitro or in vivo were delineated without analysis of the HDACs involved or of whether the HDACIs used were modulating non-histone proteins or chromatin remodelling. The broad effects of HDACIs on inflammatory and immune responses were primarily determined in rodents or using cultured human cells, as summarized in TABLE 1.
Table 1. Effects of histone deacetylase inhibitors in inflammatory and autoimmune diseases.
| Disease model | Species | Inhibitor | Effects |
|---|---|---|---|
| Endotoxin | Human, mouse |
SAHA | Reduced cytokine production (TNF- α, IL-1β, IL-6, IFN-γ) and lethality in vivo in mice77-79; decreased cytokine production in vitro using human PBMC78 |
| ConA hepatitis | Mouse | SAHA | Decreased liver injury78 |
| Airway hypersensitivity | Mouse | TsA | Decreased allergen-induced airway inflammation in a model of asthma, with decreased airway hyperresponsiveness, mucus production, leukocyte infiltration and cytokine production93 |
| Experimental autoimmune encephalomyelitis |
Mouse | TsA | Decreased demyelination, leukocyte infiltration and TH1 cytokine and chemokine production in relapsing phase of experimental autoimmune encephalomyelitis94 |
| Stroke | Mouse | SAHA, TsA | Decreased infarct size post-middle cerebral artery occlusion103 |
| Neurodegenerative diseases |
Mouse | Butyrate106,107, SAHA105 |
Decreased disease progression in models of Huntington’s chorea105, spinal and muscular dystrophy106, amyotrophic lateral sclerosis107 |
| Colitis | Mouse | SAHA, TsA | Decreased weight loss, histological injury, leukocyte infiltration and cytokine production in dextran sodium sulphate- and,4,6- trinitrobenzene sulphonic acid-induced colitis and adoptive transfer models7,26,96 |
| Glomerulonephritis | Mouse | SAHA, TsA | Decreased proteinuria in MRL/lpr model of systemic lupus erythematosus, and decreased leukocyte infiltration and production of inflammatory mediators95 |
| Arthritis | Mouse, rat | FK22897, SAHA and MS27598, VPA19, |
Decreased joint swelling and bone and cartilage destruction in antibody-97 and collagen-induced28,98 models of arthritis, in conjunction with decreased overall leukocyte infiltration and TH1 cytokine and chemokine production, but increased Foxp3+ Treg accumulation19 |
| Graft-versus-host disease |
Mouse | SAHA | Decreased graft-versus-host disease99 and decreased CD3 monoclonal antibody-induced cytokine storm during bone marrow conditioning100 |
| Transplant rejection | Mouse | SAHA, TsA, | Permanent engraftment of cardiac and islet allografts and Foxp3+ Treg-dependent tolerance induction7 |
| Haemorrhage and resuscitation |
Human, mouse, rat, pig |
Butyrate, SAHA, TsA, VPA |
Optimal protective effects with SAHA compared with TsA or VPA in a rat model109; SAHA-induced suppression of TNF-α production in rats but variable effects in humans110; VPA-associated increased expression of multiple protective genes in additional studies using pig111 and rat112 models, including BCL2 and HSP70 (REF. 111) |
CD3, cluster of differentiation 3; HSP70, heat shock protein 70; IFN, interferon; IL, interleukin; PBMC, peripheral blood mononuclear cell; SAHA, suberoylanilide hydroxamic acid; TNF, tumour necrosis factor; Treg, regulatory T cell; TH1, type 1 helper T cell; TsA, trichostatin A (TsA); VPA, valproic acid.
With regard to acute inflammation, treatment of mice with SAHA78 or NVP-LAQ824 (REF. 77), two hydroxamic acid pan-HDACIs, suppressed lipopoly-saccaride-induced production of the cytokines TNF-α, IL-1β, IL-6 and IFN-γ in dendritic cells both in vitro and in vivo77-79. Consistent with these findings, HDACI therapy is known to impair activation of NF-κB in dendritic cells and macrophages, although the mechanisms remain controversial14, and to impair their differentiation and maturation80,81. Recently, pan-HDACI exposure was shown to promote STAT3 acetylation82 and indoleamine 2,3-dioxygenase (IDO) production by dendritic cells83. As IDO catabolizes tryptophan, an amino acid that is essential for T cell activation, induction of IDO blocks T cell activation and the development of murine graft-versus-host disease post-bone marrow transplantation83. In vivo use of TsA or SAHA also inhibited T cell cytokine production and proliferation74,84,85 and promoted T cell anergy86, in conjunction with altered chromatin remodelling at the IL-2 promoter and acetylation of key transcription factors, including NF-κB84,85.
With regard to chronic inflammation, TsA or SAHA decreased fibroblast proliferation and extracellular matrix production in murine models of fibrosis87-89, and both in vitro and in vivo TsA or SAHA blocked transforming growth factor beta (TGF-β)-induced differentiation of fibroblasts into myofibroblasts and impaired epithelial–mesenchymal transformation89,90. Selective HDAC targeting might also be a useful strategy to limit inflammation, as in cultured epithelial cells, HDAC6 promotes TGF-β-induced epithelial–mesenchymal transformation by deacetylating SMAD3 or SMAD3-interacting proteins91, and in vitro, HDAC4 can inhibit expression of a TGF-β repressor, TGIF92.
The effects of HDACIs on antigen-presenting cell-induced T cell activation and differentiation are summarized in FIG. 1. In murine models of T cell-dependent disease, therapy with TsA or SAHA decreased the severity of ConA-hepatitis78, TH2-associated lung airway hyper-sensitivity responses93; experimental allergic encephalo–myelitis94; renal disease in MRL/lpr mice95; colitis96; arthritis97,98; graft-versus-host disease post-bone marrow transplantation99 and the ‘cytokine storm’ induced by the CD3 monoclonal antibody therapy used in a bone marrow transplant conditioning regimen100. The underlying mechanisms responsible for the beneficial effects of pan-HDACI therapy in these models were usually attributed, by extrapolation from the cancer literature, to effects on effector T cell apoptosis96 and no specific HDACs were identified as key targets for therapy.
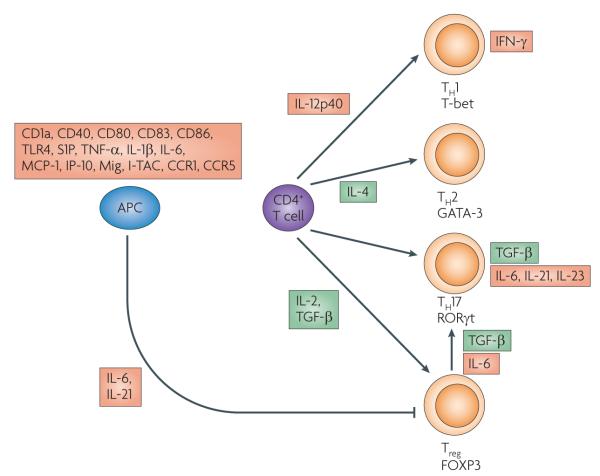
Figure 1. Main anti-inflammatory effects of histone deacetylase inhibitors (HDACls) in leukocytes.
Key pathways for differentiation of activated CD4+ T cells are shown, along with lineage-specific transcription factors (T-bet, GATA-3, RORγt and FOXP3). HDACI use decreases the production of proteins shown in red boxes. Many anti-inflammatory effects of HDACIs are mediated through effects on antigen-presenting cells (APCs), including dendritic cells and monocytes. HDACI-induced suppression of the production of multiple cytokines impairs the differentiation of CD4+ T cells into TH1 cells characterized by T-bet expression and TH17 cells characterized by RORγt expression. However, HDACI treatment does not impair development of TH2 cells expressing GATA-3 or regulatory T cells (Tregs) expressing FOXP3. HDACI use also prevents the conversion of Tregs into TH17 cells. The effects of HDACIs on other leukocytic cell types, including granulocytes, natural killer cells and non-malignant B cells, are poorly understood. CCR, C-C receptor; CD, cluster of differentiation; IFN, interferon; IL, interleukin; IP-10, inducible protein-10; I-TAC, inducible-T-cell activating chemokine; MCP, monocyte chemoattractant protein; Mig, monokine induced by interferon γ; S1P, sphingosine-1-phosphate; TGF, transforming growth factor; TNF, tumour necrosis factor; TLR, Toll-like receptor.
T cell-independent and non-pro-apoptotic beneficial effects of pan-HDACI administration were noted in studies of organ ischaemia/reperfusion injury in mice, and class II HDACIs proved especially useful, albeit with different HDACs being important in different models. TsA use prevented hypoxia-inducible factor 1 alpha (HIF1α)-induced upregulation of a number of target genes during myocardial ischaemia and reperfusion, and limited myocardial infarct size by more than 50%101. Importantly, a therapeutic benefit was observed even when a single injection of HDACI was made one hour after ischaemia/reperfusion. Moreover, individual knockdown studies in cultured myocytes pointed to a key role of HDAC4 in regulating myocyte expression of HIF1α. Surprisingly little is known about the effects of HDACI therapy on renal ischaemia/reperfusion injury, but in vitro data using cultured tubular cells suggest that bone morphogenetic protein 7 (BMP7), which is normally inhibited by HDAC5, plays an important part in the regenerative response102. Within the central nervous system, pan-HDACI therapy proved beneficial in limiting the extent of acute brain injury in mice after a stroke, even when administered post-injury, through direct neuroprotective and anti-inflammatory effects103. Pan-HDACI use was also beneficial in models of neurodegenerative diseases involving oxidative stress104, slowing disease progression in models of Huntington’s chorea105, spinal and muscular atrophy106 and amyotrophic lateral sclerosis107. Inhibition of HDAC6 promotes acetylation of peroxiredoxins and might be responsible for much of the benefits of pan-HDACI or tubacin therapy in models of neurodegenerative diseases108.
Lastly, surgically-oriented studies have examined the balance between HAT and HDAC functions in various organs, and the effects of HDACI therapy following haemorrhage and resuscitation in mice, rats, pigs and with human cells109-112. TsA or SAHA administration proved beneficial even in the absence of conventional fluid replacement, and was associated with patterns of increased acetylation that varied between organs. Optimal effects of pan-HDACI use were seen in conjunction with hyperacetylation, especially of histone 3 and a series of non-histone proteins, and increased DNA binding of acetylated β-catenin and increased expression of multiple protective genes, including BCL2 and heat shock protein 70 (HSP70) (REFS 109-112). In addition, HDACI use attenuated organ injury, and inhibited expression of a variety of pro-inflammatory cytokines.
Clinical trials
Collectively, these studies indicate that HDACIs can act through multiple mechanisms that encompass effects on chromatin remodelling and also increased acetylation of non-histone proteins. However, most studies were performed with HDACIs that are not being developed for clinical application as anti-inflammatory agents, including TsA, which is relatively toxic; SAHA and depsipeptide, which have been developed for treatment of cutaneous T cell lymphoma and potentially other oncological applications; or agents with weak potency, such as butyrate and VPA. An exception is the benzamide HDACI MS275 that is in Phase II trials for cancer, but which is also proposed for development, after further optimization is achieved, for the treatment of psoriasis and rheumatoid arthritis (TABLE 2).
Table 2. Histone deacetylase inhibitors in development for the treatment of inflammatory and autoimmune diseases.
| Company (and partner) |
Compound(s); structural group |
Target effects | Indication(s) | Development stage* |
|---|---|---|---|---|
| Acetylon | Multiple hydroxamic acid derivatives |
HDAC6-selective | Rheumatoid arthritis | Discovery |
| Chipscreen Biosciences | CS0240-CS0600 | Series of subclass-selective HDACIs | Neurodegenerative diseases, viral infections, immuno-modulation |
Preclinical |
| Chroma Therapeutics (and GlaxoSmithKline) |
CHR3996; hydroxamic acid |
Orally active class I-selective HDACI that blocks macrophage production of TNF-α |
Inflammatory disease, for example, rheumatoid arthritis |
Discovery |
| Envivo Pharmaceuticals (and Methylgene) |
EVP-0334 | HDACI that blocks TNF-α production | Neurodegenerative diseases | Phase I |
| Italfarmaco | ITF2357 (Givinostat); hydroxamic acid |
Orally active pan-HDACI that reduces the production and activity of multiple pro-inflammatory cytokines (IL-1α, IL-1β, TNF-α, IL-6, IL-12, IL-18 and IFN-γ) |
Inflammatory diseases, including juvenile arthritis113 |
Phase II |
| Karus Therapeutics and EOS Pharmaceutical Corporation |
OS-HDI and other depsipeptide derivatives |
OS-HDI is an HDAC1-selective inhibitor | Rheumatoid arthritis, psoriasis and transplantation |
Preclinical |
| Pharmacyclics | PCI-34051; hydroxamic acid |
HDAC8-selective inhibitor that blocks production of multiple cytokine s (IL-1β, IL-18) |
Psoriasis and rheumatoid arthritis | Preclinical |
| Syndax | SYDX-275 (etinostat, MS275); benzamide |
HDAC1-3 inhibition | Psoriasis and rheumatoid arthritis98 |
Preclinical |
Information on company website. HDAC, histone deacetylase; HDACI, histone deacetylase inhibitor; IFN, interferon; IL, interleukin; TNF, tumour necrosis factor.
At least an additional seven companies are developing HDACIs for treatment of inflammation and/or autoimmunity, with most attention being given to identification of hydroxamic acid derivatives. Interestingly, even within this class of compounds, some pan-HDACIs and class I HDACIs have been identified, as well as agents with individual HDAC selectivity. The latter include hydroxamates selective for HDAC6 (class IIb) or HDAC8 (class I), indicating the robust range of potential inhibitory actions of this broad class of compounds. one such hydroxamate, ITF2357 (also known as givinostat) has shown clinical benefit and safety in Phase II trials in children with active systemic onset juvenile idiopathic arthritis113. Givinostat significantly reduced the systemic feature score and number of painful joints.
HDACIs and FOXP3+ Tregs
Over the last decade, roles for FOXP3+ Tregs in maintaining immune homeostasis have been identified and this cell type has emerged as a prime target for therapeutic manipulation in new efforts to curb autoimmunity and transplant rejection23. HDACI therapy has been shown to increase the production and suppressive functions of Tregs in vitro and in vivo, leading to the concept that HDACI use might provide a pharmacological means to exploit the actions of this recently recognized cell type. This section summarizes the biology of FOXP3+ Tregs and discusses the broad effects of HDACIs on Tregs, including whether all or just selected HDACs should be targeted.
Defects in FOXP3+ Treg cells induce inflammatory and autoimmune diseases
Thymectomy of neonatal mice has been shown to lead to autoimmune gastritis; however, disease was prevented by adoptive transfer of CD4+CD25+ T cells from normal mice114. In 2001, it was reported that 5–10% of mammalian CD4+ T cells express an X-linked gene encoding FOXP3, which serves as a lineage specification factor for the development and function of CD4+CD25+ Tregs17,18. The dominant role of Tregs in maintaining immune homeostasis is illustrated by the fatal autoimmune disease found in patients with immunodysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome, and in mice lacking functional FOxp3 (REFS 17-21).
Naturally occurring Tregs arise during thymic development and enter the periphery, surviving for up to 70 days115. Whereas replacement of dying peripheral Tregs in juveniles occurs by thymic emigration, in adults, peripheral conversion of CD4+CD25− cells into CD4+CD25+ Tregs116,117 and extrathymic development probably takes place in gut-associated lymphoid tissues under the trophic effects of gut metabolites118,119-121. FOXP3 is considered key to Treg development19, given its ability to modulate the expression of hundreds of genes when transfected into T cells. As Tregs cannot produce IL-2, they need a local source of IL-2 and must express an IL-2 receptor (CD25). Their development is facilitated by exposure to TGF-β116, retinoic acid119,120 and by T cell receptor activation, and is impaired by exposure to IL-6 and IL-21 (REFS 122-124).
Tregs exert suppressive effects on adjacent cells through multiple mechanisms, only some of which seem to be active using in vitro assays of Treg function125. For example, Tregs are the main source of IL-10 in the colon, yet splenic or lymph node Tregs, which are typically used to obtain cells for Treg assays, produce little IL-10 (REF. 126). Tregs express inhibitory surface proteins, such as cytotoxic T lymphocyte-associated antigen 4 (CTLA4), tumour necrosis factor receptor superfamily member 18 (TNR18) and tumour necrosis factor ligand superfamily member 14 (TNF14), and secrete inhibitory cytokines such as IL-10, TGF-β and IL-35 (REFS 125-128). They also express membrane ecto-ATPases such as CD39 and CD73 that may contribute to the inhibition of immune activation by affecting local adenosine levels129,130. Consumption of essential amino acids may be an important mechanism of action of FOXP3+ Tregs in vivo through competition with other immune cells131.
FOXP3 structure–function relationships
FOX family members show only low-affinity binding for DNA in the absence of dimerization or co-factor binding132. Insights into FOXP3 interactions are largely derived from overexpression in cells that do not necessarily express the same regulatory molecules as Tregs. Bearing in mind these caveats, studies show that FOXP3 forms homo-oligomers at the prolinerich N-terminal repressor domain, the zinc-finger leucine zipper domain and the C-terminal forkhead domain133,134, and forms heterodimers with FOXP1 (REF. 133). FOXP3 also interacts with multiple transcription factors, including NFAT135,136, NF-κB135,137, RUNX1138, JUN139 and CReB137. FOXP3 alters the binding of these transcription factors to the promoters of target genes either by binding to immediately adjacent critical residues, or by binding to the transcription factors themselves, as with NFAT and NK-κB135,136. NFAT and NF-κB interact with the N-terminal domain of FOXP3 (REFS 135,136), whereas the zinc-finger leucine zipper domain is important for protein–protein interactions122, and the C-terminal domain promotes nuclear localization and DNA binding125. Thus, FOXP3 functions as a transcription repressor, and probably as a transcriptional activator, through the formation of both DNA-protein and protein–protein interactions.
Post-translational modifications of FOXP3
The binding of human6 and murine7 FOXP3 to chromatin and its effects on gene expression in Tregs are enhanced by FOXP3 acetylation. Moreover, biochemical fractionation shows that FOXP3 exists as part of one or more supra-molecular complexes in Tregs133,140. A higher-molecular-weight complex has been shown to contain FOXP3 and several chromatin remodelling enzymes (for example, SMCA4, ATP-dependent DNA helicases and MBD3)133,140, and a lower-molecular-weight complex contained FOXP3 and additional transcription factors, including FOXP1 and NFATc2. In addition, co-immunoprecipitation studies showed a physical association between FOXP3, a histone acetyltransferase (for example, TIP60) and one or more deacetylases (for example, HDAC7, HDAC9)6, as well as between FOXP3 and HSP70 (REF. 26). These data indicate the potential to regulate FOXP3-dependent Treg functions through modulation of the acetylation of FOXP3.
FOXP3+ Treg cells as a target of HDACIs
Promoting FOXP3 acetylation seems to be a useful strategy for decreasing autoimmunity and transplant rejection, and can be achieved pharmacologically with HDACI therapy7,27,141. The main classes of HDACIs have recently been assessed for effects on FOXP3+ Treg function in vitro142. Pan-HDACIs of the hydroxamic acid series, including TsA, SAHA, M344 and Scriptaid, boosted Treg suppression in vitro when used at nanomolar concentrations, and the short-chain fatty acids, phenylbutyrate and VPA, enhanced Treg function when used at micromolar and millimolar levels, respectively. Hydroxamates also increased FOXP3 mRNA expression and promoted peripheral conversion upon adoptive transfer of T cells into immunodeficient mice7. By contrast, quinolone (NSC3852) and benzamide (MS275, MS1293) compounds had no effect on FOXP3 expression or FOXP3+ Treg function when assayed at micromolar levels. Small-molecule inhibitors showing efficacy in vitro also increased Treg function when injected into normal mice. Comparison of the effects of the pan-HDACI compounds, TsA and SAHA, with the class I HDACI MS275 in colitis models also showed these differences in modulation of Treg function, and lack of HDACI efficacy in Treg-depleted mice26. TsA was shown to prevent the differentiation of FOXP3+ Tregs into TH17 cells143, and pan-HDACI therapy enhanced Treg function and decreased inflammatory responses in arthritis27 and renal transplant rejection144.
It should be noted that although class I-specific HDACIs fail to enhance Treg function in our studies, these agents still have important dose-dependent anti-inflammatory effects in vivo. For example, in collagen-induced arthritis, SAHA was far less efficacious than MS275 in decreasing joint destruction98. By contrast, TsA was highly effective in a second model of adjuvant-arthritis145. Whether this reflects different drug potencies, different sets of genes being induced in each model or other factors, is unknown16. In any case, the finding that pan-HDACIs but not class I-selective HDACIs enhance Treg production and function points to a role for class II HDACs in the regulation of Treg biology. Moreover, although there is biochemical evidence of HDAC7 and HDAC9 in association with FOXP3 in large supra-molecular complexes, given the typically weak deacetylase activity of such class IIa HDACs, the pharmacological evidence points to the potential value of targeting class IIb HDACs.
Class IIa-specific HDAC targeting
HDAC class IIa-selective inhibitors have been reported but are not widely available and there are little data on their efficacy in vivo64,146-149. As noted, class IIa HDACs are considered enzymatically inactive when tested in isolation and their catalytic activity after immunoprecipitation may be due to co-precipitated HDAC3 (REF. 31,150). This assessment becomes more nuanced in the light of studies showing basal activity against standard acetyl-lysine substrates but increased activity upon mutation of a key histidine to tyrosine in the active site32,48, and proposals that these HDACs might be active against other substrates. For example, HDAC9 was recently shown to deacetylate the transcription factor USF1 and thereby regulate activation of fatty acid synthase151. Class IIa-selective HDACIs might thereby act only when the inhibitor is complexed to a class I HDAC, interfere with class I or class IIa complex formation, or block activity of class IIa HDACs against non-standard substrates.
So far, only genetic evidence is available as to the value of selective class IIa targeting to promote Treg function in vitro and in vivo. A model of class IIa HDAC function in Tregs is shown in FIG. 2. HDAC9-deficient mice are immunocompetent and FOXP3+ Tregs isolated from these mice show increased suppressive functions in vitro and in vivo, including in models of inflammatory bowel disease7,26. HDAC9−/− Tregs have elevated levels of HSP70 and are resistant to pro-apoptotic conditions26, such that the development of small molecules to block HDAC9 or promote HSP70 expression in Tregs could be valuable.
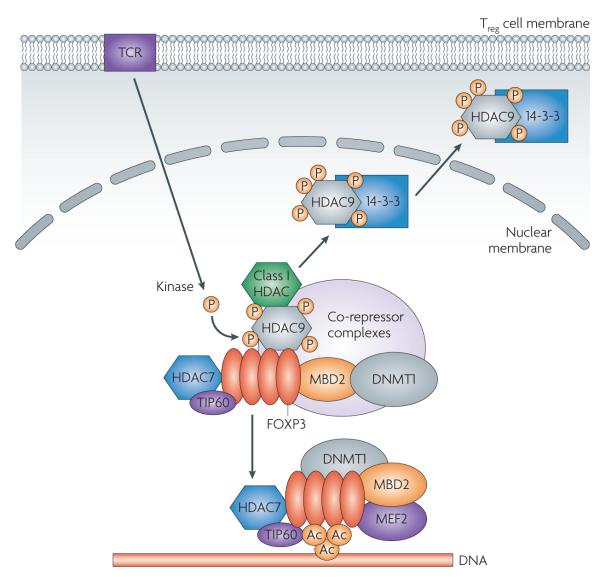
Figure 2. HDAC/FOXP3 complex in regulatory T cells.
Within the nucleus, the forkhead transcription factor FOXP3 undergoes homo- and hetero-oligomerization (the latter with FOXP1) and recruits the histone acetyl transferase (HAT) TIP60 and the class IIa histone deacetylase (HDAC) HDAC7 to a region between 100 and 200 amino acids from the amino-terminus. FOXP3 binding to DNA is impaired by its interaction with a second class IIa HDAC, HDAC9, that acts as a scaffold for assembly of a complex that includes DNA methyltransferase 1 (DNMT1), a class I HDAC (for example, HDAC3), methyl-binding domain 2 (MBD2) and co-repressor complexes, for example, silencing mediator of retinoid and thyroid hormone receptors/nuclear hormone receptor co-repressor (SMRT/NCoR). T cell receptor (TCR) stimulation activates one or more kinases that phosphorylate class II HDACs; candidate kinases include protein kinase D (PKD), calcium/calmodulin-dependent kinase (CaMK) and salt-inducible kinase (SIK). Phosphorylation of HDAC9 acts as an ‘off’ switch, as phosphorylated HDAC9 undergoes a conformational change and dissociates from FOXP3, removing inhibitory complexes and leading to de-repression of FOXP3 and its interaction with other proteins (for example, myocyte enhancing factor 2 (MEF2)) and DNA. Phosphorylated HDAC9 is exported from the nucleus in conjunction with the exportin 14-3-3. In the cytoplasm, phosphorylated HDAC9 is either degraded by the proteasome or might be dephosphorylated and re-enter the nucleus to re-establish binding to FOXP3, limiting its action again (‘on’ switch). Upon removal of HDAC9 and its associated inhibitory complexes, FOXP3 is acetylated by one or more HATs, for example, PCAF and p300, and binds to the promoter regions of target genes in the DNA. Ac denotes acetylation; P denotes phosphorylation.
Preliminary data also showed that HDAC7 targeting enhances Treg suppression in vitro and in vivo. In biochemical studies with human6 and murine152 Tregs, immunoprecipitation of FOXP3 co-precipitated HDAC7. Deletion of HDAC7 by mating mice expressing floxed HDAC7 and CD4–Cre transgenes did not markedly affect peripheral CD4, CD8 or Treg numbers, but enhanced Treg suppression in vitro. Increased Treg function was not associated with significant changes in expression (as assessed by microarray or quantitative PCR) of other HDACs or repression of IL-2, IL-6 or IFN-γ, but was associated with increased expression of IL-10 and CTLA4. In addition, whereas C57BL/6 mice rejected BALB/c cardiac allo-grafts within 14 days despite use of low-dose rapamycin for the same period, similarly treated HDAC7-deficient hosts accepted their cardiac allografts indefinitely (>100 days) and without development of chronic rejection. The presence or absence of HDAC7 as a key feature of Treg function was confirmed in immunodeficient recipients of cardiac allografts adoptively transferred with varying ratios of wild-type or HDAC7−/− Treg to wild-type T effector cells.
Lastly, although HDAC7 displays only minor catalytic activity using standard assays, the catalytic domain is likely to be important for recruiting repressive SMRT/NCoR/HDAC3 complexes. HDAC7 dominant-negative mice bearing a transgene with four point mutations in the catalytic domain of HDAC7 under the control of the lymphocyte-specific protein tyrosine kinase (Lck) promoter showed essentially normal peripheral CD4, CD8 and Treg numbers, but had enhanced Treg suppression in vitro. By contrast to wild-type controls, these mice accepted fully major histocompatibility complex (MHC)-disparate cardiac allografts long-term when treated for 14 days with low-doses of rapamycin152. Hence, HDAC7 functions as an important brake on normal FOXP3-dependent Treg function, and HDAC7 targeting might be of considerable therapeutic utility in autoimmunity and transplantation.
Class IIb-specific HDAC targeting
Ongoing analysis of HDAC expression and functions in FOXP3+ Treg cells showed TCR-activated Tregs had 5-6 fold more HDAC6 mRNA than corresponding resting Treg or non-Treg cells153. In various cell types, HDAC6 deacetylates alpha-tubulin, cortactin and HSP90, abrogates formation of the aggresome, and blocks the unfolded protein response, although nothing is known regarding these pathways in Tregs. An HDAC6-specific inhibitor, tubacin (but not the control compound niltubacin)66, increased Treg suppressive function in vitro, in association with increased expression of CTLA, IL-10, TNR18, programmed cell death protein 1 (PDCD1) and other Treg-associated genes (p<0.05), and increased Treg FOXP3 protein (but not mRNA) expression. Tubacin enhanced the conversion of CD4+CD25− cells into CD4+ FOXP3+ Tregs in vitro, and globally decreased cytokine production, with the exception of IL-10 and IL-17 mRNA. Comparable and dose-dependent effects were seen using the HSP90 inhibitor, geldanamycin, suggesting that the effects of HDAC6 inhibition were mediated, at least in part, by blocking the chaperone effect of HSP90.
Use of tubacin in vivo significantly decreased the severity of colitis in two murine inflammatory bowel disease models, dextran sodium sulphate-induced colitis and the CD4+CD62Lhigh adoptive transfer model of colitis, as assessed by clinical and histological criteria. In addition, 14 days combined use of tubacin and a sub-therapeutic dosage of rapamycin led to significantly prolonged cardiac allograft survival compared with use of either agent alone153. Hence, use of a selective HDACI has important therapeutic effects, including enhancing the production and suppressive function of Tregs. Ongoing studies are directed towards unravelling the interactions of HDAC6-dependent pathways and Treg functions, as the current data indicate the importance of understanding the functions of HDACs to the development of new ways to regulate host immune responses. A model of FOXP3–HDAC6–HDAC9 interactions is shown in FIG. 3.
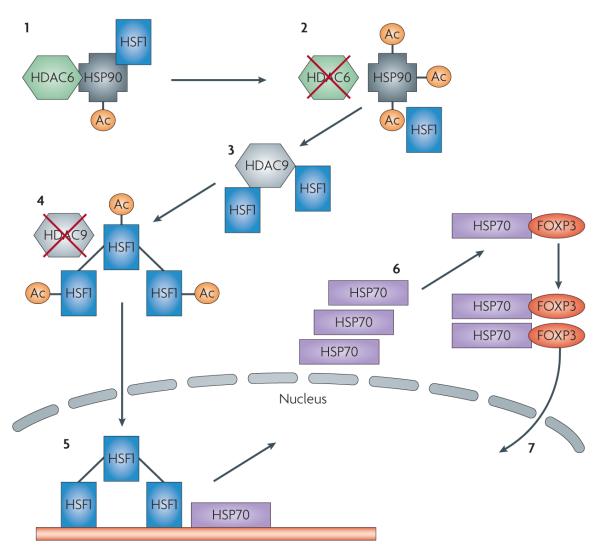
Figure 3. HDAC6 and HDAC9 as synergistic targets for Treg-based histone deacetylase inhibitor (HDACI) therapy.
In the presence of functional HDAC6, heat shock protein 90 (HSP90) exists in a state of basal acetylation and binds multiple client proteins, including heat shock factor HSF1 (1). With inhibition of HDAC6, HSP90 acetylation is markedly enhanced by the actions of one or more unknown histone acetyl transferases and client proteins are released. Whereas many client proteins undergo ubiquitination and proteasomal degradation, others, including HSF1, are functional (2). HDAC9 and SIRT1 may bind HSF1 and differentially maintain its deacetylation (3). Inhibition of HDAC9 promotes HSF1 acetylation, probably by p300, and its trimerization (4). Trimeric HSF1 undergoes phosphorylation by POLO1 or other kinases and nuclear translocation to induce the expression of other heat shock proteins such as HSP70 and HSP27 (5). HSF1-induced HSP70 binds to and serves as a chaperone for many proteins, including FOXP3 (6). HSP70 may promote the maturation of newly synthesized FOXP3 and possibly nuclear translocation and DNA interactions (7). Ac denotes acetylation.
Challenges for Treg-based therapies
Extrapolation of data from mice to humans always has limitations, including with Treg biology. For example, the cytokine IL-35 is constitutively expressed by murine FOXP3+ Tregs and promotes their suppressive function, but it is not expressed in corresponding human Tregs154. Likewise, the conversion of murine T cells to Tregs that occurs on activation and culture in the presence of IL-2 and TGF-β does not occur reliably in human cells155. Moreover, it may not always be desirable to try and increase Treg suppression, given data from the cancer field showing that the recruitment and function of Tregs may counteract host anti-tumour immune responses156. Lastly, T cells may develop increased resistance to Treg-mediated suppression, as seen clinically157 and in murine models of type 1 diabetes158. These considerations reinforce the need to develop effective strategies to control Treg functions, to validate their use with human Tregs, and to judiciously pick applications for their use.
Challenges and future directions
Available data indicate that HDACIs can have important anti-inflammatory and immunosuppressive effects through direct effects on cells of the innate and adaptive immune systems, including FOXP3+ Treg cells. However, the momentum in drug discovery is only gradually moving towards development of HDACIs that might selectively block individual HDAC isoforms. This hesitation reflects the existing one-size-fits-all standard in the field, at least from an oncological perspective, difficulties in developing screens to identify individual HDAC blocking agents, and the as yet fragmentary biology available to stimulate these efforts. This article emphasizes the potential for these agents, especially as they might harness the functions of FOXP3+ Tregs. However, additional challenges remain.
Potential adverse effects of HDACIs
There is a potential for several serious adverse affects of HDACIs, which may limit their use beyond cancer. Use of several classes of HDACI in preclinical studies and clinical trials in patients with various tumours was linked with increased electrocardiographic abnormalities, including QT interval prolongation159. This is a serious adverse drug reaction as it can potentially lead to ventricular arrhythmia and sudden death. Whether this is a class effect that is common to all HDACIs is currently unclear. In addition, clinical use of members of any of the four main classes of HDACI is accompanied by dose-limiting rates of nausea, vomiting and fatigue, and in some cases, bone marrow suppression, although the mechanisms involved are unclear160-163. At least theoretically, HDACIs may also reduce the anti-inflammatory effects of corticosteroids in patients with severe chronic obstructive airway disease by blocking the deacetylating activity of HDAC2 on NF-κB164,165, and could promote reactivation of latent viruses (for example, HIV)166. Interestingly, the reactivation of HIV in infected T cells may provide a mechanism for the purging of latently infected T lymphocytes in HIV patients and hence may prove of benefit if appropriately monitored. Lastly, selective targeting of HDAC9 might promote cardiac hypertrophy as seen in aging HDAC9−/− mice, although this phenotype was not reproduced by long-term pan-HDACI therapy in wild-type mice37.
How many HDACs should be targeted?
A one-size-fits-all approach has largely prevailed with HDACI therapy in oncology settings, but as noted here, there are data indicating that a more selective approach might be useful, at least in non-oncology settings. Given that pan-HDACIs are effective anti-inflammatory agents in some models in which class I-selective agents are ineffective, coupled with the weak deacetylase activity of HDAC class IIa members, a focus on targeting HDAC class IIb members is logical. Moreover, initial data from pharmacological and genetic targeting of HDAC6 is encouraging, although more data from additional models are needed. Lastly, selective targeting of HDAC class IIa members may prove useful in enhancing Treg functions, if selective small molecules are identified.
HDACIs in combination with other agents
In addition, taking a cue from oncology studies167, assessment of potentially synergistic interactions of HDACIs with other compounds needs to be explored in inflammatory and immune models. We have noted marked synergy in suppressing transplant rejection and promoting tolerance induction in experimental models by combining pan-HDACI therapy with subtherapeutic regimens of rapamycin7 or DNA methyltransferase inhibitors168, and are currently exploring the use of pan-HDACIs and proteasome or HSP90 inhibitors.
Future directions
It remains to be determined whether HDACIs can restore optimal regulation clinically by enhancing FOXP3 functions and/or by direct effects on effector T cells, dendritic cells and other cellular components of the inflammatory cascade. Ongoing studies are directed towards the characterization of key pathways that can control FOXP3-dependent Treg functions, with the intent of identifying and testing selective inhibitors of these pathways. Although the field is at an early stage, the ongoing analysis of HDAC structure–function relationships and the development of new and selective HDACIs may provide a major application of HDACIs and associated agents in the treatment of immuno-inflammatory disorders.
Footnotes
Histone deacetylase (HDAC). Enzyme that catalyses removal of acetyl groups from lysines in histone tails or non-histone proteins; such deacetylation usually decreases gene expression or protein function. Classical HDACs are those which are zinc-dependent and include HDACs 1–11. Sirtuins are a distinctly different family of HDACs (SIRT1–7) that are NAD-dependent and are mainly involved in cell metabolism.
FOXP3 Forkhead or winged helix transcription factor that is expressed primarily in regulatory T cells and controls their functions; its DNA binding and interactions with other proteins are promoted by acetylation and impaired by deacetylation. Mutations in FOXP3 are responsible for life-threatening autoimmunity in patients and experimental animals.
Regulatory T cell (Treg). These cells express FOXP3 and dampen immune responses mediated by T and B cells. They may also modulate functions of cells of the innate immune system. They function by multiple mechanisms, including direct membrane–membrane effects, the release of soluble products and catabolism of essential amino acids.
Histone acetyltransferase (HAT). Enzymes that catalyse acetylation of key lysines in histone tails and various non-histone proteins; such acetylation usually promotes gene expression or protein function.
Aggresome An intracellular inclusion body that stores misfolded intracellular proteins and recruits motor proteins to transport misfolded or aggregated proteins to chaperones and proteasomes for subsequent destruction.
References
- 1.Kouzarides T. Acetylation: a regulatory modification to rival phosphorylation? EMBO J. 2000;19:1176–1179. doi: 10.1093/emboj/19.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nature Rev. Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 3.Sadoul K, Boyault C, Pabion M, Khochbin S. Regulation of protein turnover by acetyltransferases and deacetylases. Biochimie. 2008;90:306–312. doi: 10.1016/j.biochi.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Spange S, Wagner T, Heinzel T, Kramer OH. Acetylation of non-histone proteins modulates cellular signalling at multiple levels. Int. J. Biochem. Cell Biol. 2009;41:185–198. doi: 10.1016/j.biocel.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 5.Choudhary C, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. Eye-opening landmark paper illustrating the extent of acetylation of non-histone proteins.
- 6.Li B, et al. FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc. Natl Acad. Sci. USA. 2007;104:4571–4576. doi: 10.1073/pnas.0700298104. Important study that highlighted the dynamic complexing of HATs and HDACs with FOXP3.
- 7.Tao R, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nature Med. 2007;13:1299–1307. doi: 10.1038/nm1652. This paper shows the potential for regulation of Treg function by modulation of FOXP3 acetylation in vitro and in vivo using HDACIs.
- 8.Yang XJ, Seto E. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nature Rev. Mol. Cell Biol. 2008;9:206–218. doi: 10.1038/nrm2346. Superb overview of class I and class II HDAC structure and function.
- 9.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nature Rev. Genet. 2009;10:32–42. doi: 10.1038/nrg2485. Latest of a fine series of reviews from this group highlighting the involvement of HDACs in diseases other than cancer.
- 10.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nature Rev. Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 11.Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins — novel therapeutic targets to treat age-associated diseases. Nature Rev. Drug Discov. 2008;7:841–853. doi: 10.1038/nrd2665. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, et al. The type III histone deacetylase Sirt1 is essential for maintenance of T cell tolerance in mice. J. Clin. Invest. 2009 Sep 1; doi: 10.1172/JCI38902. doi:10.1172/JCI38902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villagra A, et al. The histone deacetylase HDAC11 regulates the expression of interleukin 10 and immune tolerance. Nature Immunol. 2009;10:92–100. doi: 10.1038/ni.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanchard F, Chipoy C. Histone deacetylase inhibitors: new drugs for the treatment of inflammatory diseases? Drug Discov. Today. 2005;10:197–204. doi: 10.1016/S1359-6446(04)03309-4. [DOI] [PubMed] [Google Scholar]
- 15.Karagiannis TC, El-Osta A. Will broad-spectrum histone deacetylase inhibitors be superseded by more specific compounds? Leukemia. 2007;21:61–65. doi: 10.1038/sj.leu.2404464. [DOI] [PubMed] [Google Scholar]
- 16.Adcock IM. HDAC inhibitors as anti-inflammatory agents. Br. J. Pharmacol. 2007;150:829–831. doi: 10.1038/sj.bjp.0707166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunkow ME, et al. Disruption of a new forkhead/ winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nature Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 18.Bennett CL, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nature Genet. 27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 19.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor FOXP3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 20.Fontenot JD, Gavin MA, Rudensky AY. FOXP3 programs the development and function of CD4+CD25+ regulatory T cells. Nature Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 21.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nature Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 22.Chai JG, et al. in vitro expansion improves in vivo regulation by CD4+CD25+ regulatory T cells. J. Immunol. 2008;180:858–869. doi: 10.4049/jimmunol.180.2.858. [DOI] [PubMed] [Google Scholar]
- 23.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30:656–665. doi: 10.1016/j.immuni.2009.04.006. State-of-the art review of cellular therapy using human Tregs.
- 24.Chen C, Rowell EA, Thomas RM, Hancock WW, Wells AD. Transcriptional regulation by FOXP3 is associated with direct promoter occupancy and modulation of histone acetylation. J. Biol. Chem. 2006;281:36828–36834. doi: 10.1074/jbc.M608848200. Important study that first noted the need for TCR-activation to drive FOXP3-associated chromatin remodelling and the upregulation, as well as the more recognized downregulation, of gene expression by Tregs.
- 25.Samanta A, et al. TGF-β and IL-6 signals modulate chromatin binding and promoter occupancy by acetylated FOXP3. Proc. Natl Acad. Sci. USA. 2008;105:14023–14027. doi: 10.1073/pnas.0806726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Zoeten EF, Wang L, Sai H, Hancock WW. HDAC9 as a therapeutic target in murine colitis. Gastroenterology. in the press. Shows the importance of HDAC9 as a regulator of FOXP3-dependent functions, and the functionally significant physical association of FOXP3 with HSP70.
- 27.Reilly CM, et al. The histone deacetylase inhibitor trichostatin A upregulates regulatory T cells and modulates autoimmunity in NZB/W F1 mice. J. Autoimmun. 2008;31:123–130. doi: 10.1016/j.jaut.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 28.Saouaf SJ, et al. Deacetylase inhibition increases regulatory T cell function and decreases incidence and severity of collagen-induced arthritis. Exp. Mol. Pathol. 2009;87:99–104. doi: 10.1016/j.yexmp.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDonel P, Costello I, Hendrich B. Keeping things quiet: roles of NuRD and Sin3 co-repressor complexes during mammalian development. Int. J. Biochem. Cell Biol. 2009;41:108–116. doi: 10.1016/j.biocel.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grozinger CM, Hassig CA, Schreiber SL. Three proteins define a class of human histone deacetylases related to yeast Hda1p. Proc. Natl Acad. Sci. USA. 1999;96:4868–4873. doi: 10.1073/pnas.96.9.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischle W, et al. Enzymatic activity associated with class II HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR. Mol. Cell. 2002;9:45–57. doi: 10.1016/s1097-2765(01)00429-4. [DOI] [PubMed] [Google Scholar]
- 32.Lahm A, et al. Unraveling the hidden catalytic activity of vertebrate class IIa histone deacetylases. Proc. Natl Acad. Sci. USA. 2007;104:17335–17340. doi: 10.1073/pnas.0706487104. Fascinating explanation of why class IIa HDACs lack significant HDAC activity.
- 33.Jones P, et al. Probing the elusive catalytic activity of vertebrate class IIa histone deacetylases. Bioorg Med. Chem. Lett. 2008;18:1814–1819. doi: 10.1016/j.bmcl.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 34.Lemercier C, et al. Class II histone deacetylases are directly recruited by BCL6 transcriptional repressor. J. Biol. Chem. 2002;277:22045–22052. doi: 10.1074/jbc.M201736200. [DOI] [PubMed] [Google Scholar]
- 35.Zhang CL, McKinsey TA, Lu JR, Olson EN. Association of COOH-terminal-binding protein (CtBP) and MEF2-interacting transcription repressor (MITR) contributes to transcriptional repression of the MEF2 transcription factor. J. Biol. Chem. 2001;276:35–39. doi: 10.1074/jbc.M007364200. [DOI] [PubMed] [Google Scholar]
- 36.Zhang CL, McKinsey TA, Olson EN. Association of class II histone deacetylases with heterochromatin protein 1: potential role for histone methylation in control of muscle differentiation. Mol. Cell Biol. 2002;22:7302–7312. doi: 10.1128/MCB.22.20.7302-7312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang CL, et al. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell. 2002;110:479–488. doi: 10.1016/s0092-8674(02)00861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang S, Bezprozvannaya S, Li S, Olson EN. An expression screen reveals modulators of class II histone deacetylase phosphorylation. Proc. Natl Acad. Sci. USA. 2005;102:8120–8125. doi: 10.1073/pnas.0503275102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berdeaux R, et al. SIK1 is a class II HDAC kinase that promotes survival of skeletal myocytes. Nature Med. 2007;13:597–603. doi: 10.1038/nm1573. [DOI] [PubMed] [Google Scholar]
- 40.Grozinger CM, Schreiber SL. Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14-3-3-dependent cellular localization. Proc. Natl Acad. Sci. USA. 2000;97:7835–7840. doi: 10.1073/pnas.140199597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han A, He J, Wu Y, Liu JO, Chen L. Mechanism of recruitment of class II histone deacetylases by myocyte enhancer factor-2. J. Mol. Biol. 2005;345:91–102. doi: 10.1016/j.jmb.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 42.McKinsey TA, Zhang CL, Olson EN. Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14-3-3 to histone deacetylase 5. Proc. Natl Acad. Sci. USA. 2000;97:14400–14405. doi: 10.1073/pnas.260501497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Angelelli C, et al. Differentiation-dependent lysine 4 acetylation enhances MEF2C binding to DNA in skeletal muscle cells. Nucleic Acids Res. 2008;36:915–928. doi: 10.1093/nar/gkm1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paroni G, et al. PP2A regulates HDAC4 nuclear import. Mol. Biol. Cell. 2008;19:655–667. doi: 10.1091/mbc.E07-06-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z, et al. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell. 2009;138:1019–1031. doi: 10.1016/j.cell.2009.06.049. Major study showing the widespread regulation of gene expression by interacting HATs and HDACs.
- 46.Valenzuela-Fernandez A, Cabrero JR, Serrador JM, Sanchez-Madrid F. HDAC6: a key regulator of cytoskeleton, cell migration and cell-cell interactions. Trends Cell Biol. 2008;18:291–297. doi: 10.1016/j.tcb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Richon VM, O’Brien JP. Histone deacetylase inhibitors: a new class of potential therapeutic agents for cancer treatment. Clin. Cancer Res. 2002;8:662–664. [PubMed] [Google Scholar]
- 48.Bottomley MJ, et al. Structural and functional analysis of the human HDAC4 catalytic domain reveals a regulatory structural zinc-binding domain. J. Biol. Chem. 2008;283:26694–26704. doi: 10.1074/jbc.M803514200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schuetz A, et al. Human HDAC7 harbors a class IIa histone deacetylase-specific zinc binding motif and cryptic deacetylase activity. J. Biol. Chem. 2008;283:11355–11363. doi: 10.1074/jbc.M707362200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Somoza JR, et al. Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure. 2004;12:1325–1334. doi: 10.1016/j.str.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 51.Vannini A, et al. Crystal structure of a eukaryotic zinc-dependent histone deacetylase, human HDAC8, complexed with a hydroxamic acid inhibitor. Proc. Natl Acad. Sci. USA. 2004;101:15064–15069. doi: 10.1073/pnas.0404603101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nature Rev. Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 53.Walkinshaw DR, Yang XJ. Histone deacetylase inhibitors as novel anticancer therapeutics. Curr. Oncol. 2008;15:237–243. doi: 10.3747/co.v15i5.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mai A, Altucci L. Epi-drugs to fight cancer: From chemistry to cancer treatment, the road ahead. Int. J. Biochem. Cell Biol. 2009;41:199–213. doi: 10.1016/j.biocel.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 55.Gibson PR. The intracellular target of butyrate’s actions: HDAC or HDON’T? Gut. 2000;46:447–448. doi: 10.1136/gut.46.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005;46:1724–1743. doi: 10.1111/j.1528-1167.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 57.Rasheed W, Bishton M, Johnstone RW, Prince HM. Histone deacetylase inhibitors in lymphoma and solid malignancies. Expert Rev. Anticancer Ther. 2008;8:413–432. doi: 10.1586/14737140.8.3.413. [DOI] [PubMed] [Google Scholar]
- 58.Lee MJ, Kim YS, Kummar S, Giaccone G, Trepel JB. Histone deacetylase inhibitors in cancer therapy. Curr. Opin. Oncol. 2008;20:639–649. doi: 10.1097/CCO.0b013e3283127095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nature Rev. Drug Discov. 2002;1:287–299. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 60.Richon VM, Garcia-Vargas J, Hardwick JS. Development of vorinostat: Current applications and future perspectives for cancer therapy. Cancer Lett. 2009 doi: 10.1016/j.canlet.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 61.Hu E, et al. Identification of novel isoform-selective inhibitors within class I histone deacetylases. J. Pharmacol. Exp. Ther. 2003;307:720–728. doi: 10.1124/jpet.103.055541. [DOI] [PubMed] [Google Scholar]
- 62.Jones P, et al. A novel series of potent and selective ketone histone deacetylase inhibitors with antitumor activity in vivo. J. Med. Chem. 2008;51:2350–2353. doi: 10.1021/jm800079s. [DOI] [PubMed] [Google Scholar]
- 63.Massa S, et al. 3-(4-aroyl-1H-pyrrol-2-yl)-N-hydroxy-2-propenamides, a new class of synthetic histone deacetylase inhibitors. J. Med. Chem. 2001;44:2069–2072. doi: 10.1021/jm015515v. [DOI] [PubMed] [Google Scholar]
- 64.Mai A, et al. Class II (IIa)-selective histone deacetylase inhibitors. 1. Synthesis and biological evaluation of novel (aryloxopropenyl)pyrrolyl hydroxyamides. J. Med. Chem. 2005;48:3344–3353. doi: 10.1021/jm049002a. [DOI] [PubMed] [Google Scholar]
- 65.Muraglia E, et al. 2-Trifluoroacetylthiophene oxadiazoles as potent and selective class II human histone deacetylase inhibitors. Bioorg Med. Chem. Lett. 2008;18:6083–6087. doi: 10.1016/j.bmcl.2008.09.076. [DOI] [PubMed] [Google Scholar]
- 66.Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc. Natl Acad. Sci. USA. 2003;100:4389–4394. doi: 10.1073/pnas.0430973100. This paper presents the first evidence of a selective pharmacological inhibitor of a single HDAC isoform, HDAC6.
- 67.Balasubramanian S, et al. A novel histone deacetylase 8 (HDAC8)-specific inhibitor PCI-34051 induces apoptosis in T-cell lymphomas. Leukemia. 2008;22:1026–1034. doi: 10.1038/leu.2008.9. [DOI] [PubMed] [Google Scholar]
- 68.Kramer OH, et al. The histone deacetylase inhibitor valproic acid selectively induces proteasomal degradation of HDAC2. EMBO J. 2003;22:3411–3420. doi: 10.1093/emboj/cdg315. The first of various studies to highlight additional mechanisms of action of HDACIs than simply blocking HDAC catalytic activity.
- 69.Dokmanovic M, et al. Histone deacetylase inhibitors selectively suppress expression of HDAC7. Mol. Cancer Ther. 2007;6:2525–2534. doi: 10.1158/1535-7163.MCT-07-0251. [DOI] [PubMed] [Google Scholar]
- 70.Scognamiglio A, et al. HDAC-class II specific inhibition involves HDAC proteasome-dependent degradation mediated by RANBP2. Biochim. Biophys. Acta. 2008;1783:2030–2038. doi: 10.1016/j.bbamcr.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 71.Backs J, Backs T, Bezprozvannaya S, McKinsey TA, Olson EN. Histone deacetylase 5 acquires calcium/calmodulin-dependent kinase II responsiveness by oligomerization with histone deacetylase 4. Mol. Cell Biol. 2008;28:3437–3445. doi: 10.1128/MCB.01611-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu. Rev. Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- 73.Sobel RE, Cook RG, Perry CA, Annunziato AT, Allis CD. Conservation of deposition-related acetylation sites in newly synthesized histones H3 and H4. Proc. Natl Acad. Sci. USA. 1995;92:1237–1241. doi: 10.1073/pnas.92.4.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moreira JM, Scheipers P, Sorensen P. The histone deacetylase inhibitor Trichostatin A modulates CD4+ T cell responses. BMC Cancer. 2003;3:30. doi: 10.1186/1471-2407-3-30. Classic and comprehensive study of the effects of TsA on T cells.
- 75.Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J. Mol. Biol. 2004;338:17–31. doi: 10.1016/j.jmb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 76.Chen CS, Weng SC, Tseng PH, Lin HP. Histone acetylation-independent effect of histone deacetylase inhibitors on Akt through the reshuffling of protein phosphatase 1 complexes. J. Biol. Chem. 2005;280:38879–38887. doi: 10.1074/jbc.M505733200. [DOI] [PubMed] [Google Scholar]
- 77.Brogdon JL, et al. Histone deacetylase activities are required for innate immune cell control of Th1 but not Th2 effector cell function. Blood. 2007;109:1123–1130. doi: 10.1182/blood-2006-04-019711. [DOI] [PubMed] [Google Scholar]
- 78.Leoni F, et al. The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proc. Natl Acad. Sci. USA. 2002;99:2995–3000. doi: 10.1073/pnas.052702999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Backdahl L, Bushell A, Beck S. Inflammatory signalling as mediator of epigenetic modulation in tissue-specific chronic inflammation. Int. J. Biochem. Cell Biol. 2009;41:176–184. doi: 10.1016/j.biocel.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 80.Nencioni A, et al. Histone deacetylase inhibitors affect dendritic cell differentiation and immunogenicity. Clin. Cancer Res. 2007;13:3933–3941. doi: 10.1158/1078-0432.CCR-06-2903. [DOI] [PubMed] [Google Scholar]
- 81.Bosisio D, et al. Blocking TH17-polarizing cytokines by histone deacetylase inhibitors in vitro and in vivo. J. Leukoc. Biol. 2008;84:1540–1548. doi: 10.1189/jlb.0708401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun Y, et al. Cutting edge: negative regulation of dendritic cells through acetylation of the nonhistone protein STAT-3. J. Immunol. 2009;182:5899–5903. doi: 10.4049/jimmunol.0804388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reddy P, et al. Histone deacetylase inhibition modulates indoleamine 2, 3-dioxygenase-dependent DC functions and regulates experimental graft-versus-host disease in mice. J. Clin. Invest. 2008;118:2562–2573. doi: 10.1172/JCI34712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matsuoka H, et al. Disruption of HDAC4/N-CoR complex by histone deacetylase inhibitors leads to inhibition of IL-2 gene expression. Biochem. Pharmacol. 2007;74:465–476. doi: 10.1016/j.bcp.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 85.Matsuoka H, Fujimura T, Mori H, Aramori I, Mutoh S. Mechanism of HDAC inhibitor FR235222-mediated IL-2 transcriptional repression in Jurkat cells. Int. Immunopharmacol. 2007;7:1422–1432. doi: 10.1016/j.intimp.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 86.Edens RE, Dagtas S, Gilbert KM. Histone deacetylase inhibitors induce antigen specific anergy in lymphocytes: a comparative study. Int. Immunopharmacol. 2006;6:1673–1681. doi: 10.1016/j.intimp.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 87.Huber LC, et al. Trichostatin A prevents the accumulation of extracellular matrix in a mouse model of bleomycin-induced skin fibrosis. Arthritis Rheum. 2007;56:2755–2764. doi: 10.1002/art.22759. [DOI] [PubMed] [Google Scholar]
- 88.Zhou Q, et al. Histone deacetylase inhibitors blocked activation and caused senescence of corneal stromal cells. Mol. Vis. 2008;14:2556–2565. [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Z, et al. SAHA-a potential epigenetic therapeutic agent for lung fibrosis? Eur. Respir. J. 2009 Feb 12; doi: 10.1183/09031936.00084808. doi:10.1183/09031936.00084808. [DOI] [PubMed] [Google Scholar]
- 90.Yoshikawa M, Hishikawa K, Marumo T, Fujita T. Inhibition of histone deacetylase activity suppresses epithelial-to-mesenchymal transition induced by TGF-β1 in human renal epithelial cells. J. Am. Soc. Nephrol. 2007;18:58–65. doi: 10.1681/ASN.2005111187. [DOI] [PubMed] [Google Scholar]
- 91.Shan B, et al. Requirement of HDAC6 for transforming growth factor-β1-induced epithelial–mesenchymal transition. J. Biol. Chem. 2008;283:21065–21073. doi: 10.1074/jbc.M802786200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Glenisson W, Castronovo V, Waltregny D. Histone deacetylase 4 is required for TGFβ1-induced myofibroblastic differentiation. Biochim. Biophys. Acta. 2007;1773:1572–1582. doi: 10.1016/j.bbamcr.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 93.Choi JH, et al. Trichostatin A attenuates airway inflammation in mouse asthma model. Clin. Exp. Allergy. 2005;35:89–96. doi: 10.1111/j.1365-2222.2004.02006.x. [DOI] [PubMed] [Google Scholar]
- 94.Camelo S, et al. Transcriptional therapy with the histone deacetylase inhibitor trichostatin A ameliorates experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2005;164:10–21. doi: 10.1016/j.jneuroim.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 95.Mishra N, Reilly CM, Brown DR, Ruiz P, Gilkeson GS. Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse. J. Clin. Invest. 2003;111:539–552. doi: 10.1172/JCI16153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Glauben R, et al. Histone hyperacetylation is associated with amelioration of experimental colitis in mice. J. Immunol. 2006;176:5015–5022. doi: 10.4049/jimmunol.176.8.5015. [DOI] [PubMed] [Google Scholar]
- 97.Nishida K, et al. Histone deacetylase inhibitor suppression of autoantibody-mediated arthritis in mice via regulation of p16INK4a and p21(WAF1/Cip1) expression. Arthritis Rheum. 2004;50:3365–3376. doi: 10.1002/art.20709. [DOI] [PubMed] [Google Scholar]
- 98.Lin HS, et al. Anti-rheumatic activities of histone deacetylase (HDAC) inhibitors in vivo in collagen-induced arthritis in rodents. Br. J. Pharmacol. 2007;150:862–872. doi: 10.1038/sj.bjp.0707165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reddy P, et al. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. Proc. Natl Acad. Sci. USA. 2004;101:3921–3926. doi: 10.1073/pnas.0400380101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li N, et al. HDAC inhibitor reduces cytokine storm and facilitates induction of chimerism that reverses lupus in anti-CD3 conditioning regimen. Proc. Natl Acad. Sci. USA. 2008;105:4796–4801. doi: 10.1073/pnas.0712051105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Granger A, et al. Histone deacetylase inhibition reduces myocardial ischemia-reperfusion injury in mice. FASEB J. 2008;22:3549–3560. doi: 10.1096/fj.08-108548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marumo T, Hishikawa K, Yoshikawa M, Fujita T. Epigenetic regulation of BMP7 in the regenerative response to ischemia. J. Am. Soc. Nephrol. 2008;19:1311–1320. doi: 10.1681/ASN.2007091040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim HJ, et al. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J. Pharmacol. Exp. Ther. 2007;321:892–901. doi: 10.1124/jpet.107.120188. [DOI] [PubMed] [Google Scholar]
- 104.Ryu H, et al. Histone deacetylase inhibitors prevent oxidative neuronal death independent of expanded polyglutamine repeats via an Sp1-dependent pathway. Proc. Natl Acad. Sci. USA. 2003;100:4281–4286. doi: 10.1073/pnas.0737363100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hockly E, et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington’s disease. Proc. Natl Acad. Sci. USA. 2003;100:2041–2046. doi: 10.1073/pnas.0437870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Minamiyama M, et al. Sodium butyrate ameliorates phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Hum. Mol. Genet. 2004;13:1183–1192. doi: 10.1093/hmg/ddh131. [DOI] [PubMed] [Google Scholar]
- 107.Petri S, et al. Additive neuroprotective effects of a histone deacetylase inhibitor and a catalytic antioxidant in a transgenic mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2006;22:40–49. doi: 10.1016/j.nbd.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 108.Parmigiani RB, et al. HDAC6 is a specific deacetylase of peroxiredoxins and is involved in redox regulation. Proc. Natl Acad. Sci. USA. 2008;105:9633–9638. doi: 10.1073/pnas.0803749105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lin T, et al. Histone deacetylase as therapeutic target in a rodent model of hemorrhagic shock: effect of different resuscitation strategies on lung and liver. Surgery. 2007;141:784–794. doi: 10.1016/j.surg.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 110.Sailhamer EA, et al. Acetylation: a novel method for modulation of the immune response following trauma/hemorrhage and inflammatory second hit in animals and humans. Surgery. 2008;144:204–216. doi: 10.1016/j.surg.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 111.Alam HB, et al. Impact of resuscitation strategies on the acetylation status of cardiac histones in a swine model of hemorrhage. Resuscitation. 2008;76:299–310. doi: 10.1016/j.resuscitation.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 112.Li Y, et al. Cell protective mechanism of valproic acid in lethal hemorrhagic shock. Surgery. 2008;144:217–224. doi: 10.1016/j.surg.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 113.Vojinovic J, Dinarello CA, Damjanov N, Oldoni T. Safety and efficacy of oral ITF 2357 in patients with active systemic onset juvenile idopathic arthritis (SOJIA) — results of a phase II open label, international, multicentre clinical trial. Arthritis Rheum. 2008;58:S943. [Google Scholar]
- 114.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 115.Fisson S, et al. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J. Exp. Med. 2003;198:737–746. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen W, et al. Conversion of peripheral CD4+CD25+ naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor FOXP3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liang S, et al. Conversion of CD4+ CD25− cells into CD4+CD25+ regulatory T cells in vivo requires B7 costimulation, but not the thymus. J. Exp. Med. 2005;201:127–137. doi: 10.1084/jem.20041201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Almeida AR, Zaragoza B, Freitas AA. Competition controls the rate of transition between the peripheral pools of CD4+CD25− and CD4+CD25+ T cells. Int. Immunol. 2006;18:1607–1613. doi: 10.1093/intimm/dxl093. [DOI] [PubMed] [Google Scholar]
- 119.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces FOXP3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of FOXP3 T reg cells via retinoic acid. J. Exp. Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Heinonen KM, Perreault C. Development and functional properties of thymic and extrathymic T lymphocytes. Crit. Rev. Immunol. 2008;28:441–466. doi: 10.1615/critrevimmunol.v28.i5.40. [DOI] [PubMed] [Google Scholar]
- 122.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 123.Dominitzki S, et al. Cutting edge: trans-signaling via the soluble IL-6R abrogates the induction of FOXP3 in naive CD4+CD25 T cells. J. Immunol. 2007;179:2041–2045. doi: 10.4049/jimmunol.179.4.2041. [DOI] [PubMed] [Google Scholar]
- 124.Korn T, et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into FOXP3+ regulatory T cells. Proc. Natl Acad. Sci. USA. 2008;105:18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shevach EM. Mechanisms of FOXP3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 126.Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu. Rev. Immunol. 2009;27:313–338. doi: 10.1146/annurev.immunol.021908.132657. [DOI] [PubMed] [Google Scholar]
- 127.Wing K, et al. CTLA-4 control over FOXP3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 128.Tao R, Wang L, Murphy KM, Fraser CC, Hancock WW. Regulatory T cell expression of herpesvirus entry mediator suppresses the function of B and T lymphocyte attenuator-positive effector T cells. J. Immunol. 2008;180:6649–6655. doi: 10.4049/jimmunol.180.10.6649. [DOI] [PubMed] [Google Scholar]
- 129.Deaglio S, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Borsellino G, et al. Expression of ectonucleotidase CD39 by FOXP3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 131.Cobbold SP, et al. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc. Natl Acad. Sci. USA. 2009;106:12055–12060. doi: 10.1073/pnas.0903919106. Fascinating extension of the concept that Tregs can act by competing for essential nutrients by moving beyond the tryptophan/IDO concept to use multiple signalling pathways.
- 132.Stroud JC, et al. Structure of the forkhead domain of FOXP2 bound to DNA. Structure. 2006;14:159–166. doi: 10.1016/j.str.2005.10.005. Landmark structure–function modelling of FOXP2–DNA binding that has influenced the view of FOXP3 functions.
- 133.Li B, et al. FOXP3 is a homo-oligomer and a component of a supramolecular regulatory complex disabled in the human XLAAD/IPEX autoimmune disease. Int. Immunol. 2007;19:825–835. doi: 10.1093/intimm/dxm043. Further biochemical analysis by this group of muticomponent FOXP3 complexes.
- 134.Zhou Z, Song X, Li B, Greene MI. FOXP3 and its partners: structural and biochemical insights into the regulation of FOXP3 activity. Immunol. Res. 2008;42:19–28. doi: 10.1007/s12026-008-8029-x. [DOI] [PubMed] [Google Scholar]
- 135.Bettelli E, Dastrange M, Oukka M. FOXP3 interacts with nuclear factor of activated T cells and NF-kappa B to repress cytokine gene expression and effector functions of T helper cells. Proc. Natl Acad. Sci. USA. 2005;102:5138–5143. doi: 10.1073/pnas.0501675102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu Y, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 137.Grant C, et al. FOXP3 represses retroviral transcription by targeting both NF-kappa B and CREB pathways. PLoS Pathog. 2006;2:e33. doi: 10.1371/journal.ppat.0020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ono M, et al. FOXP3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature. 2007;446:685–689. doi: 10.1038/nature05673. [DOI] [PubMed] [Google Scholar]
- 139.Lee SM, Gao B, Fang D. FOXP3 maintains Treg unresponsiveness by selectively inhibiting the promoter DNA-binding activity of AP-1. Blood. 2008;111:3599–3606. doi: 10.1182/blood-2007-09-115014. [DOI] [PubMed] [Google Scholar]
- 140.Li B, Greene MI. FOXP3 actively represses transcription by recruiting the HAT/HDAC complex. Cell Cycle. 2007;6:1432–1436. [PubMed] [Google Scholar]
- 141.Lucas JL, et al. Induction of FOXP3+ regulatory T cells with histone deacetylase inhibitors. Cell. Immunol. 2009;257:97–104. doi: 10.1016/j.cellimm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 142.Wang L, Tao R, Hancock WW. Using histone deacetylase inhibitors to enhance FOXP3+ regulatory T-cell function and induce allograft tolerance. Immunol. Cell Biol. 2009;87:195–202. doi: 10.1038/icb.2008.106. [DOI] [PubMed] [Google Scholar]
- 143.Koenen HJ, et al. Human CD25highFOXP3+ regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340–2352. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 144.Kinugasa F, et al. Effect of the immunosuppressant histone deacetylase inhibitor FR276457 in a canine renal transplant model. Transpl. Immunol. 2009;21:198–202. doi: 10.1016/j.trim.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 145.Chung YL, Lee MY, Wang AJ, Yao LF. A therapeutic strategy uses histone deacetylase inhibitors to modulate the expression of genes involved in the pathogenesis of rheumatoid arthritis. Mol. Ther. 2003;8:707–717. doi: 10.1016/s1525-0016(03)00235-1. [DOI] [PubMed] [Google Scholar]
- 146.Mai A, et al. Discovery of (aryloxopropenyl)pyrrolyl hydroxyamides as selective inhibitors of class IIa histone deacetylase homologue HD1-A. J. Med. Chem. 2003;46:4826–4829. doi: 10.1021/jm034167p. [DOI] [PubMed] [Google Scholar]
- 147.Jones P, et al. 2-Trifluoroacetylthiophenes, a novel series of potent and selective class II histone deacetylase inhibitors. Bioorg Med. Chem. Lett. 2008;18:3456–3461. doi: 10.1016/j.bmcl.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 148.Estiu G, et al. Structural origin of selectivity in class II-selective histone deacetylase inhibitors. J. Med. Chem. 2008;51:2898–2906. doi: 10.1021/jm7015254. [DOI] [PubMed] [Google Scholar]
- 149.Mai A, et al. New pyrrole-based histone deacetylase inhibitors: Binding mode, enzyme- and cell-based investigations. Int. J. Biochem. Cell Biol. 2009;41:235–247. doi: 10.1016/j.biocel.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 150.Sengupta N, Seto E. Regulation of histone deacetyalse activities. J. Cell Biochem. 2004;93:57–67. doi: 10.1002/jcb.20179. [DOI] [PubMed] [Google Scholar]
- 151.Wong RH, et al. A role of DNA-PK for the metabolic gene regulation in response to insulin. Cell. 2009;136:1056–1072. doi: 10.1016/j.cell.2008.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang L, et al. HDAC7 targeting enhances FOXP3+ Treg function and induces long-term allograft survival. Am. J. Transplant. 2009;9:S621. [Google Scholar]
- 153.Hancock WW, Wang L, de Zoeten EF, Bradner JE, Mazitschel R. HDAC6 is a key new epigenetic target for the enhancement of Treg production and function in vitro and in vivo. Am. J. Transplant. 2008;8:S223. [Google Scholar]
- 154.Bardel E, Larousserie F, Charlot-Rabiega P, Coulomb-L’Hermine A, Devergne O. Human CD4+ CD25+ FOXP3+ regulatory T cells do not constitutively express IL-35. J. Immunol. 2008;181:6898–6905. doi: 10.4049/jimmunol.181.10.6898. [DOI] [PubMed] [Google Scholar]
- 155.Shevach EM. Mechanisms of FOXP3+ T Regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 156.Gallimore AM, Simon AK. Positive and negative influences of regulatory T cells on tumour immunity. Oncogene. 2008;27:5886–5893. doi: 10.1038/onc.2008.269. [DOI] [PubMed] [Google Scholar]
- 157.Lawson JM, et al. Increased resistance to CD4+CD25hi regulatory T cell-mediated suppression in patients with type 1 diabetes. Clin. Exp. Immunol. 2008;154:353–359. doi: 10.1111/j.1365-2249.2008.03810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.D’Alise AM, et al. The defect in T-cell regulation in NOD mice is an effect on the T-cell effectors. Proc. Natl Acad. Sci. USA. 2008;105:19857–19862. doi: 10.1073/pnas.0810713105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Strevel EL, Ing DJ, Siu LL. Molecularly targeted oncology therapeutics and prolongation of the QT interval. J. Clin. Oncol. 2007;25:3362–3371. doi: 10.1200/JCO.2006.09.6925. [DOI] [PubMed] [Google Scholar]
- 160.Carducci MA, et al. A Phase I clinical and pharmacological evaluation of sodium phenylbutyrate on an 120-h infusion schedule. Clin. Cancer Res. 2001;7:3047–3055. [PubMed] [Google Scholar]
- 161.Kelly WK, et al. Phase I clinical trial of histone deacetylase inhibitor: suberoylanilide hydroxamic acid administered intravenously. Clin. Cancer Res. 2003;9:3578–3588. [PubMed] [Google Scholar]
- 162.Ryan QC, et al. Phase I and pharmacokinetic study of MS-275, a histone deacetylase inhibitor, in patients with advanced and refractory solid tumors or lymphoma. J. Clin. Oncol. 2005;23:3912–3922. doi: 10.1200/JCO.2005.02.188. [DOI] [PubMed] [Google Scholar]
- 163.Klimek VM, et al. Tolerability, pharmacodynamics, and pharmacokinetics studies of depsipeptide (romidepsin) in patients with acute myelogenous leukemia or advanced myelodysplastic syndromes. Clin. Cancer Res. 2008;14:826–832. doi: 10.1158/1078-0432.CCR-07-0318. [DOI] [PubMed] [Google Scholar]
- 164.Barnes PJ. Role of HDAC2 in the Pathophysiology of COPD. Annu. Rev. Physiol. 2008 doi: 10.1146/annurev.physiol.010908.163257. [DOI] [PubMed] [Google Scholar]
- 165.Adenuga D, Yao H, March TH, Seagrave J, Rahman I. Histone deacetylase 2 is phosphorylated, ubiquitinated and degraded by cigarette smoke. Am. J. Respir. Cell. Mol. Biol. 2009;40:464–473. doi: 10.1165/rcmb.2008-0255OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Archin NM, et al. Expression of latent HIV induced by the potent HDAC inhibitor suberoylanilide hydroxamic acid. AIDS Res. Hum. Retroviruses. 2009;25:207–212. doi: 10.1089/aid.2008.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bots M, Johnstone RW. Rational combinations using HDAC inhibitors. Clin. Cancer Res. 2009;15:3970–3977. doi: 10.1158/1078-0432.CCR-08-2786. [DOI] [PubMed] [Google Scholar]
- 168.Wang L, Tao R, Friedlander JA, Hancock WW. Tolerance without immunosuppression: Exploiting epigenetic regulation as a new approach to achieving donor-specific allograft tolerance. Am. J. Transplant. 2008;8:S203. [Google Scholar]