Abstract
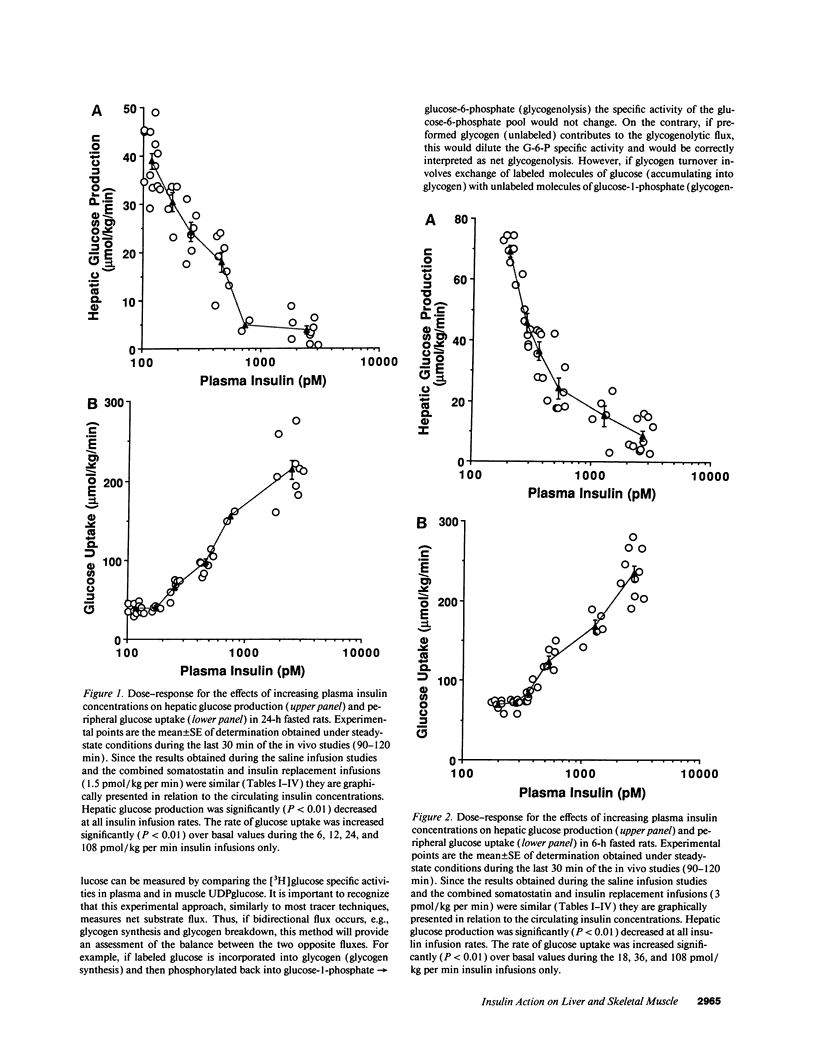
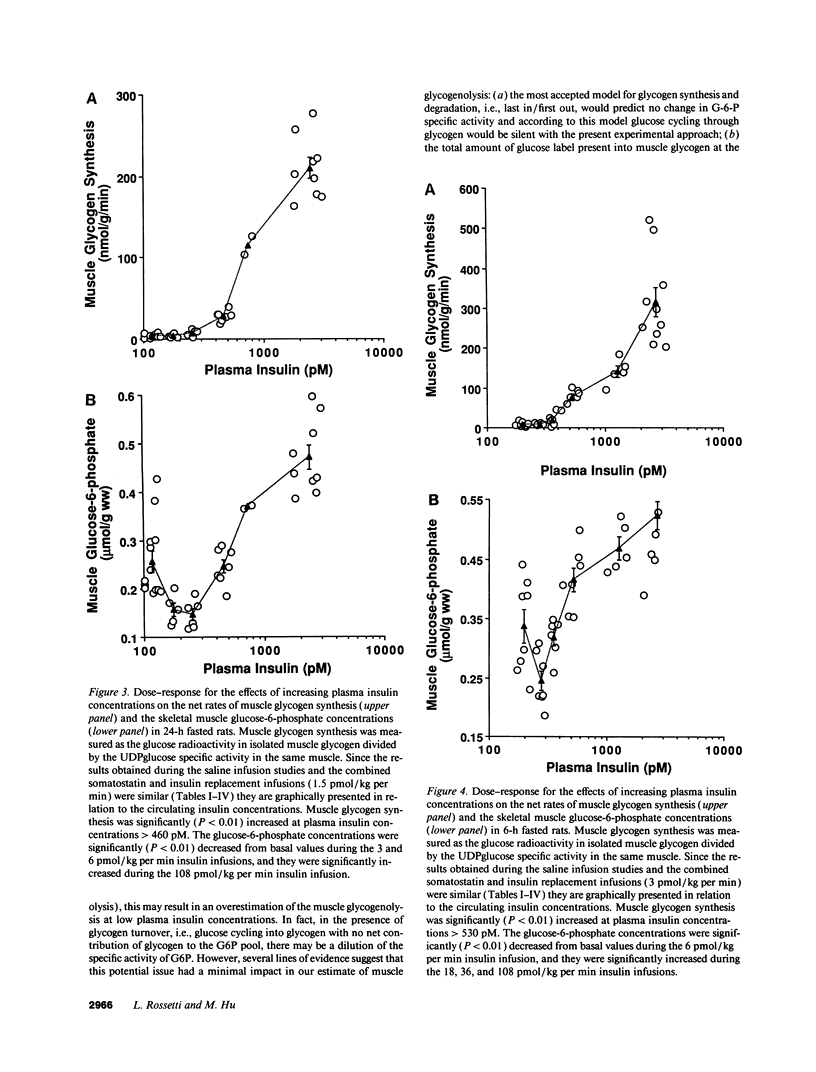
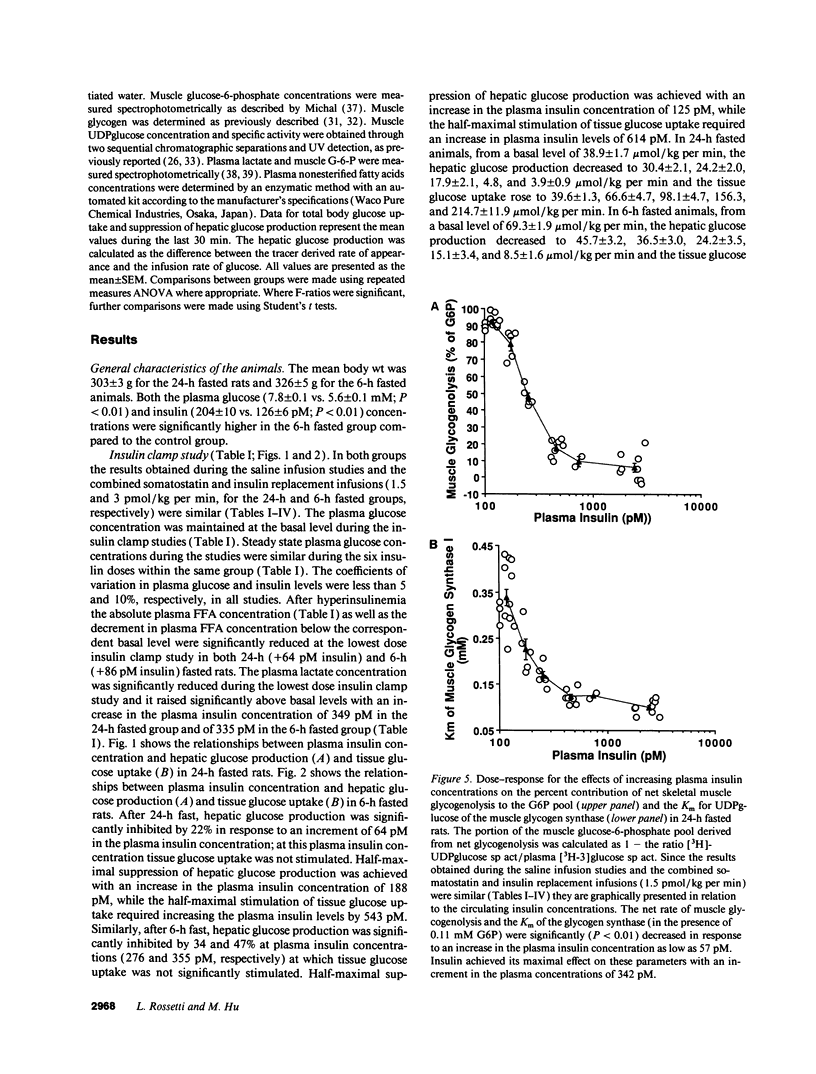
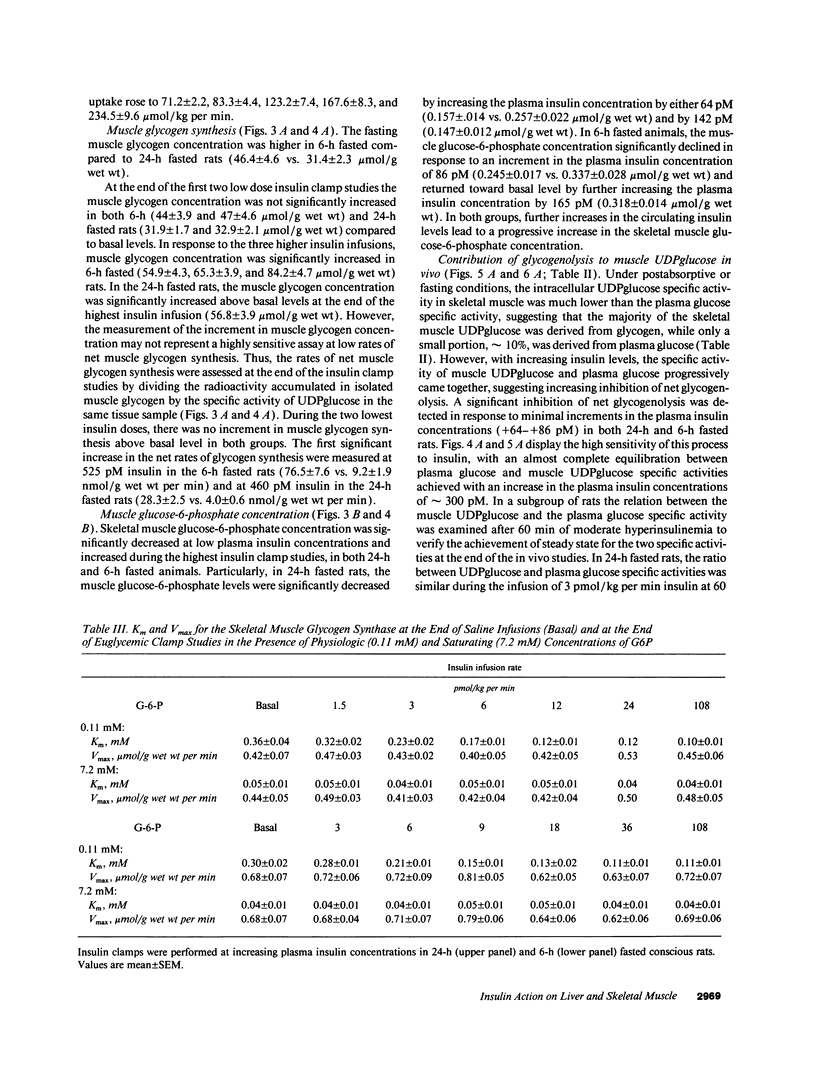
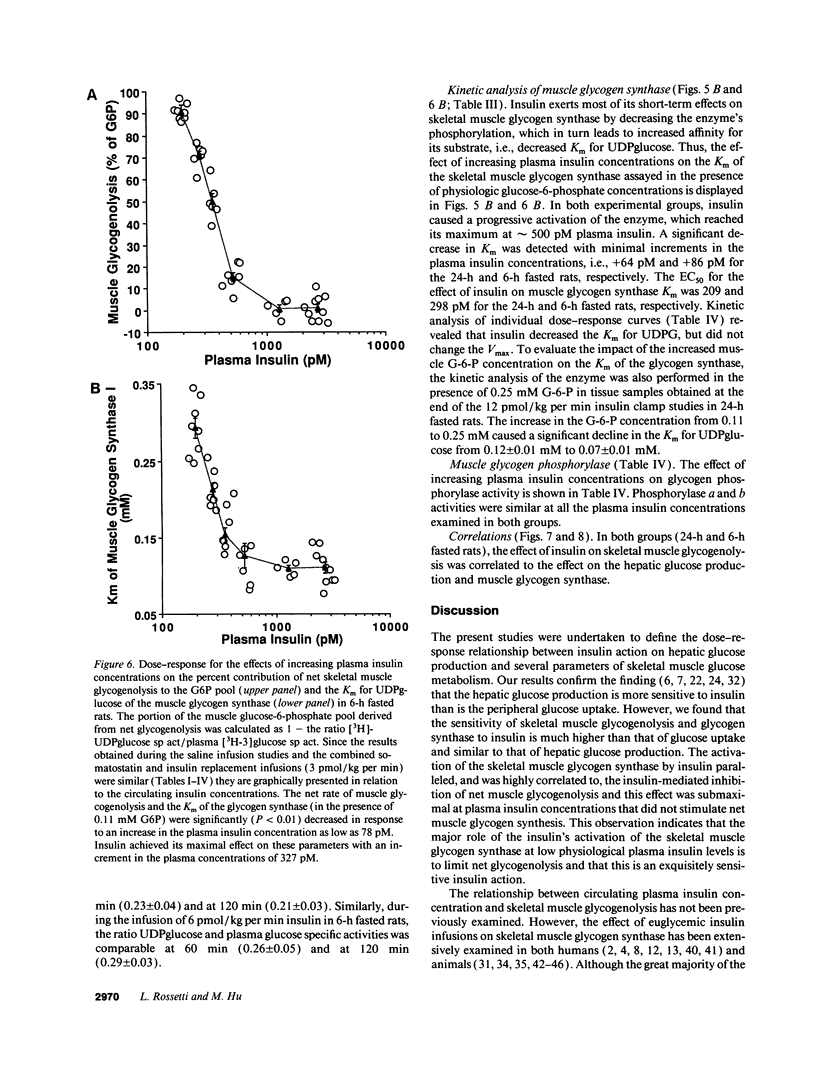
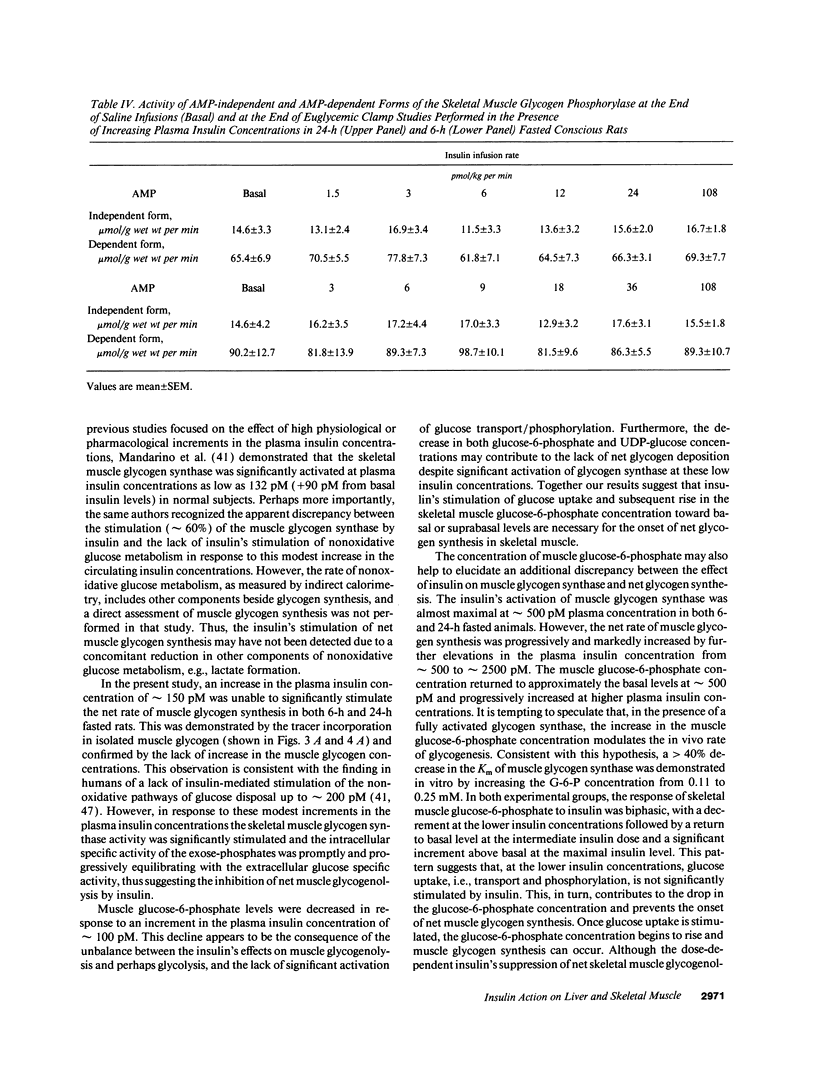
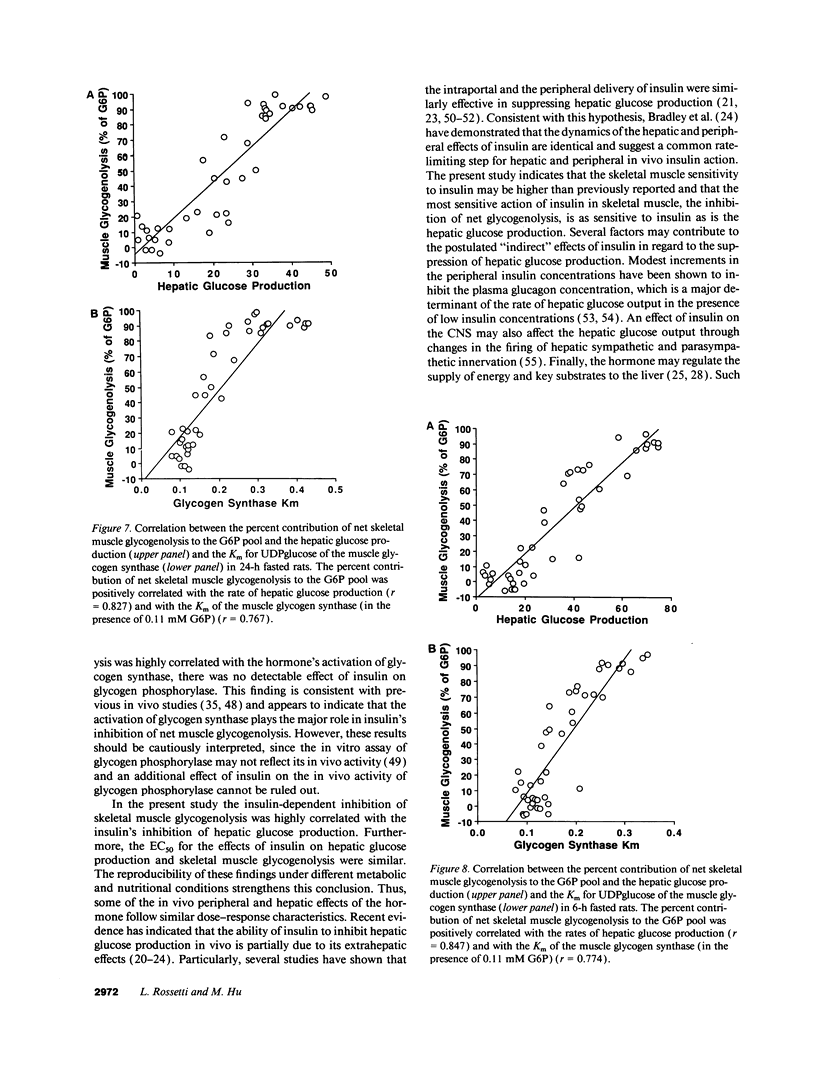
The effects of minimal increments in plasma insulin concentrations on hepatic glucose production and glucose uptake, skeletal muscle net glycogen synthesis and glycogenolysis, glycogen synthase and phosphorylase activity, glucose-6-phosphate and uridinediphosphoglucose (UDPG) concentrations were examined in 24-h and in 6-h fasted conscious rats. Insulin was infused for 120 min at rates of 1.5, 3, 6, 12, 24, and 108 pmol/kg per min in 24-h fasted rats and at rates of 3, 6, 9, 12, 36, and 108 pmol/kg per min in 6-h fasted rats while endogenous insulin release was inhibited by SRIF infusion and plasma glucose was maintained at the basal level. All rats received an infusion of [3-3H]glucose. The portion of the muscle glucose-6-phosphate (G6P) pool derived from net glycogenolysis was estimated from the ratio of specific activities of muscle UDPG and plasma glucose. Minimal increments in the circulating insulin levels, which did not stimulate glucose uptake, caused: (a) the increase in skeletal muscle glycogen synthase activity and the decrease in the rate of muscle glycogenolysis and in the G6P concentration; (b) the inhibition of hepatic glucose production. Net muscle glycogen synthesis was not stimulated despite submaximal activation of glycogen synthase, and its onset correlated with the rise in muscle G6P levels. Thus, insulin's inhibition of muscle glycogenolysis is the most sensitive insulin action on skeletal muscle and its dose-response characteristics resemble those for the inhibition of hepatic glucose production. These findings indicate that skeletal muscle glycogen synthase may play a major role in carbohydrate homeostasis even under postabsorptive (basal insulin) conditions and support the notion that insulin may exert some of its effects on the liver through an indirect or peripheral mechanism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ader M., Bergman R. N. Peripheral effects of insulin dominate suppression of fasting hepatic glucose production. Am J Physiol. 1990 Jun;258(6 Pt 1):E1020–E1032. doi: 10.1152/ajpendo.1990.258.6.E1020. [DOI] [PubMed] [Google Scholar]
- Adkins B. A., Myers S. R., Hendrick G. K., Stevenson R. W., Williams P. E., Cherrington A. D. Importance of the route of intravenous glucose delivery to hepatic glucose balance in the conscious dog. J Clin Invest. 1987 Feb;79(2):557–565. doi: 10.1172/JCI112847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogardus C., Lillioja S., Stone K., Mott D. Correlation between muscle glycogen synthase activity and in vivo insulin action in man. J Clin Invest. 1984 Apr;73(4):1185–1190. doi: 10.1172/JCI111304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. C., Poulin R. A., Bergman R. N. Dynamics of hepatic and peripheral insulin effects suggest common rate-limiting step in vivo. Diabetes. 1993 Feb;42(2):296–306. doi: 10.2337/diab.42.2.296. [DOI] [PubMed] [Google Scholar]
- Butler P. C., Kryshak E. J., Marsh M., Rizza R. A. Effect of insulin on oxidation of intracellularly and extracellularly derived glucose in patients with NIDDM. Evidence for primary defect in glucose transport and/or phosphorylation but not oxidation. Diabetes. 1990 Nov;39(11):1373–1380. doi: 10.2337/diab.39.11.1373. [DOI] [PubMed] [Google Scholar]
- Butler P. C., Kryshak E. J., Schwenk W. F., Haymond M. W., Rizza R. A. Hepatic and extrahepatic responses to insulin in NIDDM and nondiabetic humans. Assessment in absence of artifact introduced by tritiated nonglucose contaminants. Diabetes. 1990 Feb;39(2):217–225. doi: 10.2337/diab.39.2.217. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Liljenquist J. E., Shulman G. I., Williams P. E., Lacy W. W. Importance of hypoglycemia-induced glucose production during isolated glucagon deficiency. Am J Physiol. 1979 Mar;236(3):E263–E271. doi: 10.1152/ajpendo.1979.236.3.E263. [DOI] [PubMed] [Google Scholar]
- Consoli A., Nurjhan N., Reilly J. J., Jr, Bier D. M., Gerich J. E. Mechanism of increased gluconeogenesis in noninsulin-dependent diabetes mellitus. Role of alterations in systemic, hepatic, and muscle lactate and alanine metabolism. J Clin Invest. 1990 Dec;86(6):2038–2045. doi: 10.1172/JCI114940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Simonson D., Ferrannini E. Hepatic and peripheral insulin resistance: a common feature of type 2 (non-insulin-dependent) and type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1982 Oct;23(4):313–319. doi: 10.1007/BF00253736. [DOI] [PubMed] [Google Scholar]
- Farrace S., Rossetti L. Hyperglycemia markedly enhances skeletal muscle glycogen synthase activity in diabetic, but not in normal conscious rats. Diabetes. 1992 Nov;41(11):1453–1463. doi: 10.2337/diab.41.11.1453. [DOI] [PubMed] [Google Scholar]
- Firth R. G., Bell P. M., Marsh H. M., Hansen I., Rizza R. A. Postprandial hyperglycemia in patients with noninsulin-dependent diabetes mellitus. Role of hepatic and extrahepatic tissues. J Clin Invest. 1986 May;77(5):1525–1532. doi: 10.1172/JCI112467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U., Rizza R. A., Hall L. D., Westland R. E., Haymond M. W., Clemens A. H., Gerich J. E., Service F. J. Comparison of peripheral and portal venous insulin administration on postprandial metabolic responses in alloxan-diabetic dogs. Effects of identical preprogrammed complex insulin infusion waveforms. Diabetes. 1982 Jul;31(7):579–584. doi: 10.2337/diab.31.7.579. [DOI] [PubMed] [Google Scholar]
- Giacca A., Fisher S. J., Shi Z. Q., Gupta R., Lickley H. L., Vranic M. Importance of peripheral insulin levels for insulin-induced suppression of glucose production in depancreatized dogs. J Clin Invest. 1992 Nov;90(5):1769–1777. doi: 10.1172/JCI116051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaccari A., Rossetti L. Isocratic high-performance liquid chromatographic determination of the concentration and specific radioactivity of phosphoenolpyruvate and uridine diphosphate glucose in tissue extracts. J Chromatogr. 1989 Dec 29;497:69–78. doi: 10.1016/0378-4347(89)80006-4. [DOI] [PubMed] [Google Scholar]
- Giaccari A., Rossetti L. Predominant role of gluconeogenesis in the hepatic glycogen repletion of diabetic rats. J Clin Invest. 1992 Jan;89(1):36–45. doi: 10.1172/JCI115583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groop L. C., Kankuri M., Schalin-Jäntti C., Ekstrand A., Nikula-Ijäs P., Widén E., Kuismanen E., Eriksson J., Franssila-Kallunki A., Saloranta C. Association between polymorphism of the glycogen synthase gene and non-insulin-dependent diabetes mellitus. N Engl J Med. 1993 Jan 7;328(1):10–14. doi: 10.1056/NEJM199301073280102. [DOI] [PubMed] [Google Scholar]
- Herrera M. G., Kamm D., Ruderman N., Cahill Non-hormonal factors in the control of gluconeogenesis. Adv Enzyme Regul. 1966;4:225–235. doi: 10.1016/0065-2571(66)90017-3. [DOI] [PubMed] [Google Scholar]
- Jackson R. A., Peters N., Advani U., Perry G., Rogers J., Brough W. H., Pilkington T. R. Forearm glucose uptake during the oral glucose tolerance test in normal subjects. Diabetes. 1973 Jun;22(6):442–458. doi: 10.2337/diab.22.6.442. [DOI] [PubMed] [Google Scholar]
- Kelley D. E., Mandarino L. J. Hyperglycemia normalizes insulin-stimulated skeletal muscle glucose oxidation and storage in noninsulin-dependent diabetes mellitus. J Clin Invest. 1990 Dec;86(6):1999–2007. doi: 10.1172/JCI114935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D., Mitrakou A., Marsh H., Schwenk F., Benn J., Sonnenberg G., Arcangeli M., Aoki T., Sorensen J., Berger M. Skeletal muscle glycolysis, oxidation, and storage of an oral glucose load. J Clin Invest. 1988 May;81(5):1563–1571. doi: 10.1172/JCI113489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruszynska Y. T., Home P. D., Alberti K. G. Comparison of portal and peripheral insulin delivery on carbohydrate metabolism in streptozotocin-diabetic rats. Diabetologia. 1985 Mar;28(3):167–171. doi: 10.1007/BF00273866. [DOI] [PubMed] [Google Scholar]
- Kruszynska Y. T., Home P. D., Alberti K. G. In vivo regulation of liver and skeletal muscle glycogen synthase activity by glucose and insulin. Diabetes. 1986 Jun;35(6):662–667. doi: 10.2337/diab.35.6.662. [DOI] [PubMed] [Google Scholar]
- Kruszynska Y. T., Home P. D. Liver and muscle insulin sensitivity, glycogen concentration and glycogen synthase activity in a rat model of non-insulin-dependent diabetes. Diabetologia. 1988 May;31(5):304–309. doi: 10.1007/BF00277412. [DOI] [PubMed] [Google Scholar]
- Kryshak E. J., Butler P. C., Marsh C., Miller A., Barr D., Polonsky K., Perkins J. D., Rizza R. A. Pattern of postprandial carbohydrate metabolism and effects of portal and peripheral insulin delivery. Diabetes. 1990 Feb;39(2):142–148. doi: 10.2337/diab.39.2.142. [DOI] [PubMed] [Google Scholar]
- Laughlin M. R., Petit W. A., Jr, Dizon J. M., Shulman R. G., Barrett E. J. NMR measurements of in vivo myocardial glycogen metabolism. J Biol Chem. 1988 Feb 15;263(5):2285–2291. [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Freychet P. Regulation of glycogen synthase activity in the isolated mouse soleus muscle. Effect of insulin, epinephrine, glucose and anti-insulin receptor antibodies. Biochim Biophys Acta. 1981 Sep 18;677(1):13–22. [PubMed] [Google Scholar]
- Lillioja S., Mott D. M., Zawadzki J. K., Young A. A., Abbott W. G., Bogardus C. Glucose storage is a major determinant of in vivo "insulin resistance" in subjects with normal glucose tolerance. J Clin Endocrinol Metab. 1986 May;62(5):922–927. doi: 10.1210/jcem-62-5-922. [DOI] [PubMed] [Google Scholar]
- Lins P. E., Wajngot A., Adamson U., Vranic M., Efendić S. Minimal increases in glucagon levels enhance glucose production in man with partial hypoinsulinemia. Diabetes. 1983 Jul;32(7):633–636. doi: 10.2337/diab.32.7.633. [DOI] [PubMed] [Google Scholar]
- Mandarino L. J., Wright K. S., Verity L. S., Nichols J., Bell J. M., Kolterman O. G., Beck-Nielsen H. Effects of insulin infusion on human skeletal muscle pyruvate dehydrogenase, phosphofructokinase, and glycogen synthase. Evidence for their role in oxidative and nonoxidative glucose metabolism. J Clin Invest. 1987 Sep;80(3):655–663. doi: 10.1172/JCI113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårin P., Högh-Kristiansen I., Jansson S., Krotkiewski M., Holm G., Björntorp P. Uptake of glucose carbon in muscle glycogen and adipose tissue triglycerides in vivo in humans. Am J Physiol. 1992 Sep;263(3 Pt 1):E473–E480. doi: 10.1152/ajpendo.1992.263.3.E473. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Caudwell F. B., Cohen P. Glycogen synthase from rabbit skeletal muscle; effect of insulin on the state of phosphorylation of the seven phosphoserine residues in vivo. Eur J Biochem. 1983 Jan 17;130(1):227–234. doi: 10.1111/j.1432-1033.1983.tb07140.x. [DOI] [PubMed] [Google Scholar]
- Prager R., Wallace P., Olefsky J. M. Direct and indirect effects of insulin to inhibit hepatic glucose output in obese subjects. Diabetes. 1987 May;36(5):607–611. doi: 10.2337/diab.36.5.607. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Farrace S., Choi S. B., Giaccari A., Sloan L., Frontoni S., Katz M. S. Multiple metabolic effects of CGRP in conscious rats: role of glycogen synthase and phosphorylase. Am J Physiol. 1993 Jan;264(1 Pt 1):E1–10. doi: 10.1152/ajpendo.1993.264.1.E1. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Giaccari A. Relative contribution of glycogen synthesis and glycolysis to insulin-mediated glucose uptake. A dose-response euglycemic clamp study in normal and diabetic rats. J Clin Invest. 1990 Jun;85(6):1785–1792. doi: 10.1172/JCI114636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L., Lauglin M. R. Correction of chronic hyperglycemia with vanadate, but not with phlorizin, normalizes in vivo glycogen repletion and in vitro glycogen synthase activity in diabetic skeletal muscle. J Clin Invest. 1989 Sep;84(3):892–899. doi: 10.1172/JCI114250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L., Smith D., Shulman G. I., Papachristou D., DeFronzo R. A. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987 May;79(5):1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalin-Jäntti C., Härkonen M., Groop L. C. Impaired activation of glycogen synthase in people at increased risk for developing NIDDM. Diabetes. 1992 May;41(5):598–604. doi: 10.2337/diab.41.5.598. [DOI] [PubMed] [Google Scholar]
- Shimazu T. Neuronal regulation of hepatic glucose metabolism in mammals. Diabetes Metab Rev. 1987 Jan;3(1):185–206. doi: 10.1002/dmr.5610030109. [DOI] [PubMed] [Google Scholar]
- Shulman G. I., Rothman D. L., Jue T., Stein P., DeFronzo R. A., Shulman R. G. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990 Jan 25;322(4):223–228. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- Thorburn A. W., Gumbiner B., Bulacan F., Brechtel G., Henry R. R. Multiple defects in muscle glycogen synthase activity contribute to reduced glycogen synthesis in non-insulin dependent diabetes mellitus. J Clin Invest. 1991 Feb;87(2):489–495. doi: 10.1172/JCI115022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn A. W., Gumbiner B., Bulacan F., Wallace P., Henry R. R. Intracellular glucose oxidation and glycogen synthase activity are reduced in non-insulin-dependent (type II) diabetes independent of impaired glucose uptake. J Clin Invest. 1990 Feb;85(2):522–529. doi: 10.1172/JCI114468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhing R. J., Shikama H., Exton J. H. Effects of insulin on the phosphate content and kinetics of glycogen synthase in perfused rat hindlimb muscle. FEBS Lett. 1981 Nov 16;134(2):185–188. doi: 10.1016/0014-5793(81)80598-4. [DOI] [PubMed] [Google Scholar]
- Vaag A., Henriksen J. E., Beck-Nielsen H. Decreased insulin activation of glycogen synthase in skeletal muscles in young nonobese Caucasian first-degree relatives of patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1992 Mar;89(3):782–788. doi: 10.1172/JCI115656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard H., Bjørbaek C., Andersen P. H., Bak J. F., Pedersen O. Impaired expression of glycogen synthase mRNA in skeletal muscle of NIDDM patients. Diabetes. 1991 Dec;40(12):1740–1745. doi: 10.2337/diab.40.12.1740. [DOI] [PubMed] [Google Scholar]
- Vranic M. Banting Lecture: glucose turnover. A key to understanding the pathogenesis of diabetes (indirect effects of insulin). Diabetes. 1992 Sep;41(9):1188–1206. doi: 10.2337/diab.41.9.1188. [DOI] [PubMed] [Google Scholar]
- Wright K. S., Beck-Nielsen H., Kolterman O. G., Mandarino L. J. Decreased activation of skeletal muscle glycogen synthase by mixed-meal ingestion in NIDDM. Diabetes. 1988 Apr;37(4):436–440. doi: 10.2337/diab.37.4.436. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H., Helve E., Sane T., Nurjhan N., Taskinen M. R. Insulin inhibition of overnight glucose production and gluconeogenesis from lactate in NIDDM. Am J Physiol. 1989 Jun;256(6 Pt 1):E732–E739. doi: 10.1152/ajpendo.1989.256.6.E732. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H., Mott D., Young A. A., Stone K., Bogardus C. Regulation of glycogen synthase and phosphorylase activities by glucose and insulin in human skeletal muscle. J Clin Invest. 1987 Jul;80(1):95–100. doi: 10.1172/JCI113069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn J. H., Bergman R. N. Enhancement of hepatic glycogen by gluconeogenic precursors: substrate flux or metabolic control? Am J Physiol. 1990 Jun;258(6 Pt 1):E899–E906. doi: 10.1152/ajpendo.1990.258.6.E899. [DOI] [PubMed] [Google Scholar]
- Zawadzki J. K., Wolfe R. R., Mott D. M., Lillioja S., Howard B. V., Bogardus C. Increased rate of Cori cycle in obese subjects with NIDDM and effect of weight reduction. Diabetes. 1988 Feb;37(2):154–159. doi: 10.2337/diab.37.2.154. [DOI] [PubMed] [Google Scholar]


