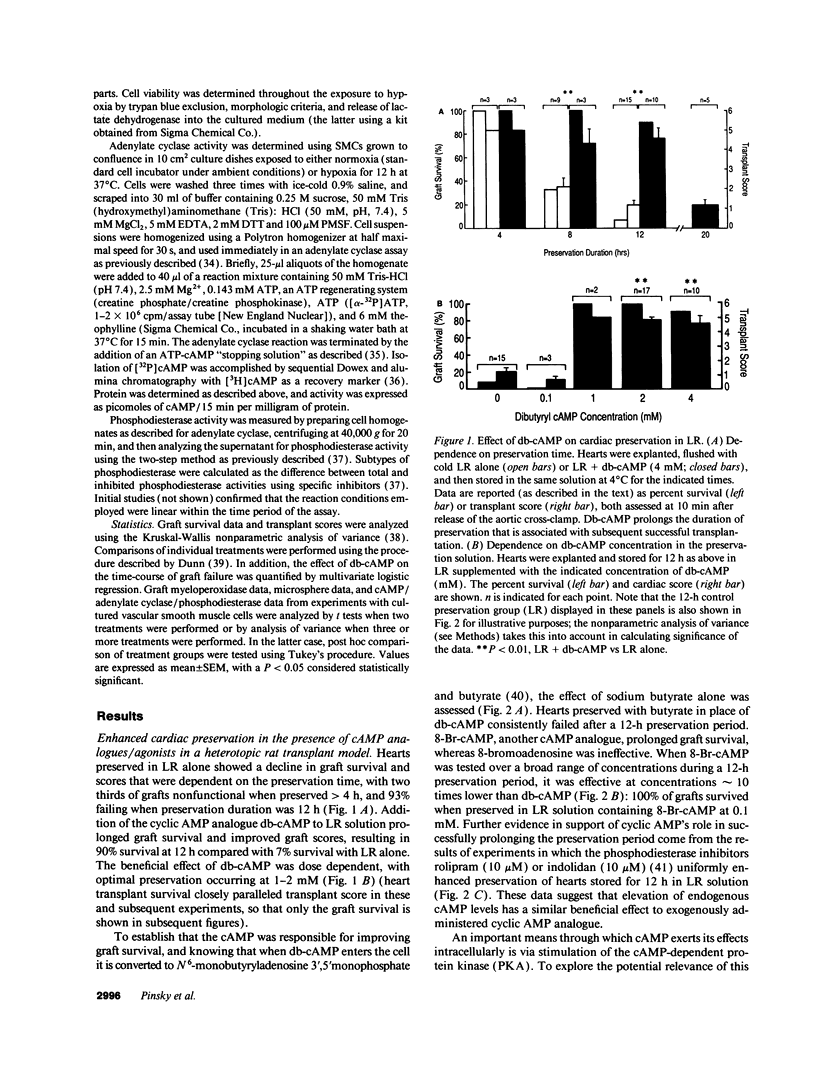
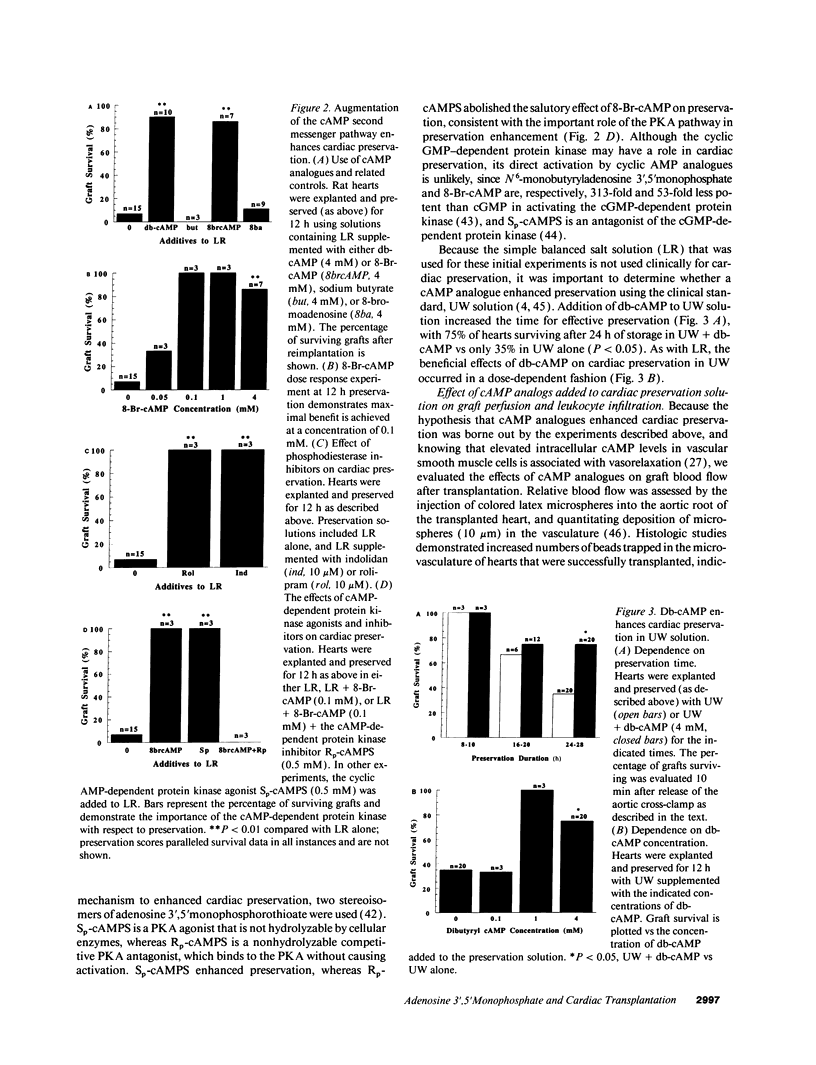
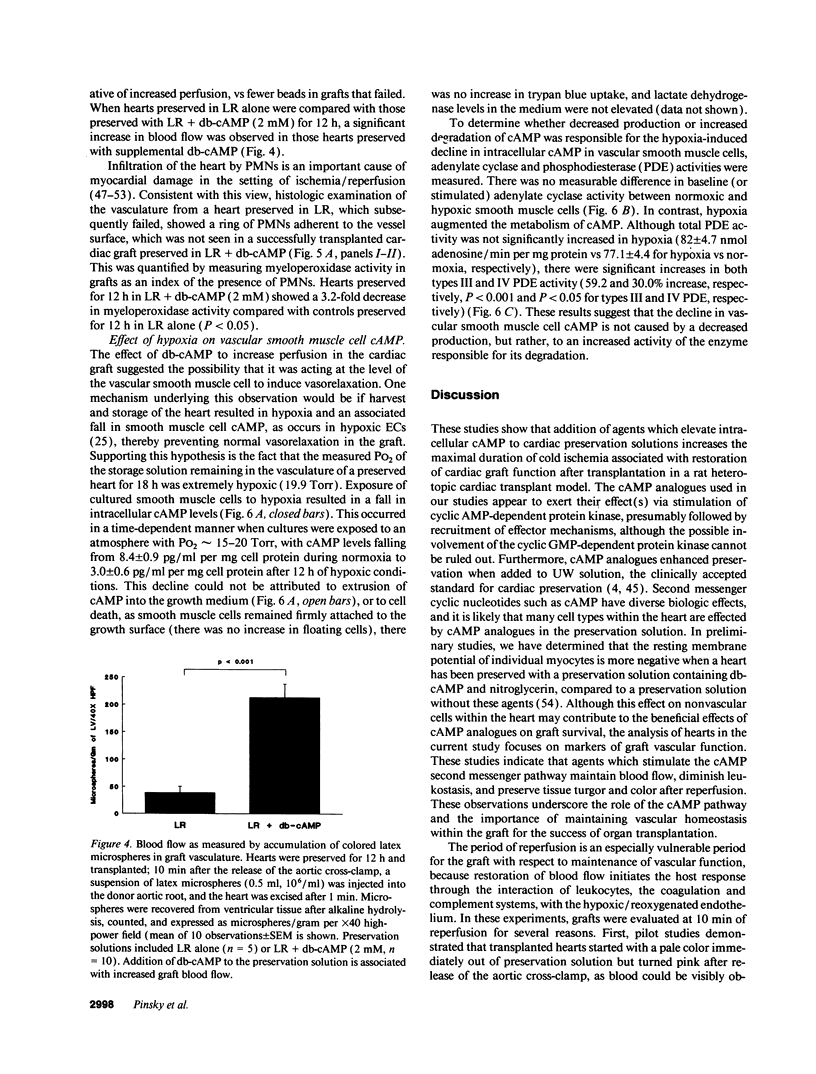
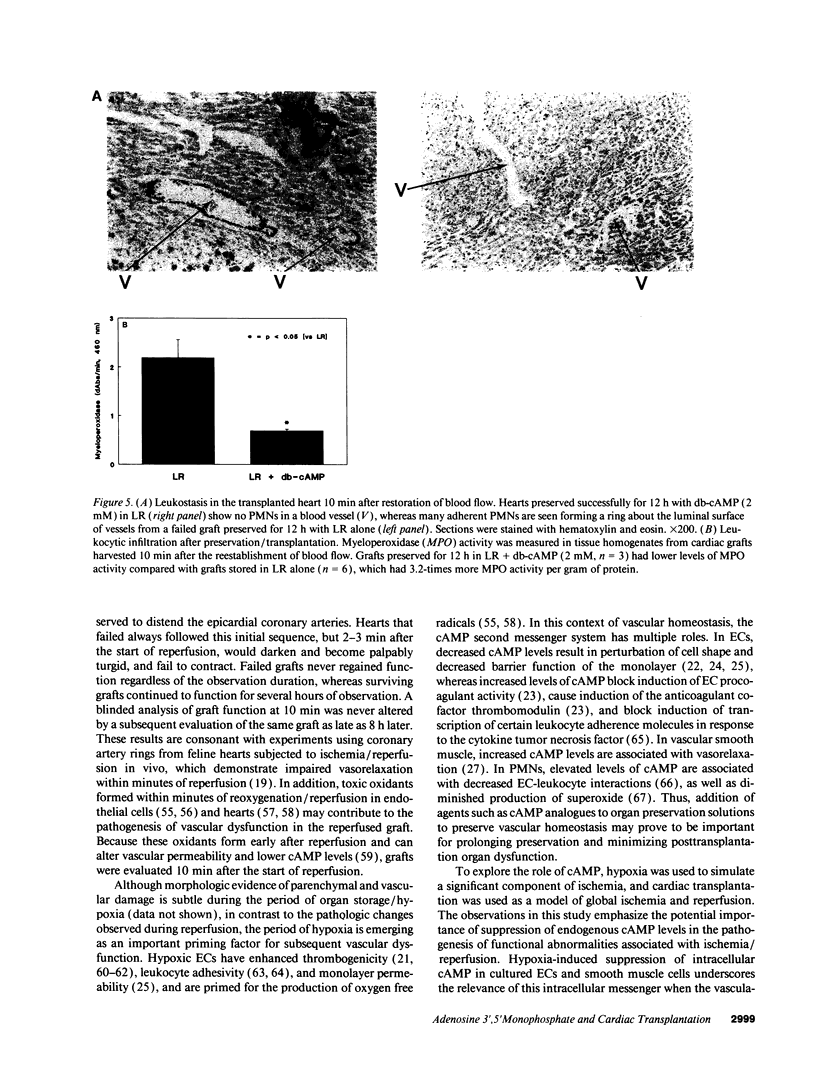
Abstract
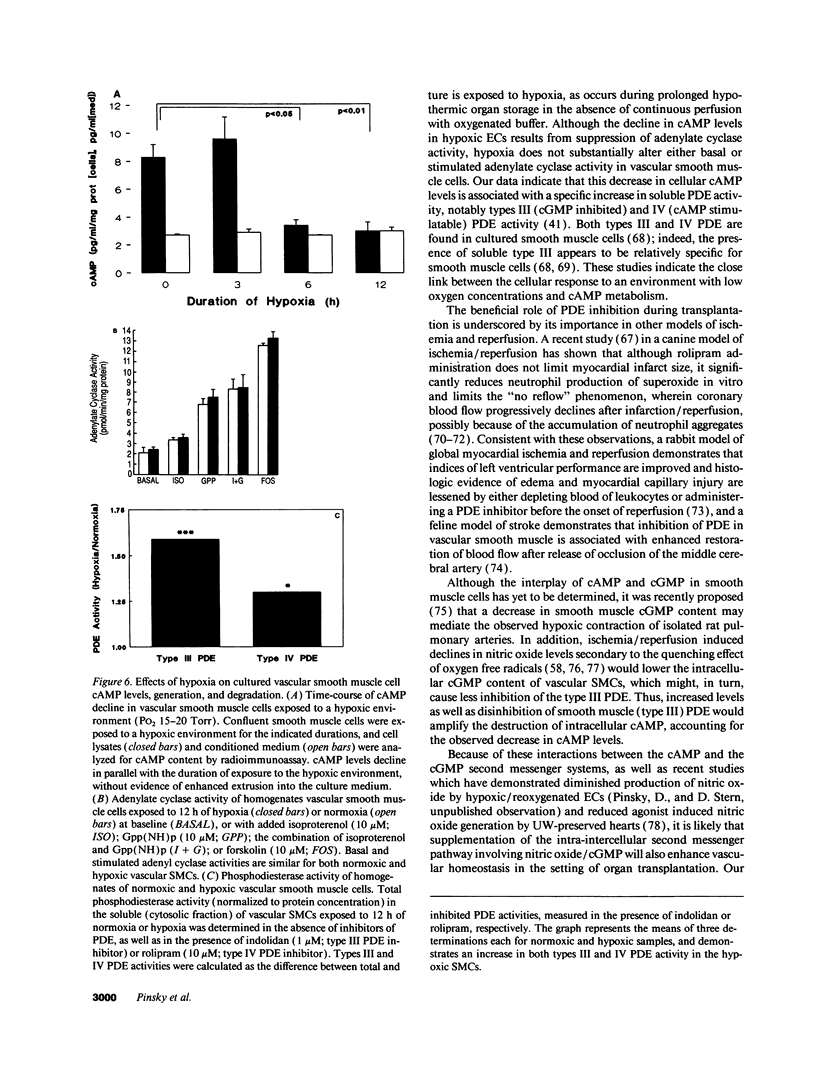
Current organ preservation strategies subject graft vasculature to severe hypoxia (PO2 approximately 20 Torr), potentially compromising vascular function and limiting successful transplantation. Previous work has shown that cAMP modulates endothelial cell (EC) antithrombogenicity, barrier function, and leukocyte/EC interactions, and that hypoxia suppresses EC cAMP levels. To explore the possible benefits of cAMP analogs/agonists in organ preservation, we used a rat heterotopic cardiac transplant model; dibutyryl cAMP added to preservation solutions was associated with a time- and dose-dependent increase in the duration of cold storage associated with successful graft function. Preservation was also enhanced by 8-bromo-cAMP, the Sp isomer of adenosine 3',5'monophosphorothioate, and types III (indolidan) and IV (rolipram) phosphodiesterase inhibitors. Neither butyrate alone nor 8-bromoadenosine were effective, and the cAMP-dependent protein kinase antagonist Rp isomer of adenosine 3',5'monophosphorothioate prevented preservation enhancement induced by 8-bromo-cAMP. Grafts stored with dibutyryl cAMP demonstrated a 5.5-fold increase in blood flow and a 3.2-fold decreased neutrophil infiltration after transplantation. To explore the role of cAMP in another cell type critical for vascular homeostasis, vascular smooth muscle cells were subjected to hypoxia, causing a time-dependent decline in cAMP levels. Although adenylate cyclase activity was unchanged, diminished oxygen tensions were associated with enhanced phosphodiesterase activity (59 and 30% increase in soluble types III and IV activity, respectively). These data suggest that hypoxia or graft ischemia disrupt vascular homeostasis, at least in part, by perturbing the cAMP second messenger pathway. Supplementation of this pathway provides a new approach for enhancing cardiac preservation, promoting myocardial function, and maintaining vascular homeostatic properties.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babbs C. F., Cregor M. D., Turek J. J., Badylak S. F. Endothelial superoxide production in the isolated rat heart during early reperfusion after ischemia. A histochemical study. Am J Pathol. 1991 Nov;139(5):1069–1080. [PMC free article] [PubMed] [Google Scholar]
- Belzer F. O., Southard J. H. Principles of solid-organ preservation by cold storage. Transplantation. 1988 Apr;45(4):673–676. doi: 10.1097/00007890-198804000-00001. [DOI] [PubMed] [Google Scholar]
- Bilezikian J. P., Dornfeld A. M., Gammon D. E. Structure-binding-activity analysis of beta-adrenergic amines--I. Binding to the beta receptor and activation of adenylate cyclase. Biochem Pharmacol. 1978 May 15;27(10):1445–1454. doi: 10.1016/0006-2952(78)90100-4. [DOI] [PubMed] [Google Scholar]
- Boggs B. R., Torchiana D. F., Geffin G. A., Titus J. S., Redonnett B. E., O'Keefe D. D., Newell J. B., Daggett W. M. Optimal myocardial preservation with an acalcemic crystalloid cardioplegic solution. J Thorac Cardiovasc Surg. 1987 Jun;93(6):838–846. [PubMed] [Google Scholar]
- Boxer L. A., Allen J. M., Baehner R. L. Diminished polymorphonuclear leukocyte adherence. Function dependent on release of cyclic AMP by endothelial cells after stimulation of beta-receptors by epinephrine. J Clin Invest. 1980 Aug;66(2):268–274. doi: 10.1172/JCI109853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan B. E., Roeder T. L., Shasby D. M. Insight into the nature and site of oxygen-centered free radical generation by endothelial cell monolayers using a novel spin trapping technique. Blood. 1992 Feb 1;79(3):699–707. [PubMed] [Google Scholar]
- Chang-Chun C., Matsuda H., Sawa Y., Kaneko M., Sakagoshi N., Nishimura M., Kuratani T., Amemiya A., Kawashima Y. Effect of a cyclic adenosine monophosphate phosphodiesterase inhibitor, DN-9693, on myocardial reperfusion injury. Ann Thorac Surg. 1991 Sep;52(3):495–499. doi: 10.1016/0003-4975(91)90911-9. [DOI] [PubMed] [Google Scholar]
- Colucci W. S., Brock T. A., Gimbrone M. A., Jr, Alexander R. W. Nonlinear relationship between alpha 1-adrenergic receptor occupancy and norepinephrine-stimulated calcium flux in cultured vascular smooth muscle cells. Mol Pharmacol. 1985 May;27(5):517–524. [PubMed] [Google Scholar]
- Corbin J. D., Ogreid D., Miller J. P., Suva R. H., Jastorff B., Døskeland S. O. Studies of cGMP analog specificity and function of the two intrasubunit binding sites of cGMP-dependent protein kinase. J Biol Chem. 1986 Jan 25;261(3):1208–1214. [PubMed] [Google Scholar]
- Crawford M. H., Grover F. L., Kolb W. P., McMahan C. A., O'Rourke R. A., McManus L. M., Pinckard R. N. Complement and neutrophil activation in the pathogenesis of ischemic myocardial injury. Circulation. 1988 Dec;78(6):1449–1458. doi: 10.1161/01.cir.78.6.1449. [DOI] [PubMed] [Google Scholar]
- Das D. K., Russell J. C., Jones R. M. Reduction of cold injury by superoxide dismutase and catalase. Free Radic Res Commun. 1991;12-13 Pt 2:653–662. doi: 10.3109/10715769109145843. [DOI] [PubMed] [Google Scholar]
- Engler R. L., Dahlgren M. D., Morris D. D., Peterson M. A., Schmid-Schönbein G. W. Role of leukocytes in response to acute myocardial ischemia and reflow in dogs. Am J Physiol. 1986 Aug;251(2 Pt 2):H314–H323. doi: 10.1152/ajpheart.1986.251.2.H314. [DOI] [PubMed] [Google Scholar]
- Engler R. L., Schmid-Schönbein G. W., Pavelec R. S. Leukocyte capillary plugging in myocardial ischemia and reperfusion in the dog. Am J Pathol. 1983 Apr;111(1):98–111. [PMC free article] [PubMed] [Google Scholar]
- Entman M. L., Michael L., Rossen R. D., Dreyer W. J., Anderson D. C., Taylor A. A., Smith C. W. Inflammation in the course of early myocardial ischemia. FASEB J. 1991 Aug;5(11):2529–2537. doi: 10.1096/fasebj.5.11.1868978. [DOI] [PubMed] [Google Scholar]
- Goldblum S. E., Wu K. M., Jay M. Lung myeloperoxidase as a measure of pulmonary leukostasis in rabbits. J Appl Physiol (1985) 1985 Dec;59(6):1978–1985. doi: 10.1152/jappl.1985.59.6.1978. [DOI] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M., Gordon D., Lu P. L. A smooth muscle-specific monoclonal antibody recognizes smooth muscle actin isozymes. J Cell Biol. 1985 Mar;100(3):807–813. doi: 10.1083/jcb.100.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale S. L., Alker K. J., Kloner R. A. Evaluation of nonradioactive, colored microspheres for measurement of regional myocardial blood flow in dogs. Circulation. 1988 Aug;78(2):428–434. doi: 10.1161/01.cir.78.2.428. [DOI] [PubMed] [Google Scholar]
- Hoek J. B. Intracellular signal transduction and the control of endothelial permeability. Lab Invest. 1992 Jul;67(1):1–4. [PubMed] [Google Scholar]
- Hofmann F., Gensheimer H. P., Landgraf W., Hullin R., Jastorff B. Diastereomers of adenosine 3',5'-monothionophosphate (cAMP[S]) antagonize the activation of cGMP-dependent protein kinase. Eur J Biochem. 1985 Jul 1;150(1):85–88. doi: 10.1111/j.1432-1033.1985.tb08991.x. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Ross G., Tillisch J. Pharmacology of endothelium-derived nitric oxide and nitrovasodilators. West J Med. 1991 Jan;154(1):51–62. [PMC free article] [PubMed] [Google Scholar]
- Iyengar J., George A., Russell J. C., Das D. K. The effects of an iron chelator on cellular injury induced by vascular stasis caused by hypothermia. J Vasc Surg. 1990 Nov;12(5):545–551. [PubMed] [Google Scholar]
- Jeevanandam V., Barr M. L., Auteri J. S., Sanchez J. A., Ott G. Y., Schenkel F. A., Marboe C., Smith C. R., Rose E. A. University of Wisconsin solution for human donor heart preservation: initial clinical experience. Ann Thorac Surg. 1991 Dec;52(6):1213–1216. doi: 10.1016/0003-4975(91)90003-9. [DOI] [PubMed] [Google Scholar]
- Kaukel E., Mundhenk K., Hilz H. N 6 -monobutyryladenosine 3':5'-mono phosphate as the biologically active derivative of dibutyryladenosine 3':5'-monophosphate in HeLa S3 cells. Eur J Biochem. 1972 May;27(1):197–200. doi: 10.1111/j.1432-1033.1972.tb01826.x. [DOI] [PubMed] [Google Scholar]
- Keravis T. M., Wells J. N., Hardman J. G. Cyclic nucleotide phosphodiesterase activities from pig coronary arteries. Lack of interconvertibility of major forms. Biochim Biophys Acta. 1980;613(1):116–129. doi: 10.1016/0005-2744(80)90198-9. [DOI] [PubMed] [Google Scholar]
- Kloner R. A. No reflow revisited. J Am Coll Cardiol. 1989 Dec;14(7):1814–1815. doi: 10.1016/0735-1097(89)90037-5. [DOI] [PubMed] [Google Scholar]
- Koga S., Ogawa S., Kuwabara K., Brett J., Leavy J. A., Ryan J., Koga Y., Plocinski J., Benjamin W., Burns D. K. Synthesis and release of interleukin 1 by reoxygenated human mononuclear phagocytes. J Clin Invest. 1992 Sep;90(3):1007–1015. doi: 10.1172/JCI115913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lefer A. M., Tsao P. S., Lefer D. J., Ma X. L. Role of endothelial dysfunction in the pathogenesis of reperfusion injury after myocardial ischemia. FASEB J. 1991 Apr;5(7):2029–2034. doi: 10.1096/fasebj.5.7.2010056. [DOI] [PubMed] [Google Scholar]
- Liu X. K., Engelman R. M., Iyengar J., Cordis G. A., Das D. K. Amiloride enhances postischemic ventricular recovery during cardioplegic arrest. A possible role of Na(+)-Ca2+ exchange. Ann N Y Acad Sci. 1991;639:471–474. doi: 10.1111/j.1749-6632.1991.tb17338.x. [DOI] [PubMed] [Google Scholar]
- Lowenstein C. J., Snyder S. H. Nitric oxide, a novel biologic messenger. Cell. 1992 Sep 4;70(5):705–707. doi: 10.1016/0092-8674(92)90301-r. [DOI] [PubMed] [Google Scholar]
- Lucchesi B. R., Mullane K. M. Leukocytes and ischemia-induced myocardial injury. Annu Rev Pharmacol Toxicol. 1986;26:201–224. doi: 10.1146/annurev.pa.26.040186.001221. [DOI] [PubMed] [Google Scholar]
- Ma X. L., Tsao P. S., Lefer A. M. Antibody to CD-18 exerts endothelial and cardiac protective effects in myocardial ischemia and reperfusion. J Clin Invest. 1991 Oct;88(4):1237–1243. doi: 10.1172/JCI115427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowka L., Zerbe T. R., Chapman F., Qian S. G., Sun H., Murase N., Kormos R., Snyder J., Starzl T. E. Prolonged rat cardiac preservation with UW lactobionate solution. Transplant Proc. 1989 Feb;21(1 Pt 2):1350–1352. [PMC free article] [PubMed] [Google Scholar]
- Malhorta D., Zhou H. Z., Kong Y. L., Shapiro J. I., Chan L. Improvement in experimental cardiac preservation based on metabolic considerations. Transplantation. 1991 Dec;52(6):1004–1008. doi: 10.1097/00007890-199112000-00013. [DOI] [PubMed] [Google Scholar]
- Marivet M. C., Bourguignon J. J., Lugnier C., Mann A., Stoclet J. C., Wermuth C. G. Inhibition of cyclic adenosine-3',5'-monophosphate phosphodiesterase from vascular smooth muscle by rolipram analogues. J Med Chem. 1989 Jul;32(7):1450–1457. doi: 10.1021/jm00127a009. [DOI] [PubMed] [Google Scholar]
- Mathew R., Omar H. A., Cherry P. D., Gewitz M. H., Wolin M. S. Role of cGMP mechanisms in response of rat pulmonary arteries to hypoxia. Am J Physiol. 1992 Jul;263(1 Pt 2):H141–H146. doi: 10.1152/ajpheart.1992.263.1.H141. [DOI] [PubMed] [Google Scholar]
- Minnear F. L., Johnson A., Malik A. B. Beta-adrenergic modulation of pulmonary transvascular fluid and protein exchange. J Appl Physiol (1985) 1986 Jan;60(1):266–274. doi: 10.1152/jappl.1986.60.1.266. [DOI] [PubMed] [Google Scholar]
- Morris S. A., Tanowitz H., Factor S. M., Bilezikian J. P., Wittner M. Myocardial adenylate cyclase activity in acute murine Chagas' disease. Circ Res. 1988 Apr;62(4):800–810. doi: 10.1161/01.res.62.4.800. [DOI] [PubMed] [Google Scholar]
- Mullane K. M., Read N., Salmon J. A., Moncada S. Role of leukocytes in acute myocardial infarction in anesthetized dogs: relationship to myocardial salvage by anti-inflammatory drugs. J Pharmacol Exp Ther. 1984 Feb;228(2):510–522. [PubMed] [Google Scholar]
- Ogawa S., Gerlach H., Esposito C., Pasagian-Macaulay A., Brett J., Stern D. Hypoxia modulates the barrier and coagulant function of cultured bovine endothelium. Increased monolayer permeability and induction of procoagulant properties. J Clin Invest. 1990 Apr;85(4):1090–1098. doi: 10.1172/JCI114540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S., Koga S., Kuwabara K., Brett J., Morrow B., Morris S. A., Bilezikian J. P., Silverstein S. C., Stern D. Hypoxia-induced increased permeability of endothelial monolayers occurs through lowering of cellular cAMP levels. Am J Physiol. 1992 Mar;262(3 Pt 1):C546–C554. doi: 10.1152/ajpcell.1992.262.3.C546. [DOI] [PubMed] [Google Scholar]
- Oksendal A. N., Jynge P. Protection by verapamil in the calcium paradox: dependence on micromolar calcium. Cardiovasc Res. 1986 Nov;20(11):845–852. doi: 10.1093/cvr/20.11.845. [DOI] [PubMed] [Google Scholar]
- Ono K., Lindsey E. S. Improved technique of heart transplantation in rats. J Thorac Cardiovasc Surg. 1969 Feb;57(2):225–229. [PubMed] [Google Scholar]
- Rascón A., Lindgren S., Stavenow L., Belfrage P., Andersson K. E., Manganiello V. C., Degerman E. Purification and properties of the cGMP-inhibited cAMP phosphodiesterase from bovine aortic smooth muscle. Biochim Biophys Acta. 1992 Mar 16;1134(2):149–156. doi: 10.1016/0167-4889(92)90038-d. [DOI] [PubMed] [Google Scholar]
- Rothermel J. D., Parker Botelho L. H. A mechanistic and kinetic analysis of the interactions of the diastereoisomers of adenosine 3',5'-(cyclic)phosphorothioate with purified cyclic AMP-dependent protein kinase. Biochem J. 1988 May 1;251(3):757–762. doi: 10.1042/bj2510757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W., Engler R. L. Granulocytes as active participants in acute myocardial ischemia and infarction. Am J Cardiovasc Pathol. 1987 Jan;1(1):15–30. [PubMed] [Google Scholar]
- Seibert A. F., Thompson W. J., Taylor A., Wilborn W. H., Barnard J., Haynes J. Reversal of increased microvascular permeability associated with ischemia-reperfusion: role of cAMP. J Appl Physiol (1985) 1992 Jan;72(1):389–395. doi: 10.1152/jappl.1992.72.1.389. [DOI] [PubMed] [Google Scholar]
- Shatos M. A., Doherty J. M., Hoak J. C. Alterations in human vascular endothelial cell function by oxygen free radicals. Platelet adherence and prostacyclin release. Arterioscler Thromb. 1991 May-Jun;11(3):594–601. doi: 10.1161/01.atv.11.3.594. [DOI] [PubMed] [Google Scholar]
- Shatos M. A., Doherty J. M., Orfeo T., Hoak J. C., Collen D., Stump D. C. Modulation of the fibrinolytic response of cultured human vascular endothelium by extracellularly generated oxygen radicals. J Biol Chem. 1992 Jan 5;267(1):597–601. [PubMed] [Google Scholar]
- Shatos M. A., Doherty J. M., Stump D. C., Thompson E. A., Collen D. Oxygen radicals generated during anoxia followed by reoxygenation reduce the synthesis of tissue-type plasminogen activator and plasminogen activator inhibitor-1 in human endothelial cell culture. J Biol Chem. 1990 Nov 25;265(33):20443–20448. [PubMed] [Google Scholar]
- Shreeniwas R., Koga S., Karakurum M., Pinsky D., Kaiser E., Brett J., Wolitzky B. A., Norton C., Plocinski J., Benjamin W. Hypoxia-mediated induction of endothelial cell interleukin-1 alpha. An autocrine mechanism promoting expression of leukocyte adhesion molecules on the vessel surface. J Clin Invest. 1992 Dec;90(6):2333–2339. doi: 10.1172/JCI116122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreeniwas R., Ogawa S., Cozzolino F., Torcia G., Braunstein N., Butura C., Brett J., Lieberman H. B., Furie M. B., Joseph-Silverstein J. Macrovascular and microvascular endothelium during long-term hypoxia: alterations in cell growth, monolayer permeability, and cell surface coagulant properties. J Cell Physiol. 1991 Jan;146(1):8–17. doi: 10.1002/jcp.1041460103. [DOI] [PubMed] [Google Scholar]
- Simpson P. J., Schelm J. A., Smallwood J. K., Clay M. P., Lindstrom T. D. Inhibition of granulocyte cAMP-phosphodiesterase by rolipram in vivo is not sufficient to protect the canine myocardium from reperfusion injury. J Cardiovasc Pharmacol. 1992 Jun;19(6):987–995. doi: 10.1097/00005344-199206000-00022. [DOI] [PubMed] [Google Scholar]
- Stelzner T. J., Weil J. V., O'Brien R. F. Role of cyclic adenosine monophosphate in the induction of endothelial barrier properties. J Cell Physiol. 1989 Apr;139(1):157–166. doi: 10.1002/jcp.1041390122. [DOI] [PubMed] [Google Scholar]
- Stewart J. R., Frist W. H., Merrill W. H. Oxygen scavengers in myocardial preservation during transplantation. Methods Enzymol. 1990;186:742–748. doi: 10.1016/0076-6879(90)86173-s. [DOI] [PubMed] [Google Scholar]
- Suttorp N., Weber U., Welsch T., Schudt C. Role of phosphodiesterases in the regulation of endothelial permeability in vitro. J Clin Invest. 1993 Apr;91(4):1421–1428. doi: 10.1172/JCI116346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson D. K., Pasaoglu I., Berkoff H. A., Southard J. A., Hegge J. O. Improved heart preservation with UW preservation solution. J Heart Transplant. 1988 Nov-Dec;7(6):456–467. [PubMed] [Google Scholar]
- Tanaka K., Gotoh F., Fukuuchi Y., Amano T., Uematsu D., Kawamura J., Yamawaki T., Itoh N., Obara K., Muramatsu K. Effects of a selective inhibitor of cyclic AMP phosphodiesterase on the pial microcirculation in feline cerebral ischemia. Stroke. 1989 May;20(5):668–673. doi: 10.1161/01.str.20.5.668. [DOI] [PubMed] [Google Scholar]
- Thompson W. J. Cyclic nucleotide phosphodiesterases: pharmacology, biochemistry and function. Pharmacol Ther. 1991;51(1):13–33. doi: 10.1016/0163-7258(91)90039-o. [DOI] [PubMed] [Google Scholar]
- Wahlberg J. A., Southard J. H., Belzer F. O. Development of a cold storage solution for pancreas preservation. Cryobiology. 1986 Dec;23(6):477–482. doi: 10.1016/0011-2240(86)90056-8. [DOI] [PubMed] [Google Scholar]
- Warnick C. T., Lazarus H. M. Adenine nucleotides during organ storage. Transplant Proc. 1977 Sep;9(3):1575–1577. [PubMed] [Google Scholar]
- Wickens D. G., Li M. K., Atkins G., Fuller B. J., Hobbs K. E., Dormandy T. L. Free radicals in hypothermic rat heart preservation--prevention of damage by mannitol and desferrioxamine. Free Radic Res Commun. 1987;4(3):189–195. doi: 10.3109/10715768709088104. [DOI] [PubMed] [Google Scholar]
- Zimmer S. D., Uğurbil K., Michurski S. P., Mohanakrishnan P., Ulstad V. K., Foker J. E., From A. H. Alterations in oxidative function and respiratory regulation in the post-ischemic myocardium. J Biol Chem. 1989 Jul 25;264(21):12402–12411. [PubMed] [Google Scholar]
- Zweier J. L., Kuppusamy P., Lutty G. A. Measurement of endothelial cell free radical generation: evidence for a central mechanism of free radical injury in postischemic tissues. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4046–4050. doi: 10.1073/pnas.85.11.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier J. L. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J Biol Chem. 1988 Jan 25;263(3):1353–1357. [PubMed] [Google Scholar]




